Abstract
Flexible and small-scale energy storage technologies are critical for future applications such as wearable electronics. In this context, paper has emerged as a lightweight, low-cost, and ecologically friendly flexible substrate for energy storage devices. This paper presents a simple method for fabricating a micro-supercapacitor (MSC) using a wax printing approach that can achieve high resolution without requiring complex processes. In addition, the developed MnO2–Au-paper (MAP) microelectrodes with high porosity exhibit enhanced charge and ion transfer while maintaining a stable flexibility. The solid-state micro-supercapacitor delivers a very large areal capacitance of 624.32 mF cm−2 at 0.2 mA cm−2 and is stable under external stress. The highest energy and power densities obtained for the present MSCs (55.5 μWh cm−2 and 1.59 mW cm−2, respectively), are much larger than those previously reported for pseudocapacitive MSCs, including flexible ones.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lightweight, flexible, highly efficient, and miniature energy storage devices are essential for any future application of sensors, nanorobotics, medical implants, and wearable electronics. Research in this area has been accelerated by the recent rapid development of portable electronic devices [1,2,3]. Supercapacitors (SCs) have been thoroughly investigated as energy sources, owing to their fast charging/discharging rate, long cycle life, and higher power density compared to batteries [4, 5]. Conventional SCs, however, are challenging to scale down and also not appropriate for the planar geometries of integrated manufacturing methods. In-plane micro-supercapacitors (MSCs) have recently shown the ability to provide successful integration for on-chip applications. The interdigital structure of MSCs not only improves charge transfer by decreasing the ion diffusion lengths, but also reduces the complexity of conventional SCs with vertically layered structure, making them suitable for on-chip devices [6]. Several studies have aimed to improve the performance of MSCs by increasing their active areas and enabling good electrical conduction.
Because of its surface properties, paper is an excellent substrate for the above purposes. In particular, paper might be an attractive alternative to silicone elastomers and plastics as flexible substrate, because it is more environmentally friendly, less expensive, and lighter. The paper surface is known to be markedly porous and rough, owing to its composition of cellulose fibers with a 3-dimensional hierarchical arrangement, which sets it apart from common flexible substrates. In particular, these surface characteristics can help to enhance the performance of micro-supercapacitors. Paper is an ideal substrate for use as the structural unit of conductive active materials, owing to its high porosity and abundant hydroxyl functional groups. Furthermore, the cellulose fibers of paper have capability to absorb significant amounts of electrolyte, acting as electrolyte reservoirs to assist in the transportation of ions [7,8,9].
Herein, we present a simple wax printing approach, for achieving MSC interdigital structure with high resolution by printing hydrophobic wax barriers on paper using a commonly accessible printer [10]. Many micropatterning technologies, including photolithography [11,12,13], inkjet printing [14], screen printing [15, 16], and laser scribing [17], have been used for creating the interdigital structure of MSCs. Although photolithography provides high resolution, it necessitates the use of hazardous compounds. Screen and inkjet printing require appropriate precursor ink and complex processes, which result in high manufacturing costs [18, 19]. In order to solve these issues, we utilized wax printing technology, which is the cost-effective and simple process to manufacture multiple MSCs in a single batch [20, 21]. The wettability and capillary action of paper prevent micropatterning of metal electrodes to be achieved through a solution-based process [20]. A wax barrier acts as a mask by patterning metal seeds deposited on the paper surface by thermal evaporation into interdigital structures. An additional solution-based process was used to enhance the patterned metal seeds, resulting in the formation of a porous gold interdigital electrode on the fiber surface.
MnO2-Au-paper (MAP) microelectrodes were designed to obtain high-performance MSCs. In contrast with carbon materials, transition metal oxides, undergo a pseudo-capacitive process involving a redox reaction with a greater specific capacitance. Manganese oxides have several advantages among pseudocapacitive materials, such as high theoretical-specific capacitance (1370 F g−1), abundance, environmental friendliness, and low-cost [22,23,24]. In order to enhance the pseudocapacitive properties of MnO2, we added a thin layer of highly conductive Au onto the paper as a current collector. This resulted in an augmented contact area between the MnO2 active layer and the Au current collector [18]. Finally, a porous MnO2–Au thin film structure was fabricated on paper, resulting in flexible electrodes with high areal capacitance and rate capability, as well as excellent cycle stability [25].
