Abstract
In this research, an innovative Z-scheme vanadium pentoxide (V2O5)/graphitic carbon nitride (g-C3N4) photocatalyst was synthesised using a facile thermal treatment method, and its photodegradation performance and physicochemical properties were evaluated. The heterostructure provided high Brunauer–Emmett–Teller surface area and pore volume, which encouraged charge carrier separation and transfer, as well as supplied abundant micro-mesoporous structures and active sites for photocatalytic redox reactions. The successful incorporation of V2O5 between g-C3N4 layers can be proven by proposing the synthesis mechanism, as well as conducting morphology, crystal structure, elemental, and chemical analysis through scanning electron microscopy, X-ray diffraction, energy dispersive X-ray spectroscopy, and X-ray photoelectron spectroscopy, respectively. Using these combined photocatalysts, ciprofloxacin (CIP) was successfully degraded up to 90.17% removal efficiency in the visible-light spectrum. The superior photocatalytic activity of g-C3N4 composite over V2O5 is primarily due to its increased light absorption capacity, as well as increased surface area, pore size, and volume, effective charge transfer, and optimal band alignment between g-C3N4 and V2O5. This research provides a significant future perspective for the utilisation of Z-scheme V2O5/g-C3N4 heterojunction photocatalyst for water treatment, especially those involving endocrine-disrupting compounds and antibiotics like CIP.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Endocrine-disrupting compounds like pharmaceuticals and personal care products (PCPPs) are substances that bind to endocrine receptors in the body, activating, inhibiting, and breaking down natural hormone synthesis, resulting in aberrant hormonal signals that can impede normal endocrine function even at low dosages [1, 2]. Ciprofloxacin (CIP), a broad-spectrum antibiotic, is the most commonly used PCPP that works by stopping bacterial cell replication and restricting their growth [3, 4]. The CIP levels recorded in surface water ranged from 2.45 × 104 to 6.3 × 104 mg/L, whereas hospital effluent levels ranged from 7.0 to 0.1245 mg/L [2]. Meanwhile, global CIP concentrations in surface water have been reported to range between 0.0018 and 19,617 nmol/L [3]. The physical method of removing CIP pollutants from aqueous solutions, such as membrane filtration, is not recommended due to the need for a large process space, high operating costs, and longer processing time, and may generate secondary pollutants [4,5,6]. On the other hand, conventional chemical methods (e.g., chemical precipitation, coagulation, and flocculation) are not economically feasible due to their high ability and tendency to produce a high amount of sludge [7]. In addition, most conventional biological treatments (e.g., activated sludge and membrane bioreactor) are also ineffective due to CIP resistance to biological degradation [8].
In current trends, much attention has been drawn to advanced oxidation processes (AOPs) like Fenton, ozone, and photocatalysis, which are known as promising alternatives to the previously mentioned treatments due to rapid non-selective reactions of hydroxyl radicals (HO•) with pollutants regardless of the size and structure, as well as high reactivity of HO• to decompose organic pollutants into stable and less harmful inorganic compounds (e.g., water, carbon dioxide, and salts), making them fast and effective CIP removal treatment processes without generating additional waste [9, 10]. Moreover, AOPs are deemed infrastructure-friendly due to their non-toxicity and short lifetime of HO• [11]. Among them, photocatalysis has emerged as a viable solar-driven AOP technology, with advantages such as more effective pollutant removal at ambient temperature and pressure, no water disposal difficulties, requires a small space, and low costs [12, 13]. Various semiconductors have been investigated for use as photocatalysts in heterogeneous photocatalysis, such as TiO2, ZnO, gallium arsenide, WO3, gallium phosphide, CdS, BiPO4, GCN, Ag3PO4, BiVO4, tin oxide, vanadium oxide, and bismuth oxide [14,15,16].
Graphitic carbon nitride (g-C3N4) is highly attractive among other mentioned semiconductors above due to its beneficial properties, including efficient visible-light absorption with high photoactivity, high thermal and chemical stability, high electronic conductivity, and moderate band gap energy of 2.7 eV (460 nm) [17]. It has been revealed that g-C3N4 could exhibit photocatalytic activity for CIP degradation up to 53% for 100 min with the removal rate constant of 0.0071/min under visible-light radiation from a 300 W Xenon lamp with a cut-off filter (λ > 420 nm) [18]. However, pristine g-C3N4 suffers from a low specific surface area and high charge carrier recombination that may inhibit its photocatalytic performance in the long term [19, 20]. Among various mentioned metal oxide-based semiconductors, vanadium oxide (V2O5) has received much attention due to its outstanding properties, such as narrow band gap, strong oxidising power, long-term stability against photo and chemical decay, and high chemical stability [21]. It has been found that V2O5 could degrade 53% and 35.68% of CIP in 50 and 120 min with the constant rate of 0.12 and 0.02556/min when exposed to ultraviolet (UV) and visible light, respectively, indicating a great potential of V2O5 photocatalyst in antibiotic degradation [22, 23]. However, the maximum photoactivity of V2O5 is inhibited by the short lifespan of electron–hole pairs due to their rapid recombination [24].
Therefore, a potential approach is needed to address challenging issues occurring in both g-C3N4 and V2O5 photocatalysts for energy-efficient degradation of antibiotics. The appropriate match of the conduction band (CB) (0.47 eV) and valence band (VB) (2.73 eV) of V2O5 and the CB (− 1.2 eV) and VB (1.5 eV) of g-C3N4 resulted in the fabrication of a heterojunction with certain defects in the crystalline structure but promoting the efficiency of charge carrier separation, broad light-harvesting, and tremendous redox ability [25, 26]. Numerous researchers have reported the performance of V2O5/g-C3N4 heterojunction photocatalysts in the photodegradation of antibiotics, as summarised in Table 1. It can be seen that the combination of V2O5 and g-C3N4 can degrade antibiotic pollutants with significant photodegradation performance percentages in different photocatalysis conditions and heterojunction schemes.