Experimental
Materials
Whatman qualitative filter paper of grade 3 (thickness = 390 µm, porosity = 6 µm, 46 cm × 56 cm sheets) was purchased from Sigma-Aldrich, USA. Hydroxylamine hydrochloride (159,417, 500 g), gold(III) chloride trihydrate (520,918, 5 g), sodium sulfate (239,313, 1 kg), polyvinyl alcohol (PVA) (341,584, 1 kg, MW = 89,000 ~ 98,000 g/mol), and manganese(II) acetate tetrahydrate (221,007, 500 g) were purchased from Sigma-Aldrich, USA. Toluene (8541-4410, 18 L) was purchased from Daejung, Korea. Au pellets (diameter = 3 mm, thickness = 3 mm) were purchased from TASCO, Korea.
Paper Patterning by Wax Printing
We created interdigital patterns of hydrophobic barriers as blue lines on a white background using a drawing software (AutoCAD). The size of the printed features on the paper substrate did not exactly match the size of the designed hydrophobic barriers in AutoCAD, because of the capillary flow of the wax during printing (Fig. S1a, S1b) [15]. Solid wax was printed on the patterns of Whatman grade 3 paper using a Xerox ColorQube 8570 printer.
Deposition of Gold Clusters on Paper
Gold (Au, thickness = 10 nm) was deposited onto the paper substrate by thermal evaporation. To remove solid wax, the paper was placed in toluene on a 100 °C hotplate for 10 min. After washing with toluene and isopropyl alcohol (IPA) and drying on a 50 °C hotplate, gold clusters were observed to be deposited on the area of the paper not covered by wax.
Manufacturing Gold Electrodes on the Paper Surface
Gold clusters were deposited in a pattern along the surface of the cellulose fibers in the paper substrate. A 25 mL aliquot of a 12 mM hydroxylamine hydrochloride solution and 3 mL of a 1 wt% HAuCl4 solution were combined to create a reduction solution. The paper sample, which was deposited with gold clusters, was submerged in the mixture, followed by a gold reduction reaction carried out for 10 min, using an orbital shaker operating at 125 rpm. The interdigitated Au-paper structure, designed in a pattern, was achieved through washing with DI water and subsequent drying on a hot plate a temperature of 50 °C.
Assembly of Solid-State MnO2–Au-Paper Micro-supercapacitor
MnO2 was electrochemically deposited on the Au-paper electrode to assemble a solid-state symmetric micro-supercapacitor by anodic deposition at 1.3 V vs. Ag/AgCl from a 1 M manganese acetate solution. A PVA/Na2SO4 gel electrolyte was used. A 30 mL aliquot of a 10 wt% PVA solution and 850 mg of Na2SO4 in 5 mL of water were stirred at 120 °C until the water evaporated. Then, the PVA/Na2SO4 gel was applied to the MAP substrate, allowing it to be completely absorbed and dried under ambient conditions until it solidified.
Characterization
Images of the paper samples were acquired using an optical microscope (JENOPTIK ProgRes, Germany). Morphological images of the paper surfaces were observed under field-emission scanning electron microscopy (FE-SEM) and energy dispersive X-ray spectroscopy (EDS) (CarlZeiss, Germany). The sheet resistance was examined using a sourcemeter with a four-point probe system (SMU 2612A, Tektronix). The chemical structures of device were investigated using X-ray photoelectron spectroscopy (XPS) (K-alpha+, ThermoFisher Scientific, USA), whereas the crystal structure was characterized by X-ray diffraction (XRD) (New D8-Advance, Bruker-AXS, Germany).
Electrochemical Measurements
All electrochemical properties were conducted with a potentiostat (ZIVE SP 1 Electrochemical Workstation, WonATech, Korea). Two-electrode system was employed in air. Measurements of cyclic voltammetry (CV) and galvanostatic charge and discharge (GCD) were conducted over a potential range of 0 to 0.8 V. Electrochemical impedance spectroscopy (EIS) tests were performed, utilizing a potential amplitude of 5 mV, and covering a frequency range from 0.1 Hz to 100 kHz. The areal capacitance of the MAP MSC was calculated from the GCD curve using Eq. (1):
where ∆V is the voltage window, A is the area of the electrode, I is the discharge current, and ∆t is the discharging time corresponding to the specified potential change ∆V. The energy density (E) and power density (P) of the MAP MSC were calculated from the GCD curves according to Eqs. (2) and (3) [26]:
where CGCD is the areal capacitance, ∆t is the discharge time, and ∆V is the operating voltage.
Results and Discussion
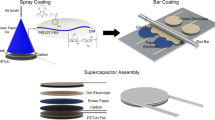
Figure 1a shows the fabrication process of MAP MSC device. First, we designed the interdigital structure with AutoCAD and printed solid wax on the surface of filter paper. After that, the wax-printed paper was separated into two sections: hydrophilic and hydrophobic (Fig. 1a-ii). The hydrophobic barrier was used as a mask for thermal evaporation to divide the electrodes and create a gap between them. The process of wax printing provides a fast, cost-effective, and flexible method for creating micro patterns [20]. Then, gold (thickness = 10 nm) was deposited on the wax-printed paper by thermal evaporation to fabricate the seeds of the Au current collector (Fig. 1a-iii). Toluene readily dissolved both the gold placed onto the wax layer and the wax layer itself. As a result, gold only remained in the area where the wax was not deposited, and seeds of Au electrodes were formed in an interdigital structure (Fig. 1a-iv). However, gold reduction is essential, because the gold clusters alone have insufficient conductivity to serve as current collector. The dimensions of the Au clusters were enlarged through catalytic reduction of gold ions present on the surfaces of the Au clusters. This was achieved by utilizing HAuCl4 as a source of gold and hydroxylamine hydrochloride as a reducing agent in the reducing solution. As shown in Fig. 1a-v, the electrodes exhibited a stronger golden color compared to that in Fig. 1a-iv. By applying manganese dioxide to the surface of the Au-paper, an active layer for a pseudocapacitive reaction-based MSC was formed (Fig. 1a-vi). Finally, the polyvinyl alcohol (PVA)/Na2SO4 gel electrolyte was applied onto the interdigital patterns to assemble the MAP MSC. A photograph and the detailed parameters of the MAP MSC device are shown in Fig. 1b, where W and L represent the width and length of the microelectrodes, respectively, whereas G is the gap separating them. The average finger width of the single interdigital MnO2–Au-based electrodes was approximately 500 μm, and their average distance was ~ 500 μm, with no evident short circuits between them.
Fabrication, digital photographs, and design of MAP MSC. a Schematic representation of the fabrication process of MSC on paper substrate: (i) bare paper; (ii) solid-wax-printed paper (wax region: hydrophobic, non-wax region: hydrophilic); (iii) gold- deposited paper by thermal evaporation; (iv) gold-deposited paper after wax removal with toluene; (v) gold reduced Au-paper; (vi) MnO2-electrodeposited paper. b The Photograph and detailed parameters of designed single microelectrode: W = 500 μm, G = 500 μm, L = 4 mm
To demonstrate the resolution of the Au micropatterning achieved by the wax printing technique, optical micrograph images of micropatterned wax-printed paper and Au microelectrodes on paper are displayed in Fig. 2a. The width of the electrode could be adjusted in increments of 100 μm and reduced to 300 μm without the electrodes being connected to one another (Fig. 2a-i). The gap for which the electrodes are not connected could be extended up to 200 μm and adjusted in steps of 50 μm (Fig. 2a-ii). The MAP MSC could be produced with the minimum W and G values of 300 and 200 μm, respectively, but was manufactured with a width of 500 μm and a gap of 500 μm based on productivity considerations. A gap of size similar to that the printed wax was created between the electrodes; this size remained constant throughout the manufacturing process. Electrodes were created in the region where wax was not printed, whose width also remained constant, as the fabrication procedure continued (Fig. S2). We found that the solid wax printing technique is one of the approaches that can be used to create micropatterns with high resolution. Only the surface of the cellulose is coated with solid wax, which is simple to remove because it dissolves readily in an organic solvent. Thermal evaporation also only deposits metal on the paper surface; thus, if wax is removed after the metal has been deposited, gold will only be present in the regions where wax was not printed (Fig. S2). Therefore, a solid wax was employed to produce a hydrophobic barrier, which acted as a mask for micro-patterning.