Table 1 presents Z-scheme and S-scheme heterojunctions in recent studies. Z-scheme heterojunction is different to S-scheme heterojunction in terms of the pairing type of photocatalysts. The oxidative and reductive photocatalysts represented by the S-scheme heterojunction are made up mostly of two n-type semiconductors and generate electron–hole pairs simultaneously after light absorption. Meanwhile, a direct Z-scheme heterojunction can also be made up of a p-type semiconductor and an n-type semiconductor and generate electron–hole pairs sequentially when light is captured [25, 27]. By referring to this literature review, no study of V2O5/g-C3N4 heterojunction photocatalysts on CIP antibiotics has been conducted, especially in heterojunction schemes that can be obtained by the incorporation of mechanisms between V2O5 and g-C3N4 during synthesis, which enables the degradation of CIP antibiotics. Thus, in this study, Z-scheme heterojunction nanocomposites were synthesised by coupling V2O5 with g-C3N4 using a simple thermal treatment to achieve a synergistic effect with highly desired surface and pore, morphological, and optical properties for photocatalysis application in antibiotic treatment. To the best of our knowledge, there is no published study regarding the use of V2O5/g-C3N4 to degrade CIP antibiotics with efficient energy under visible irradiation. The principles of the V2O5/g-C3N4 synthesis procedure and its exceptional physicochemical properties were justified. Various V2O5 loadings on g-C3N4 were also investigated to determine their influence on photocatalytic performance in degrading CIP under visible-light illumination. Finally, the potential charge carrier photogeneration and charge transfer pathway were provided for the prepared V2O5/g-C3N4. This has opened the opportunity for researchers to further study and better understand the excellent ability of this synthesis method for the preparation of efficient photocatalysts for the degradation of not only CIP but also various organic pollutants.
Experimental Section
Chemicals and Materials
Melamine and ammonium metavanadate (NH4VO3, Merck, United States) were used in the preparation of photocatalysts. The CIP reference standard was purchased from Sigma (United States). For pH adjustment purposes, sodium hydroxide and sulphuric acid were obtained from Merck (United States).
Synthesis of g-C3N4 and V2O5
First, 15 g of melamine was added to a crucible, which was then covered with aluminium foil and its lid to prepare g-C3N4. The crucible containing melamine was heated in a muffle furnace (RWF 12/5, Carbolite Gero, UK) at 550 °C with a heating rate of 3 °C/min for 120 min [30]. The products were cooled naturally to room temperature and then ground into fine powder. The sample was stored in a desiccator prior to the experiment. Meanwhile, pure V2O5 was prepared by putting 5 g of NH4VO3 into a crucible and was processed for thermal treatment in a muffle furnace. The thermal treatment was done at 550 °C with a heating rate of 5 °C/min for 60 min [29].
Synthesis of V2O5/g-C3N4
To synthesise the binary photocatalyst of V2O5/g-C3N4, different loading ratios (0.5, 1, 2, and 5 wt%) prepared using different amounts of NH4VO3 (0.005, 0.010, 0.020, and 0.050 g) were added into 1 g of g-C3N4 powder and were ground using an agate mortar. Then, the ground mixed powder was placed in a covered crucible for calcination. The crucible was heated at a calcination temperature of 500 °C based on preliminary studies in a muffle furnace at a rate of 5 °C/min for 180 min.
Characterisation
A quantitative and qualitative investigation was carried out to better understand the mechanism and photocatalytic reaction of the photocatalysts synthesised. The band gap analysis was analysed using ultraviolet–visible-near infrared diffuse reflectance spectra (UV–Vis-NIR DRS, UV-3600i Plus, Shimadzu, USA). Scanning Electron Microscopy (SEM, JSM-7600F, Jeol, USA) was used to elucidate the morphology of V2O5/g-C3N4 photocatalyst. Meanwhile, X-ray diffraction (XRD, Rigaku, SmartLab, USA) was used to analyse the crystallinity of the photocatalyst. Energy dispersive X-ray (EDX, EOL JSM-IT300LV, Jeol, USA) was used to analyse the elemental composition of V2O5/g-C3N4 together with elemental colour mapping. An X-ray photoelectron spectrometer (XPS, Axis Ultra DLD, Shimadzu, USA) was used to analyse the chemical composition of the sample. Lastly, Brunauer-Emmett -Teller (BET) method (ASTM D 4641-12, Thermo Scientific, USA) was applied to characterise the binary photocatalyst V2O5/g-C3N4 in terms of surface area, pore volume, and pore size.
Evaluation of Photocatalytic Activity
The photocatalytic activity of the synthesised V2O5/g-C3N4 was determined based on the reduction of CIP when irradiated under visible light. To prevent light from escaping, the reaction was conducted in a closed reactor complete with a visible 100 W LED lamp source. In the day photocatalytic process, 0.1 g of the as-prepared photocatalyst was dispersed into a 250 mL beaker containing 100 mL of 10 mg/L initial CIP concentration at pH 7. Firstly, the solution was stirred for 60 min in the dark to determine the maximum duration of the photocatalyst to achieve the adsorption–desorption equilibrium of CIP. This was followed by sampling every 15 min, where the sample was filtered through a 0.22 µm syringe filter, and the concentration of residual CIP was determined using a UV–Vis spectrophotometer (Lambda 35, Perkin Elmer, USA). After obtaining the maximum duration of the amount of CIP that could be adsorbed by the prepared photocatalyst, the photocatalysis was started by switching on the light. The photocatalytic activity of the photocatalyst to degrade CIP was performed for 150 min, and every 30 min, the sample was filtered and the concentration of residual CIP was determined. The measurement of photodegradation performance was determined using Eq. 1:
where C0 is the initial CIP concentration and C is the residual CIP concentration.
Results and Discussion
Photocatalyst Characterisation
The UV–Vis-NIR absorption spectra of V2O5, g-C3N4, and V2O5/g-C3N4 at different loading ratios were analysed, and the band gap energies for all samples were calculated using Tauc plot. Figure 1a depicts the absorption spectra of V2O5, g-C3N4, and V2O5/g-C3N4. It can be observed that g-C3N4 has an absorption edge around 400–430 nm, while V2O5 exhibits an absorption edge at about 550–600 nm, which is attributed to the narrow band gap of V2O5. For all V2O5/g-C3N4 photocatalysts, the absorption edge in the 450–500 nm range slightly shifted to longer wavelengths compared to g-C3N4. The absorption intensities of V2O5/g-C3N4 increased as V2O5 percentages increased, indicating that the presence of V2O5 affects their absorption intensities. It is notable that the V2O5/g-C3N4 composites exhibit a broad background absorption in the visible-light region. Figure 1b shows the band gap energy of all samples, which were calculated using the Tauc plot equation, (αhv)1/n = A(hv − Eg). The symbol α is the adsorption coefficient, h is the Plank constant, v is the photon frequency, A is a constant relative to the material, and n is the electronic transition parameter of 1/2 for direct bandgap semiconductor. The band gap energy, also known as the Eg value, was estimated by extrapolating the peak linear portion of the (αhv)2-hv curve to the energy axis. As shown in Fig. 1b, the band gaps of all samples were 2.71, 1.95, 2.61, 2.64, 2.62, and 2.73 eV for g-C3N4, V2O5, 5 wt% V2O5/g-C3N4, 2 wt% V2O5/g-C3N4, 1 wt% V2O5/g-C3N4, and 0.5 wt% V2O5/g-C3N4, respectively. The band gap analysis showed that increasing V2O5 leads to a narrower band gap value than pure g-C3N4 and correlates to higher visible-light absorption, indicating a good potential photocatalyst. It was also proved that the contribution of V2O5 to a combined sample of V2O5/g-C3N4 leads to band gap alteration. The overall performance showed that all samples have a spectral response in the visible region based on their band gap values of less than 3.0 eV, which are nearly identical to previous research [31]. Furthermore, as V2O5/g-C3N4 doping increased, the band gap narrowed down, which could be due to the band gap mixing effect of the two, as well as the interaction of the two semiconductors at the interface, or the increase in V2O5 content and the concomitant increase in functional groups, which affects the crystalline morphology of g-C3N4 and V2O5. The affected crystalline contributes to different light absorption and band gap modifications for varied loading ratios of the samples [32].
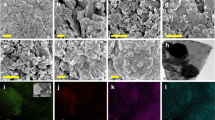
After the band gap values of all the samples have been confirmed in visible-light wavelength, only the best loading ratio of V2O5/g-C3N4 based on CIP degradation performance was used for SEM, BET, and EDX analysis together with pure V2O5 and g-C3N4. Based on the CIP degradation performance shown in Fig. 2a and b, the best loading ratio of V2O5/g-C3N4 sample is 2 wt%. Thus, only 2 wt% V2O5/g-C3N4 proceeded for SEM analysis together with V2O5 and g-C3N4. Figure 3a–c show the SEM images of g-C3N4, V2O5, and 2 wt% V2O5/g-C3N4, respectively. Figure 3a shows that the morphology of g-C3N4 is an irregular bulk structure with a rough surface [33], whereas the morphology of V2O5 is irregularly shaped with a flakes-like structure. The SEM images of the V2O5 sample are consistent with previous reports [34]. The successful combination of g-C3N4 and V2O5 in 2 wt% V2O5/g-C3N4 in Fig. 3c was proved by the presence of V2O5 flakes-like shaped, which are distributed non-uniformly on the bulk surface of g-C3N4. The SEM images of 2 wt% V2O5/g-C3N4 also showed a massive compact rough structure with pores on its surface. A stable rough surface of g-C3N4 that appeared in the SEM images helped the agglomeration of V2O5 nanoparticles on it, resulting in firm heterojunction relation between V2O5 and g-C3N4 samples during calcination [35]. The rough surface of 2 wt% V2O5/g-C3N4 is due to the polymerisation of g-C3N4 and the incorporation of V2O5 nanoparticles into g-C3N4 layers, which can be attributed to the oxide phase present during calcination [31].
The N2 adsorption/desorption analysis was performed to analyse the surface area, pore structure, and pore volume of the prepared photocatalysts, as shown in Fig. 4a and b and summarised in Table 2. According to the International Union of Pure and Applied Chemistry classification, the pristine g-C3N4, V2O5, and 2 wt% V2O5/g-C3N4 photocatalyst exhibited an H4 hysteresis loop and type III isotherm (see Fig. 4a and b), indicating the presence of a micro-mesoporous structure and that the adsorbed molecules are clustered around the most favourable sites on the surface of a non-porous or macroporous solid [36]. Adsorption increased for the 2 wt% V2O5/g-C3N4 photocatalyst at low P/P0 due to enhanced adsorbent adsorptive interactions in the narrow micropores, showing unrestricted monolayer-multilayer adsorption at high P/P0 despite being limited by the accessible micropore volume [36]. The plotted graph in differential form (dV/dD) in Fig. 4b clearly demonstrates that the combination of V2O5 and g-C3N4 has a significant effect on its differential (dV/dD) distribution data with 0.0239 cm3/g/nm when compared to V2O5 and g-C3N4 alone with 0.0001 and 0.0004 cm3/g/nm, respectively. As a result, high pore volume and mesoporous diameter are formed, which play important roles in the excellent photodegradation of CIP by V2O5/g-C3N4 photocatalyst [37]. Other research findings of the combination of V2O5 and g-C3N4 with higher surface area and pore size compared to V2O5 and g-C3N4 alone supported previous results [25, 28]. The BET surface areas of 2 wt% V2O5/g-C3N4, V2O5, and g-C3N4 were 181.00, 4.77, and 7.70 m2/g, respectively, as summarised in Table 2. The surface area of the combined materials was 25 times greater than that of V2O5 and g-C3N4 alone. The large surface area of 2 wt% V2O5/g-C3N4 resulted in excellent CIP adsorption due to the photocatalyst surface and efficient light trapping during the photocatalysis of CIP degradation [29]. The considerable contribution of 2 wt% V2O5/g-C3N4 is due to its large surface area after the combination of V2O5 and g-C3N4.