Fabrication and characteristics of Au microelectrodes. a Optical micrographs of wax barrier and Au microelectrodes: (i) resolution test of wax barriers, with lines of various sizes showing that the minimum width of the electrodes was 300 μm; (ii) resolution test showing that the minimum gap between the electrodes was 200 μm. b FE-SEM images of Au microelectrode fabricated with 10 nm Au and 0.1 wt% HAuCl4 conditions. c Sheet resistance vs. reducing agent concentration for different thicknesses of Au clusters. Resistance vs. d bending radius and e folding angle profiles under tensile and compressive loads. f Bending stability test consisting of 1000 tensile and compressive loading cycles with 0.4 cm bending radius
According to the FE-SEM images in Fig. 2b, porous Au-paper electrodes with 10 nm-thick gold clusters and a 0.1 wt% concentration of the HAuCl4 reduction solution were uniformly formed along the surface of the cellulose fibers. In comparison to the 10 nm-thick gold clusters that were deposited by thermal evaporation, the gold particles deposited on Au-paper were connected to each other (Fig. S3). Moreover, the resistivity of Au-paper also decreased with increasing duration of Au reduction process, particularly compared to that before Au reduction (Fig. S4a). To determine the optimal conditions for the application of the paper electrode as a current collector, FE-SEM images and sheet resistances were obtained at varying concentrations of the gold reduction solution and thicknesses of the gold clusters (Figs. 2c, S5, and S6). The sheet resistances of the Au-paper were averaged over samples cut into 1 cm × 3 cm, and the reducing time was 10 min. The resistance tended to decrease with increasing Au cluster thickness and HAuCl4 concentration. With a HAuCl4 concentration of 0.1 wt%, the resistance dropped from 0.62 to 0.39 Ω sq−1 as the Au cluster thickness increased from 1 to 20 nm (Fig. S4b). This revealed a decreased interfacial resistance through the grain boundaries due to the bigger grain size of Au particles at a high coverage of Au clusters [27, 28]. The corresponding FE-SEM images (Fig. S5) show the morphological changes induced by increasing thickness of the Au clusters at 0.1 wt% HAuCl4. As the thickness of the Au clusters increased, the Au particles grew and were tightly packed throughout the entire paper fiber, which improved the conductivity. We also studied the effect of the HAuCl4 concentration: the resistance decreased from 5.41 to 0.1 Ω sq−1 as the HAuCl4 concentration increased from 0.05 to 0.2 wt%. The increase in HAuCl4 concentration resulted in a decline in grain boundary density, leading to a decrease in resistance caused by a reduction in grain boundary scattering. The related FE-SEM images (Fig. S6) show how the increase in HAuCl4 concentration affected the morphology of the 10 nm-thick Au clusters. The Au-paper reduced with 0.05 wt% HAuCl4 was remarkably free of large vertical features, because coalescence of Au particles occurred at an earlier stage of seeding. In other words, in-plane growth occurred at a faster rate than that on the z-axis if Au0 was preferentially deposited between Au nanoparticles [27]. A high HAuCl4 concentration causes a rapid reaction with the seeds, which accelerates their growth and is sufficient for the growth of particles along the z-axis. We determined that a 10 nm-thick Au cluster and 0.1 wt% HAuCl4 solution were the optimal conditions based on the observed conductivity, uniformity, and gold consumption. X-ray photoelectron spectroscopy (XPS) was used to further examine the Au-paper electrode (Fig. S7). The presence of metallic Au(0) as the current collector was confirmed by the presence of the doublet peaks at binding energies of 84.58 and 88.28 eV in the Au 4f spectra.
We measured the resistance of bent and folded Au microelectrodes to test the flexibility of the MAP MSC (Fig. 2d–f). First, the microelectrodes were bent to a bending radius of 0.75–5 cm under tensile and compressive stresses (Fig. 2d). The resistance under tensile and compressive loadings gradually rose by 19% and 13%, respectively, as the bending radius decreased. Subsequently, a folding test was performed: the electrode was folded by 0–180° under tensile and compressive stresses (Fig. 2e). Gradual increases of 31% and 14% in electrode resistance under tensile and compressive stresses, respectively, were observed with an increase in folding angle. Because the gold electrodes cracked under tensile stress, the resistance increased significantly more than under compressive stress. Additionally, the Au microelectrodes were subjected to a bending stability test with a 0.4 cm bending radius over 1000 cycles of both tensile and compressive loads (Fig. 2f). The resistance retention increased by less than 50%. We thus concluded that the Au microelectrodes had a high degree of flexibility.
The Au microelectrodes were applied as flexible current collector in an energy storage device to enhance low conductivity of active material, and MnO2, a redox-active transition metal oxide, was selected as the active material [29, 30]. Using Au as an active material is inadequate because the Au electrode of our MSC has little effect on the pseudo-capacitive reaction (Fig. S8). Manganese acetate was used as a precursor for the anodic electrodeposition of MnO2 onto the Au microelectrodes. Figure 3a shows the FE-SEM images of the MAP electrode. Because of its sheet-like structure with nanoflower morphology, the MnO2 prepared by electrodeposition had a higher specific surface area and charge transfer capacity. The results clearly show that, by limiting the MnO2 deposition onto the Au microelectrodes, an interdigital structure was successfully formed, resulting in complete separation of the electrodes (cathode and anode) (Fig. 3b).
Characterization of MnO2–Au microelectrodes. a FE-SEM images of MnO2–Au microelectrode and b corresponding EDS mappings of C, O, Au, and Mn elements. Scale bar in (b): 200 μm. c XRD patterns of bare paper, 10 nm-thick gold cluster (Au deposited on paper by thermal evaporation), Au-paper, and MnO2–Au-paper. d, e XPS spectra of MnO2–Au microelectrodes
The crystallinity of MAP was studied using X-ray diffraction (XRD). Figure 3c shows a comparison of the XRD patterns of bare paper, a 10 nm-thick gold cluster, Au-paper, and MAP. The characteristic diffraction peaks of bare paper observed at 2θ = 14.7°, 16.8°, and 22.7° corresponded to the (1\({\overline{\text{1}}}\)0), (110), and (200) crystallographic planes of monolithic cellulose, respectively [31]. Owing to the amorphous nature of the gold clusters, the XRD peaks of the 10 nm-thick gold cluster on paper resembled the peaks of bare paper [32]. The pattern of the Au-paper sample displayed peaks corresponding to the (111), (200), (220), (311), and (222) reflections, associated with the cubic lattice of crystalline gold [33]. The XRD peaks of MAP were hard to distinguish, indicating an amorphous MnO2 structure with weak crystallinity. XPS analysis was used to examine the elements and valence states of MAP (Fig. 3d, e). The high resolution Mn 2p spectra displayed two significant peaks at 642.38 eV (Mn 2p3/2) and 654.18 eV (Mn 2p1/2), with a spin-energy separation of 11.8 eV, indicating the dominant presence of Mn4+ species (Fig. 3d) [34]. The O 1s spectrum of MnO2 in Fig. 3e was composed of three characteristic oxygen bond peaks at 530.08, 531.58, and 532.98 eV, corresponding to the binding energies of O 1s electrons in anhydrous (Mn–O–Mn), hydrated (Mn–O–H), and hydrous manganese oxide (H–O–H), respectively [35]. These results confirmed that amorphous MnO2 was successfully deposited and exhibited a strong pseudocapacitive performance.
The electrochemical properties of the single MAP MSC device with 0.5 mg cm−2 of MnO2 were evaluated by CV, GCD, and EIS measurements based on a two-electrode configuration in the potential window of 0 to 0.8 V (Fig. 4). The electrochemical performance of the MAP MSC was examined after fully integrating it with PVA/Na2SO4 as gel electrolyte, considering that the safety of flexible MSCs as liquid electrolytes is negatively affected by the complex packaging procedure and electrolyte leakage (Fig. 4a). CV measurements were carried out at scan rates ranging from 5 to 200 mV s−1 (Fig. 4b). The CV curves presented a near-rectangular shape with noticeably broadened peaks for all scan rates, indicating a dominant pseudocapacitive behavior [25, 36]. Galvanostatic charge–discharge curves were obtained at current densities ranging from 0.2 to 2 mA cm−2 (Fig. 4c). The GCD curves exhibited an asymmetrical triangular shape because of the decomposition of the redox-active materials and the relatively high ion diffusion resistance of the PVA-based gel electrolyte [37]. Nonetheless, the GCD curves displayed a quasi-triangular shape with a small IR drop, reflecting an ideal pseudocapacitive charge storage process. Figure 4d and e show the areal capacitances of a single MAP MSC calculated from the scan rate and current density, respectively. A high areal capacitance of 71.68 mF cm−2 was observed at a scan rate of 200 mV s−1, indicating a good retention (52.85% of the value at 5 mV s−1) (Fig. 4d). A very high areal capacitance (89.91 mF cm−2 corresponding to 67.67% of the value at 0.2 mA cm−2) was also measured at a high current density of 2.1 mA cm−2 (Fig. 4e). The performance of the solid-state device was comparable with that of the device immersed in a liquid electrolyte (1.0 M Na2SO4) (Fig. S9). Furthermore, cyclic voltammetry scans with different MnO2 mass loadings were carried out at a scan rate of 5 mV s−1 (Fig. S10). The results enabled to measure the gravimetric and areal capacitances as a function of the areal mass of MnO2 (Fig. 4f). The maximum gravimetric and areal capacitances were 217.84 F g−1 and 624.32 mF cm−2, respectively. The Nyquist curve generated from the EIS measurements (Fig. 4g) illustrates how the resistance varied with the MnO2 mass loading. Because the resistance of the diffusion layer increased with the MnO2 mass loading, the slope of the curve showed a corresponding decrease [38, 39]. The MAP MSC also had good electrochemical stability, showing similar CV curves as the number of cycles increased (Fig. S11). The MAP MSC tested at a scan rate of 500 mV s−1 retained up to 86% of its capacitance after 5000 cycles. The active material's hierarchical porous structure led to a short ion diffusion length, high specific area, and rapid ion diffusion (Fig. 4h) [40]. Further important properties to consider when assessing the effectiveness of energy storage technologies are the energy density and power density. According to Fig. 4i, the energy and power densities of the MAP MSC ranged from 44.5 to 55.5 μWh cm−2 and 1.59 to 0.32 mW cm−2, respectively. It is important to note that the MAP MSC exhibited the highest energy and power densities among all pseudocapacitive MSCs, including flexible MSCs [41,42,43,44,45,46,47,48].