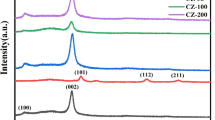
The successful incorporation of V2O5 into the g-C3N4 structure can also be determined by EDX analysis, which showed that 2 wt% V2O5/g-C3N4 has the elemental C, N, O, and V composition desired, with C and V elements dominating the photocatalyst (37% and 1.6%, respectively), as displayed in Fig. 5. The low percentage of V in 2 wt% V2O5/g-C3N4 is due to the low loading amount of V2O5 calcined together with g-C3N4 during its synthesis. Furthermore, the EDX mapping analysis in Fig. 6a–c also shows various elemental mappings of C, N, O, and V with different colour mappings. The brighter images indicated that higher concentrations of elements were well distributed in the sample. The results proved the successful formation of V2O5/g-C3N4. The XRD analysis was conducted for the samples of g-C3N4, V2O5, 0.5 wt% V2O5/g-C3N4, 1 wt% V2O5/g-C3N4, 2 wt% V2O5/g-C3N4, and 5 wt% V2O5/g-C3N4. Figure 7 shows the XRD pattern for g-C3N4 with two typical weak and strong peaks at 12.9° and 27.6° conforming to (100) in-plane structural units and the stacking layer along the (002) plane, respectively [31]. Meanwhile, the orthorhombic structure of V2O5 is well-matched to its diffraction peaks. The four diffraction peaks at 20.3°, 26.1°, 31.0°, and 47.3° are attributed to the (001), (110), (301), and (600) crystal planes of V2O5, respectively, and are consistent with those of pure V2O5. Figure 7 also demonstrates that all V2O5/g-C3N4 nanocomposites with different weight ratios (0.5, 1, 2, and 5 wt%) have well-defined diffraction peaks for V2O5 and g-C3N4 structures without impurity phase. By increasing the amount of V2O5, the XRD pattern of V2O5/g-C3N4 nanocomposites slightly shifted to a higher value of 2θ for the (002) crystal lattice of g-C3N4, hence lowering its interplanar spacing. This phenomenon can be explained by the insertion of oxygen heteroatoms from V2O5 into the graphitic structure. A similar phenomenon was discovered by Pisanu et al. [38], where g-C3N4 was oxidised by HNO3 during reflux, leading to a shift of (002) lattice to a higher angle, which corresponds to the incorporation of oxygen atoms into the g-C3N4 structure and may create electron-rich states in the oxidised sample [38]. The XRD patterns demonstrate an efficient synthesis of heterostructure nature composites, which may be the result of minor structural modifications caused by mutual interactions.
Similar results were observed in the XPS results of 2 wt% V2O5/g-C3N4 in Fig. 8a, which exhibit characteristic peaks at 289.9, 293.1, 401.4, 517.6, 524.7, and 529.9 eV corresponding to the elements of C 1 s, N 1 s, V 2p, and O 1 s, respectively, indicating the presence of C, N, V, and O elements together. Meanwhile, the V 2p peaks in Fig. 8b fitted into two peaks with binding energies of 524.7 and 517.6 eV, which can be ascribed to V 2p1/2 and V 2p3/2 of the V2O5 sample [29]. From Fig. 8c, the C 1 s peaks at 289.8 and 293.1 eV can be assigned to N–C–N coordination in the g-C3N4 phase, and N 1 s signals at 401.4 eV can be assigned to C=N–C, as shown in Fig. 8d [28]. Meanwhile, in Fig. 8e, at 529.9 eV, the O 1 s peak of V2O5 and 2 wt% V2O5/g-C3N4 can be attributed to O2 in the V–O bond [25]. Many changes in binding energy occurred after the incorporation of V2O5 into g-C3N4 layers, where V 2p and O 1 s moved into lower binding energy, whereas C 1 s and N 1 s shifted into higher binding energy. Therefore, the formation of heterojunction V2O5/g-C3N5 is related to the electron density variation, giving direct evidence for carrier migration across the interface between V2O5 and g-C3N4 [26].
It has been proposed that the synergistic impact of g-C3N4 exfoliation and the incorporation of V2O5 crystal into the g-C3N4 structure make the V2O5/g-C3N4 formation feasible with high surface area and pore volume, which can be illustrated in the form of synthesis mechanism as in Fig. 9a and b. The fundamental of V2O5 formation (Fig. 9a) is the thermal decomposition of NH4VO3 compound as a precursor above 260 °C, as stated by Twu et al. [39]. The initial stage decomposition of NH4VO3 leads to the formation of disordered structure x(NH4)2O·yV2O5 with the ratio of x:y is 1:1. A further step of this decomposition results in the random removal of NH4+ ions from the disordered structure and the decrease of x:y ratio, with changes in the V–O structure reduce the symmetry of the NH4+ ion environment and oxygen is involved in the release of H2O from the V–O network; consequently, V2O5 becomes the final product of this decomposition [40]. Subsequently, the thermal exfoliation of bulk g-C3N4 through layer-by-layer oxidation and the structural formation of V2O5 on the surface of g-C3N4 occurred simultaneously at 500 °C, as shown in Fig. 9b [41]. Finally, based on the XRD results, the V2O5/g-C3N4 heterostructure emerged, which can be distinguished by the reduction of (002) lattice spacing on the g-C3N4 structure.
Photocatalysis Performance of CIP
The photocatalytic activity of the photocatalysts was assessed for the photodegradation of CIP under visible-light LED irradiation. This is because CIP will not degrade by photolysis in the absence of a catalyst under light. To guarantee adsorption equilibrium, the as-prepared samples were immersed in the CIP solution for 60 min in the dark. As can be seen in Fig. 2a, all samples reached adsorption–desorption equilibrium in 45–60 min, while the 2 wt.% V2O5/g-C3N4 photocatalyst eliminated about 43.4% of CIP without light irradiation. The adsorption capacity of 2 wt% V2O5/g-C3N4 improved first and then declined with increasing V2O5 concentration. After 60 min of adsorption in the dark, the reactor was exposed to a visible 100 W LED lamp, and the photocatalytic breakdown of CIP by 2 wt% V2O5/g-C3N4 was performed for 150 min [23]. From Fig. 2a, the percentage removal of CIP by 2 wt% V2O5/g-C3N4 achieved the highest degradation efficiency of 90.17%. In contrast, the photodegradation rates of CIP in Fig. 2a and b for g-C3N4, V2O5, 5 wt% V2O5/g-C3N4, 2 wt% V2O5/g-C3N4, 1 wt% V2O5/g-C3N4, and 0.5 wt% V2O5/g-C3N4 photocatalyst samples at 150 min were 26%, 29%, 76%, 90.17%, 79%, and 66%, respectively. It can also be seen that an increase in V2O5 content leads to an increase in the photodegradation performance of CIP. However, any additional increase of V2O5 loading of more than 2% hindered the photodegradation of CIP, as can be seen in Fig. 2a for 5 wt% V2O5/g-C3N4. Excessive V2O5 on the surface of g-C3N4 prevents light absorption and obscures the active sites when the V2O5 level is greater than 2 wt% [29]. It is supported by a study by Li et al. [42] also reported the same pattern of photodegradation performance as an increase in the loading ratio of different photocatalysts led to lower photodegradation performance due to the inhibition of light absorption.
To further compare the photodegradation capability of the photocatalyst samples, the results of CIP degradation were fitted using the pseudo-first-order reaction kinetic model: Ln(C0/Ct) = kappt, where t and kapp are the degradation time and the apparent reaction rate constant, respectively [43]. The k constant rate values (Fig. 2c) of CIP for g-C3N4, V2O5, 5 wt% V2O5/g-C3N4, 2 wt% V2O5/g-C3N4, 1 wt% V2O5/g-C3N4, and 0.5 wt% V2O5/g-C3N4 were 0.0025, 0.0026, 0.0076, 0.0102, 0.0110, and 0.0107/min, respectively. The k value of the 2 wt% V2O5/g-C3N4 sample is 4.1 times superior to that of g-C3N4 and V2O5 samples. These results validate that the combination of g-C3N4 and V2O5 enhances visible-light utilisation and could degrade CIP efficiently compared to bare g-C3N4 and V2O5 [44]. The photodegradation performance of CIP by 2 wt% V2O5/g-C3N4 is determined to be considerable with 90.17% removal in 150 min as other studies observed similar CIP photodegradation performance. According to a report by Hunge et al. [45], the CIP photodegradation by MoS2/ZnO photocatalysts of 89% occurred in 160 min. In the meantime, Feng et al. [46] and Shen et al. [30] demonstrated CIP photodegradation performance by a g-C3N4-based photocatalyst with 87.8% and 95%, respectively. Table 3 provides a summary of CIP photodegradation performance of other photocatalysts in previous studies.
Possible CIP Photodegradation Mechanism
The VB and CB edge positions (vs. normalised hydrogen energy, NHE) of relevant V2O5 and g-C3N4 components were intended to retrieve the charge transport pathway of 2 wt% V2O5/g-C3N4. Therefore, as per the standard Mulliken electronegativity formula in Eqs. 2, 3 [53]:
where ECB, EVB, Ee, Eg and χ represent the energy levels of conduction band, energy levels of valence band, energy of free electron on the hydrogen scale, band gap energy of specified semiconductors and absolute electronegativity value respectively. Additionally, χ for g-C3N4 and V2O5 were 4.73 and 6.1 eV, respectively [31]. Comparatively, Eg of g-C3N4 and V2O5 were 2.71 and 1.95 eV, respectively, while Ee is 4.5 eV. In this case, the CB edge potential values for g-C3N4 and V2O5 were − 2.48 and − 0.35 V (vs. NHE), respectively. In addition, for g-C3N4 and V2O5 elements, the constant VB edge potential values were 0.23 and 1.6 V (vs. NHE), respectively. As the CB and VB edges are located where they are, charge carrier separation via the Z-scheme diffuses easily across the interfaces. Therefore, based on the aforementioned experimental results, the effective Z-scheme components in a 2 wt% V2O5/g-C3N4 photocatalysis system are presented in Fig. 10.
Under visible-light irradiation (λ ≥ 400 nm), electrons (e−) are excited from the VB to the CB of g-C3N4 and V2O5, leaving holes (h+) in the VB. According to the charge transfer characteristics of the Z-scheme heterojunction, the e− on the CB of V2O5 combine with the h+ on the VB of g-C3N4, thereby retaining h+ with a strong oxidation capability on the VB of V2O5 and e− with a strong reduction capability on the CB of g-C3N4. Then, the strong h+ on the VB of V2O5 could degrade the CIP pollutant in conjunction with e− on the CB of g-C3N4, reducing the surface-adsorbed O2 on the catalyst surface to the reactive species •O2−. On the other hand, they might not be oxidised at all but rather break down into various components (i.e., CO2 and H2O). As a result, more e− that persists in the CB of g-C3N4 and more h+ that persists in the VB of V2O5 react with O2 molecules and HO− to yield •O2− and HO• radicals, respectively. In addition, the charge separation might be greatly enhanced during the transfer of photoexcited e−/h+ pairs over the aforementioned two Z-scheme pathways, effectively extending the lifespan of these charges and also extending to the visible-light absorption range [54, 55]. Due to the dual Z-scheme electron transfer mechanism, e− and h+ of V2O5 were effectively separated, enhancing the photocatalytic activity and robust redox ability of the 2 wt% V2O5/g-C3N4 photocatalyst.
Regeneration of 2 wt% V2O5/g-C3N4 Photocatalyst
In the CIP photodegradation study, a regeneration test of 2 wt% V2O5/g-C3N4 photocatalyst was performed. About 0.100 g of the 2 wt% V2O5/g-C3N4 photocatalyst was used in four cycles. The photocatalyst was centrifuged with distilled water at 5000 rpm for 5 min between each cycle of the experiments and then dried at 70 °C overnight for reuse in the next cycle. Figure 11 depicts the photocatalyst's CIP photodegradation performance over multiple cycles. The 2 wt% V2O5/g-C3N4 photocatalyst still maintained an excellent photodegradation rate for CIP after four consecutive regenerations, with consistent degradation rates higher than 65–70% under visible-light exposure. In the fourth cycle, the degradation reached 65%, which was lower than 90% for the first cycle of regeneration, where the photodegradation efficiency was approximately 25% lower after three consecutive cycles. The lower photodegradation activity was caused by adsorbed intermediate by-products that blocked the active sites on the surface of 2 wt% V2O5/g-C3N4. Vignesh et al. [31] found that the photocatalytic activity was slightly reduced after five cycles of irradiation with α-Fe2O3/V2O5 and g-C3N4, while Zang et al. [32] indicated high stability in practical applications of biochar/vanadium pentoxide/graphite-like carbon nitride (BC/V2O5/g-C3N4) after five cycles with any reduction of photodegradation performance to rhodamine B dyes. Overall, although the degradation efficiency decreased in this study, the regeneration ability of spent 2 wt% V2O5/g-C3N4 was promising. The 2 wt% V2O5/g-C3N4 photocatalyst showed reasonable reusability after four consecutive cycles of CIP photodegradation. A photocatalyst is said to have excellent photochemical stability for practical applications in the degradation of various organic pollutants.