Electrochemical properties of single MAP MSC in the solid-state (PVA/Na2SO4). a Schematic illustration of single solid-state device. b CV curves measured at scan rates of 5–200 mV s−1. c Galvanostatic charging/discharging curves of single MSC measured with different current densities (0.2–2 mA cm−2). Specific areal capacitance of single MSC calculated from the discharging time at different d scan rates and e currents. f Gravimetric and areal capacitances vs. areal mass of MnO2. g Nyquist plots of single MSC with different amounts of MnO2. h Cycling stability of MSC at a scan rate of 500 mV s−1. The inset shows the CV curves measured in the initial state and after 1000, 2000, 3000, 4000, and 5000 cycles. i Ragone plots of areal energy vs. power density compared with those of previously reported devices
By tuning the structure of the microelectrodes and their mutual connection in order to control both the output operating voltage and the total capacitance, MSCs can be interconnected in series or in parallel on a single substrate. On the other hand, arranging conventional supercapacitors in either series or parallel is necessary based on the external connection, resulting in additional energy and space consumption [6, 49, 50]. Our technique facilitates the fabrication of series and parallel circuits without the need for extra space or resistance. The micro-supercapacitor devices could be easily integrated on a single piece of paper by changing the architecture of the wax barrier. By separating the devices with paraffin wax, we could infiltrate the gel electrolyte (PVA/Na2SO4) into each single micro-supercapacitor (Fig. 5a, b). The CV curves of a device with five integrated MSCs were measured at 100 mV s−1. As shown in Fig. 5c and d, the charging and discharging times of the curves of the parallel device were 5 times higher than that of a single MSC (Fig. 5c) and the overall current of the parallel device increased by a factor of 5 in comparison to that of a single MSC unit (Fig. 5d). Moreover, the series-connected MSCs showed a fivefold increase in the potential window (Fig. 5e, f).
Electrochemical properties of single MSC in an integrated device. Schematic illustrations and digital photographs of five devices connected a in parallel and b in series. c Galvanostatic charging/discharging curves of group of five MSCs connected in parallel. d CV curves of MSCs connected in parallel. e Galvanostatic charging/discharging curves of group of five MSCs connected in series. f CV curves of MSCs connected in series
Conclusion
In conclusion, a flexible MAP MSC was produced using a simple micropatterning process based on wax printing, which is a convenient, inexpensive, rapid, and scalable method. High-resolution interdigital microelectrodes could be achieved using wax barriers as a mask. The porous MnO2–Au hybrid structure increased the electrical conductivity and surface area of the active layer, improving the charge transfer and ion transport. The paper-based microelectrodes also remained stable when subjected to external stress, suggesting that the flexibility of paper was retained. Furthermore, the use of a PVA/Na2SO4 gel electrolyte enabled the fabrication of flexible all-solid-state MAP MSCs with exceptional pseudocapacitive performance. The MAP MSCs achieved a high areal capacitance of 624.32 mF cm−2 and an excellent cycling stability of 86% for 5000 cycles at 500 mV s−1. The highest energy and power densities were 55.5 Wh cm−2 and 1.59 mW cm−2, respectively. These values were significantly higher than those previously reported for flexible and pseudocapacitive MSCs. We anticipate that our wax printing technology will enable the production of MSC arrays of various sizes and shapes, which could be applied as power sources for microelectronic devices with high power and energy densities.
Data availability
Not applicable.
References
P. Zhang, F. Wang, S. Yang, G. Wang, M. Yu, X. Feng, Energy Storage Mater. 28, 160 (2020)
Y. Liu, Y. Shi, X. Xu, Energy Storage Mater. 41, 677 (2021)
A.C. Ezika, E.R. Sadiku, C.I. Idumah, S.S. Ray, Y. Hamam, J. Energy Storage. 45, 103686 (2022)
H.S. Chang, B.M. Lee, J.M. Yun, J.H. Choi, Korean J. Chem. Eng. 39, 1232 (2022)
M. Jung, I. Yang, D. Choi, J. Lee, J.C. Jung, Korean J. Chem. Eng. (2023). https://doi.org/10.1007/s11814-023-1466-3
L. Liu, Z. Niu, J. Chen, Nano Res. 10, 1524 (2017)
Z. Zhao, S. Hao, P. Hao, Y. Sang, A. Manivannan, N. Wu, H. Liu, J. Mater. Chem. A 3, 15049 (2015)
Y.Z. Zhang, Y. Wang, T. Cheng, W.Y. Lai, H. Pang, W. Huang, Chem. Soc. Rev. 44, 699 (2015)
R. Guo, J. Chen, B. Yang, L. Liu, L. Su, B. Shen, X. Yan, Adv. Funct. Mater. 27, 1702394 (2017)
I.H. Oh, E. Park, S.T. Chang, S. Lim, Adv. Mater. 35, 2300197 (2023)
J. Lin, C. Zhang, Z. Yan, Y. Zhu, Z. Peng, R.H. Hauge, D. Natelson, J.M. Tour, Nano Lett. 13, 72 (2013)
J.H. Sung, S.J. Kim, S.H. Jeong, E.H. Kim, K.H. Lee, J. Power. Sources 162, 1467 (2006)
Q. Jiang, N. Kurra, K. Maleski, Y. Lei, H. Liang, Y. Zhang, Y. Gogotsi, H.N. Alshareef, Adv. Energy Mater. 9, 1901061 (2019)
P. Sundriyal, S. Bhattacharya, ACS Appl. Energy Mater. 2, 1876 (2019)
X. Zhang, W. Zhao, L. Wei, Y. Jin, J. Hou, X. Wang, X. Guo, Energy 170, 338 (2019)
Y. Wang, Y. Shi, C.X. Zhao, J.I. Wong, X.W. Sun, H.Y. Yang, Nanotechnology 25, 094010 (2014)
L. Zhang, D. DeArmond, N.T. Alvarez, R. Malik, N. Oslin, C. McConnell, P.K. Adusei, Y.Y. Hsieh, V. Shanov, Small 13, 1603114 (2017)
H.B. Hu, Z.B. Pei, H.J. Fan, C.H. Ye, Small 12, 3059 (2016)
H. Hu, T. Hua, J. Mater. Chem. A. 5, 19639 (2017)
E. Carrilho, A.W. Martinez, G.M. Whitesides, Anal. Chem. 81, 7091 (2009)
R. Lu, W. Shi, L. Jiang, J. Qin, B. Lin, Electrophoresis 30, 1497 (2009)
G.A.M. Ali, L.L. Tan, R. Jose, M.M. Yusoff, K.F. Chong, Mater. Res. Bull. 60, 5 (2014)
M. Zhang, D. Jin, L. Zhang, X. Cui, Z. Zhang, D. Yang, J. Li, Chem. Eng. J. Adv. 6, 100098 (2021)
K. Zhang, X. Han, Z. Hu, X. Zhang, Z. Tao, J. Chen, Chem. Soc. Rev. 44, 699 (2015)
W. Si, C. Yan, Y. Chen, S. Oswald, L. Han, O.G. Schmidt, Energy Environ. Sci. 6, 3218 (2013)
C. Peng, J. Lang, S. Xu, X. Wang, RSC Adv. 4, 54662 (2014)
K.R. Brown, L.A. Lyon, A.P. Fox, B.D. Reiss, M.J. Natan, Chem. Mater. 12, 314 (2000)
M.C. Barnes, D.-Y. Kim, H.S. Ahn, C.O. Lee, N.M. Hwang, J. Cryst. Growth 213, 83 (2000)
W. Guo, C. Yu, S. Li, Z. Wang, J. Yu, H. Huang, J. Qiu, Nano Energy 57, 459 (2019)
D. Wu, X. Xie, Y. Zhang, D. Zhang, W. Du, X. Zhang, B. Wang, Front. Mater. 7, 1 (2020)
M.N. Costa, B. Veigas, J.M. Jacob, D.S. Santos, J. Gomes, P.V. Baptista, R. Martins, J. Inácio, E. Fortunato, Nanotechnology 25, 094006 (2014)