Radical Scavenging Studies
Multiple quenching experiments were carried out under optimal conditions to clarify the contribution of generated reactive species, such as e−, h+, superoxide radicals (•O2−), and hydroxyl radicals (HO•) throughout CIP photodegradation and to determine the charge transfer pathway. For this purpose, 1 mmol of Silver nitrate (AgNO3) and Ethylenediaminetetraacetic acid (EDTA) were used to scavenge e− and h+, while Benzoquinone (BZQ) and butanol at the same concentrations quenched •O2− and HO• [29]. Figure 12 depicts the prohibition effect associated with the presence of each quenching agent on CIP photodegradation. As expected, the results showed a reduction in CIP elimination efficiency in all cases to varying degrees, implying the involvement of all of the aforementioned reactive species in photodecomposition. The highest reduction in CIP photodegradation in the presence of scavenger agents was observed in the case of butanol (73.64%), which scavenged HO• radicals, while the lowest was observed in the case of BZQ (28.56%), which scavenged •O2−. Here, it can be determined that HO• radical is the lowest contributor to CIP photodegradation performance. Meanwhile, both scavenger agents AgNO3 and EDTA reduced CIP photodecomposition by 45.77% and 34.54%, respectively. It showed that e− and h+ which were scavenged by AgNO3 and EDTA, respectively, were also less likely to participate in CIP photodegradation. As a result, •O2− species is the largest contributor of radicals for CIP photodegradation, followed by positively charged holes (h+), negatively charged electrons (e−), and HO•.
Conclusions
In conclusion, a simple calcination approach was used to arrange a dual Z-scheme 2 wt% V2O5/g-C3N4 heterojunction photocatalyst. Visible-light-activated photodegradation of CIP was most effective when using the synthesised 2 wt% V2O5/g-C3N4 photocatalyst with 90.17% removal. In the case of CIP photodegradation, the k constant value for the photocatalyst composed of V2O5 and g-C3N4 was (0.0110/min), which is 4.1 times better than that of the V2O5 and g-C3N4 alone samples. The improved photodegradation was mainly due to the increased visible-light absorption ability with a lower band gap value of 2.64 eV, quicker charge separation, restrained recombination rate, and well-supported redox capability as shifted binding energy was observed in the XPS analysis of 2 wt% V2O5/g-C3N4. The synergistic interfacial contact of V2O5 with the g-C3N4 surface, as proven by the SEM, XPS, and XRD analysis, produced the dual Z-Scheme heterostructure junction. The high surface area and pore volume of 2 wt.% V2O5/g-C3N4 in the BET surface area analysis also validated the significant effect of the incorporation of V2O5 on the surface of g-C3N4 via annealing treatment. Clarification was provided on how the suggested dual Z-scheme transfer mechanism and the more conventional charge transfer method both contributed to enhanced photodegradation performance. The results from this study may provide a fresh idea for the practical synthesis of photocatalysts in treating pharmaceutical waste, particularly antibiotics in wastewater treatment plants dealing with environmental remediation.
Data availability
Data available on request from the authors.
References
X. Gao, S. Kang, R. Xiong, M. Chen, Environment-friendly removal methods for endocrine disrupting chemicals. Sustainability 12(18), 1–16 (2020). https://doi.org/10.3390/su12187615
C.A. Igwegbe, S.N. Oba, C.O. Aniagor, A.G. Adeniyi, J.O. Ighalo, Adsorption of ciprofloxacin from water: a comprehensive review. J. Ind. Eng. Chem. 93, 57–77 (2021). https://doi.org/10.1016/j.jiec.2020.09.023
R.B. Carneiro, C.A. Sabatini, Á.J. Santos-Neto, M. Zaiat, Feasibility of anaerobic packed and structured-bed reactors for sulfamethoxazole and ciprofloxacin removal from domestic sewage. Sci. Total. Environ. 678, 419–429 (2019). https://doi.org/10.1016/j.scitotenv.2019.04.437
M.K. Uddin, A review on the adsorption of heavy metals by clay minerals, with special focus on the past decade. Chem. Eng. J. 308, 438–462 (2017). https://doi.org/10.1016/j.cej.2016.09.029
T. Afzal, M.H. Isa, M. Raza Ul Mustafa, Removal of organic pollutants from produced water using Fenton oxidation. E3S Web Conf. 34, 1–7 (2018). https://doi.org/10.1051/e3sconf/20183402035
D. Zioui, H. Salazar, L. Aoudjit, P.M.M. Martins, S. Lanceros-Méndez, Polymer-based membranes for oily wastewater remediation. Polymers (Basel). (2020). https://doi.org/10.3390/polym12010042
F. Zeman, Metropolitan sustainability: understanding and improving the urban environment (Woodhead Publishing Limited, Sawston, 2012)
P. Mathur, D. Sanyal, D.L. Callahan, X.A. Conlan, F.M. Pfeffer, Treatment technologies to mitigate the harmful effects of recalcitrant fluoroquinolone antibiotics on the environ- ment and human health. Environ. Pollut. 291, 118233 (2021). https://doi.org/10.1016/j.envpol.2021.118233
J. Rodríguez-Chueca, J. Carbajo, P. García-Muñoz, Intensification of photo-assisted advanced oxidation processes for water treatment: a critical review. Catalysts 13(2), 1–36 (2023). https://doi.org/10.3390/catal13020401
A. Saravanan et al., A detailed review on advanced oxidation process in treatment of wastewater: mechanism, challenges and future outlook. Chemosphere 308(P3), 136524 (2022). https://doi.org/10.1016/j.chemosphere.2022.136524
E.M. Cuerda-Correa, M.F. Alexandre-Franco, C. Fernández-González, Advanced oxidation processes for the removal of antibiotics from water: an overview. Water 12(1), 102 (2019). https://doi.org/10.3390/w12010102
R. Saravanan, F. Gracia, A. Stephen, Basic principles, mechanism, and challenges of photocatalysis (Springer, Cham, 2017), pp.19–40