M. Schrinner, F. Polzer, Y. Mei, Y. Lu, B. Haupt, M. Ballauff, A. Göldel, M. Drechsler, J. Preussner, U. Glatzel, Macromol. Chem. Phys. 208, 1542 (2007)
X. Ren, Y. Song, A. Liu, J. Zhang, P. Yang, J. Zhang, M. An, RSC Adv. 5, 64997 (2015)
Y.W. Kim, I.H. Oh, S. Choi, I. Nam, S.T. Chang, Chem. Eng. J. 454, 140117 (2023)
H.Q. Wang, J. Chen, S.J. Hu, X.H. Zhang, X.P. Fan, J. Du, Y.G. Huang, Q.Y. Li, RSC Adv. 5, 72495 (2015)
A. Noori, M.F. El-Kady, M.S. Rahmanifar, R.B. Kaner, M.F. Mousavi, Chem. Soc. Rev. 48, 1272 (2019)
X. Li, H. Li, X. Fan, X. Shi, J. Liang, Adv. Energy Mater. 10, 1903794 (2020)
B.A. Mei, O. Munteshari, J. Lau, B. Dunn, L. Pilon, J. Phys. Chem. C 122, 194 (2018)
J.N. Broughton, M.J. Brett, Electrochim. Acta 50, 4814 (2005)
Y. Yue, Z. Yang, N. Liu, W. Liu, H. Zhang, Y. Ma, C. Yang, J. Su, L. Li, F. Long, Z. Zou, Y. Gao, ACS Nano 10, 11249 (2016)
C. Gao, J. Huang, Y. Xiao, G. Zhang, C. Dai, Z. Li, Y. Zhao, L. Jiang, L. Qu, Nat. Commun. 12, 2647 (2021)
X. Li, Y. Ma, P. Shen, C. Zhang, M. Cao, S. Xiao, J. Yan, S. Luo, Y. Gao, Adv. Mater. Technol. 5, 2000272 (2020)
L. Li, D. Chen, G. Shen, Chem. Res. Chin. Univ. 36, 694 (2020)
Y. Wang, L. Sun, D. Xiao, H. Du, Z. Yang, X. Wang, L. Tu, C. Zhao, F. Hu, B. Lu, ACS Appl. Mater. Interfaces. 12, 43864 (2020)
Y. Yuan, L. Jiang, X. Li, P. Zuo, C. Xu, M. Tian, X. Zhang, S. Wang, B. Lu, C. Shao, B. Zhao, J. Zhang, L. Qu, T. Cui, Nat. Commun. 11, 6185 (2020)
H. Park, C. Song, S.W. Jin, H. Lee, K. Keum, Y.H. Lee, G. Lee, Y.R. Jeong, J.S. Ha, Nano Energy 83, 105837 (2021)
K. Brousse, S. Pinaud, S. Nguyen, P.F. Fazzini, R. Makarem, C. Josse, Y. Thimont, B. Chaudret, P.L. Taberna, M. Respaud, P. Simon, Adv. Energy Mater. 10, 1903136 (2020)
B. Asbani, K. Robert, P. Roussel, T. Brousse, C. Lethien, Energy Storage Mater. 37, 207 (2021)
M. Fu, R. Lv, Y. Lei, M. Terrones, Small 17, 2004827 (2021)
Z. Zhu, H. Jiang, S. Guo, Q. Cheng, Y. Hu, C. Li, Sci. Rep. 5, 15936 (2015)
Acknowledgements
This research was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No. 2022R1A2C1005739), the Competency Development Program for Industry Specialists of the Korean Ministry of Trade, Industry and Energy (MOTIE), operated by Korea Institute for Advancement of Technology (KIAT) (No. P0012453, Next-generation Display Expert Training Project for Innovation Process and Equipment, Materials Engineers), and a Chung-Ang University Graduate Research Scholarship in 2022.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kim, N.Y., Oh, I.H. & Chang, S.T. Paper-Based Solid-State Micro-supercapacitors Fabricated by Hydrophobic Wax Barrier Printing. Korean J. Chem. Eng. 41, 763–772 (2024). https://doi.org/10.1007/s11814-024-00101-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-024-00101-9