H. Liu, C. Wang, G. Wang, Photocatalytic advanced oxidation processes for water treatment: recent advances and perspective. Chem. Asian J. 15(20), 3239–3253 (2020). https://doi.org/10.1002/asia.202000895
H.N.C. Dharma et al., A review of titanium dioxide (TiO2)-based photocatalyst for oilfield-produced water treatment. Membranes (Basel) 12(3), 345 (2022). https://doi.org/10.3390/membranes12030345
R. Acharya, K. Parida, A review on TiO2/g-C3N4 visible-light-responsive photocatalysts for sustainable energy generation and environmental remediation. J. Environ. Chem. Eng. 8(4), 103896 (2020). https://doi.org/10.1016/j.jece.2020.103896
R. Wang et al., Cobalt-doped V2O5 hexagonal nanosheets for superior photocatalytic toxic pollutants degradation, Cr (VI) reduction, and photoelectrochemical water oxidation performance. Environ. Res. 217, 114923 (2023). https://doi.org/10.1016/j.envres.2022.114923
M. Ismael, A review on graphitic carbon nitride (g-C3N4) based nanocomposites: Synthesis, categories, and their application in photocatalysis. J. Alloys Compd. 846, 156446 (2020). https://doi.org/10.1016/j.jallcom.2020.156446
W. Xing et al., Construction of large-scale ultrathin graphitic carbon nitride nanosheets by a hydrogen-bond-assisted strategy for improved photocatalytic hydrogen production and ciprofloxacin degradation activity. ChemCatChem 8(17), 2838–2845 (2016). https://doi.org/10.1002/cctc.201600397
X. Li, A.F. Masters, T. Maschmeyer, Photocatalytic hydrogen evolution from silica-templated polymeric graphitic carbon nitride-is the surface area important? ChemCatChem 7(1), 121–126 (2015). https://doi.org/10.1002/cctc.201402567
S. Cao, J. Low, J. Yu, M. Jaroniec, Polymeric photocatalysts based on graphitic carbon nitride. Adv. Mater. 27(13), 2150–2176 (2015). https://doi.org/10.1002/adma.201500033
Q. Liu, C. Fan, H. Tang, X. Sun, J. Yang, X. Cheng, One-pot synthesis of g-C3N4/V2O5 composites for visible light-driven photocatalytic activity. Appl. Surf. Sci. 358, 188–195 (2015). https://doi.org/10.1016/j.apsusc.2015.09.010
J. Zia, J. Kashyap, U. Riaz, Facile synthesis of polypyrrole encapsulated V2O5 nanohybrids for visible light driven green sonophotocatalytic degradation of antibiotics. J. Mol. Liq. 272, 834–850 (2018). https://doi.org/10.1016/j.molliq.2018.10.091
V. Vasanthkumar et al., MWCNT supported V2O5 quantum dot nanoparticles decorated Bi2O3 nanosheets hybrid system: efficient visible light driven photocatalyst for degradation of ciprofloxacin. Chemosphere 306, 135505 (2022). https://doi.org/10.1016/j.chemosphere.2022.135505
T. Tahir et al., Synthesis of sponge like Gd3+ doped vanadium oxide/2D MXene composites for improved degradation of industrial effluents and pathogens. Ceram. Int. 48(2), 1969–1980 (2022). https://doi.org/10.1016/j.ceramint.2021.09.282
N. Ding et al., Fluorinated inverse opal carbon nitride combined with vanadium pentoxide as a Z-scheme photocatalyst with enhanced photocatalytic activity. Chinese Chem. Lett. 33(8), 3797–3801 (2022). https://doi.org/10.1016/j.cclet.2021.11.042
M. Preeyanghaa, V. Vinesh, B. Neppolian, Construction of S-scheme 1D/2D rod-like g-C3N4/V2O5 heterostructure with enhanced sonophotocatalytic degradation for Tetracycline antibiotics. Chemosphere (2021). https://doi.org/10.1016/j.chemosphere.2021.132380
J. Liu, X. Wei, W. Sun, X. Guan, X. Zheng, J. Li, Fabrication of S-scheme CdS-g-C3N4-graphene aerogel heterojunction for enhanced visible light driven photocatalysis. Environ. Res. 197, 111136 (2021). https://doi.org/10.1016/j.envres.2021.111136
F. Hasanvandian, A. Shokri, M. Moradi, B. Kakavandi, S. Rahman Setayesh, Encapsulation of spinel CuCo2O4 hollow sphere in V2O5-decorated graphitic carbon nitride as high-efficiency double Z-type nanocomposite for levofloxacin photodegradation. J. Hazard. Mater. 423, 127090 (2022). https://doi.org/10.1016/j.jhazmat.2021.127090
S. Le et al., V2O5 nanodot-decorated laminar C3N4 for sustainable photodegradation of amoxicillin under solar light. Appl. Catal. B Environ. 303, 120903 (2021). https://doi.org/10.1016/j.apcatb.2021.120903
J.H. Shen, T.H. Chiang, C.K. Tsai, Z.W. Jiang, J.J. Horng, Mechanistic insights into hydroxyl radical formation of Cu-doped ZnO/g-C3N4composite photocatalysis for enhanced degradation of ciprofloxacin under visible light: Efficiency, kinetics, products identification and toxicity evaluation. J. Environ. Chem. Eng. 10(2), 107352 (2022). https://doi.org/10.1016/j.jece.2022.107352
S. Vignesh et al., Investigation of heterojunction between α-Fe2O3/V2O5 and g-C3N4 ternary nanocomposites for upgraded photo-degradation performance of mixed pollutants: efficient dual Z-scheme mechanism. J. Alloys Compd. 902, 163705 (2022). https://doi.org/10.1016/j.jallcom.2022.163705
Y.N. Zang et al., A biochar-promoted V2O5/g-C3N4Z-Scheme heterostructure for enhanced simulated solar light-driven photocatalytic activity. RSC Adv. 11(25), 15106–15117 (2021). https://doi.org/10.1039/d1ra02712c
Y. Hong et al., In-situ synthesis of direct solid-state Z-scheme V2O5/g-C3N4 heterojunctions with enhanced visible light efficiency in photocatalytic degradation of pollutants. Appl. Catal. B Environ. 180, 663–673 (2016). https://doi.org/10.1016/j.apcatb.2015.06.057
A.A. Yadav, Y.M. Hunge, S.W. Kang, A. Fujishima, C. Terashima, Enhanced photocatalytic degradation activity using the V2O5/RGO composite. Nanomaterials 13(2), 1–10 (2023). https://doi.org/10.3390/nano13020338
Y. Wang, J. Yu, W. Peng, J. Tian, C. Yang, Novel multilayer TiO2 heterojunction decorated by low g-C3N4 content and its enhanced photocatalytic activity under UV, visible and solar light irradiation. Sci. Rep. 9(1), 1–14 (2019). https://doi.org/10.1038/s41598-019-42438-w
M. Thommes et al., Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC technical report). Pure Appl. Chem. 87(9–10), 1051–1069 (2015). https://doi.org/10.1515/pac-2014-1117
S.S. Lam et al., Engineering pyrolysis biochar via single-step microwave steam activation for hazardous landfill leachate treatment. J. Hazard. Mater. 390, 121649 (2020). https://doi.org/10.1016/j.jhazmat.2019.121649
A. Pisanu et al., Enhanced hydrogen photogeneration by bulk g-C3N4 through a simple and efficient oxidation route. Dalt. Trans. 47(19), 6772–6778 (2018). https://doi.org/10.1039/c8dt00276b
J. Twu, C.F. Shih, T.H. Guo, K.H. Chen, Raman spectroscopic studies of the thermal decomposition mechanism of ammonium metavanadate. J. Mater. Chem. 7(11), 2273–2277 (1997). https://doi.org/10.1039/a702968c
M.E. Brown, L. Glasser, B.V. Stewart, The thermal decomposition of ammonium metavanadate. J. Therm. Anal. 6(5), 529–541 (1974). https://doi.org/10.1007/BF01911558
Y. Li et al., Tuning and thermal exfoliation graphene-like carbon nitride nanosheets for superior photocatalytic activity. Ceram. Int. 42(16), 18521–18528 (2016). https://doi.org/10.1016/j.ceramint.2016.08.190
X. Li, Y. Qiu, Z. Zhu, T. Chen, H. Zhang, D. Yin, Construction of magnetically separable dual Z-scheme g-C3N4/α-Fe2O3/Bi3TaO7 photocatalyst for effective degradation of ciprofloxacin under visible light. Chem. Eng. J. 440, 135840 (2022). https://doi.org/10.1016/j.cej.2022.135840
H. Sun, F. Guo, J. Pan, W. Huang, K. Wang, W. Shi, One-pot thermal polymerization route to prepare N-deficient modified g-C3N4 for the degradation of tetracycline by the synergistic effect of photocatalysis and persulfate-based advanced oxidation process. Chem. Eng. J. 406, 126844 (2021). https://doi.org/10.1016/j.cej.2020.126844
X. Zhang, J. Duan, Y. Tan, Y. Deng, C. Li, Z. Sun, Insight into peroxymonosulfate assisted photocatalysis over Fe2O3 modified TiO2/diatomite composite for highly efficient removal of ciprofloxacin. Sep. Purif. Technol. 293, 121123 (2022). https://doi.org/10.1016/j.seppur.2022.121123
Y.M. Hunge, A.A. Yadav, S.W. Kang, S. Jun Lim, H. Kim, Visible light activated MoS2/ZnO composites for photocatalytic degradation of ciprofloxacin antibiotic and hydrogen production. J. Photochem. Photobiol. A Chem. 434, 114250 (2023). https://doi.org/10.1016/j.jphotochem.2022.114250
J. Feng et al., Adsorption and photocatalytic synergistic removal of ciprofloxacin on mesoporous ErFeO3/g-C3N4 heterojunction. Environ. Technol. Innov. 28, 102785 (2022). https://doi.org/10.1016/j.eti.2022.102785
D. Aguilar-Ferrer, J. Szewczyk, E. Coy, Recent developments in polydopamine-based photocatalytic nanocomposites for energy production: physico-chemical properties and perspectives. Catal. Today (2021). https://doi.org/10.1016/j.cattod.2021.08.016
M. Manasa, P.R. Chandewar, H. Mahalingam, Photocatalytic degradation of ciprofloxacin & norfloxacin and disinfection studies under solar light using boron & cerium doped TiO2 catalysts synthesized by green EDTA-citrate method. Catal. Today 375, 522–536 (2021). https://doi.org/10.1016/j.cattod.2020.03.018
C. Du et al., Facile synthesis of Z-scheme ZnO/Ag/Ag3PO4 composite photocatalysts with enhanced performance for the degradation of ciprofloxacin. Mater. Chem. Phys. 260, 124136 (2021). https://doi.org/10.1016/j.matchemphys.2020.124136
J. Wang et al., Enhanced visible-light photodegradation of fluoroquinolone-based antibiotics andE. coligrowth inhibition using Ag-TiO2nanoparticles. RSC Adv. 11(23), 13980–13991 (2021). https://doi.org/10.1039/d0ra10403e
V.D. Cao et al., Degradation of ciprofloxacin antibiotic under visible light by BIVO4 photocatlyst. IOP Conf. Ser. Mater. Sci. Eng. (2020). https://doi.org/10.1088/1757-899X/736/6/062004
J. Wang, X. Chang, Y. Zhao, H. Xu, G. He, H. Chen, A novel Bi2WO6/BiOBr/RGO photocatalyst for enhanced degradation of ciprofloxacin under visible light irradiation: performance, mechanism and toxicity evaluation. Diam. Relat. Mater. 128, 109274 (2022). https://doi.org/10.1016/j.diamond.2022.109274
C. Ding et al., Ag nanoparticles decorated Z-scheme CoAl-LDH/TiO2 heterojunction photocatalyst for expeditious levofloxacin degradation and Cr(VI) reduction. Sep. Purif. Technol. 297, 121480 (2022). https://doi.org/10.1016/j.seppur.2022.121480
X. Zhang et al., V2O5/P-g-C3N4 Z-scheme enhanced heterogeneous photocatalytic removal of methyl orange from water under visible light irradiation. Colloids Surfaces A Physicochem. Eng. Asp. 608, 125580 (2021). https://doi.org/10.1016/j.colsurfa.2020.125580
C. Wang et al., All-solid-state Z-scheme photocatalysts of g-C3N4/Pt/macroporous-(TiO2@carbon) for selective boosting visible-light-driven conversion of CO2 to CH4. J. Catal. 389, 440–449 (2020). https://doi.org/10.1016/j.jcat.2020.06.026
Acknowledgements
The authors would also like to acknowledge the University Laboratory Management Unit, Universiti Teknologi Malaysia (UTM) for the analysis and characterisation services. One of the authors, Khairunissa Syairah Ahmad Sohaimi, would like to thank Universiti Malaysia Perlis (UniMAP) for the scholarship awarded.
Funding
This research was financially supported by the Ministry of Education Malaysia for the research funding under the HiCOE grant (R.J090301.7851.4J433); the Ministry of Science, Technology, and Innovation (MOSTI) under the International Collaboration Fund research scheme (R.J130000.7951.4S146) IF0120I1176; and Universiti Teknologi Malaysia for the financial funding under Hi-Tech (F4) grant Q.J130000.4609.00Q14.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sohaimi, K.S.A., Jaafar, J., Dharma, H.N.C. et al. Synthesis and Characterisation of Dual Z-Scheme V2O5/g-C3N4 Photocatalysts for Degrading Ciprofloxacin Antibiotics Under Visible Light. Korean J. Chem. Eng. 41, 893–907 (2024). https://doi.org/10.1007/s11814-024-00113-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-024-00113-5