Abstract
This study delves into the production and evaluation of cellulose acetate (CA) separators with a focus on their application in lithium-ion batteries. The primary objective is to optimize battery performance by customizing separator characteristics through the integration of diverse additives and water-pressure treatments. Three distinct categories of additives were investigated, which include hydrated metal nitrates, organic compounds, and metal compounds. The impact of these additives on pore generation and porosity was comprehensively analyzed. Among the hydrated metal nitrates, Cd(NO3)2·4H2O emerged as a highly effective plasticizer in comparison to Ni(NO3)2 and Mg(NO3)2. This superiority can be attributed to the relatively larger ionic radius of cadmium (Cd) among these three elements, facilitating the dissociation of Cd ions into cations and counteranions. Within the realm of organic compounds, glycerin proved to be more efficient in inducing the formation of abundant pores in CA polymers when compared to propylene glycol and lactic acid. As for the metal compounds, they exhibited notable effectiveness in preparing porous CA polymers for battery separators. However, these materials tend to yield larger pore sizes, potentially due to their higher dissociation energy. The findings of this investigation underscore the feasibility of employing a range of additives to craft porous cellulose acetate separators. These resulting separators exhibit varying degrees of porosity, positioning them as promising candidates for enhancing lithium-ion battery performance. Consequently, this review contributes to the ongoing advancement of cutting-edge battery technologies by tailoring separator materials to specific requirements.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The global issues on energy problems, combined with the compelling shift toward sustainable and renewable resources, has driven extensive research and technological advancements in energy storage. With scientific development, rechargeable batteries have emerged as crucial enablers for effectively harnessing and distributing intermittent energy sources like solar and wind power [1,2,3,4,5]. This importance has heightened the focus on optimizing various components of batteries, including the cathode, anode, electrolytes, and separators, to achieve the improved performance, safety, and ecological sustainability. Of these components, separators have historically held a position of great importance due to their impact on safety. By physically isolating the positive and negative electrodes while facilitating the movement of lithium ions essential during the charge/discharge processes, separators play a critical role in ensuring stable battery operation. Over time, the significance of separators has evolved beyond mere segregation, encompassing vital functions such as ion conductivity, mechanical resilience, thermal stability, and operational safety [6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31]. In particular, polypropylene (PP) have been utilized as battery separator since it has high mechanical property, electrochemical stability and easy processibility. However, although PP separators showed various advantages, they have limitations such as short circuits, and relatively modest ionic conductivity, and low thermal stability related with safety [16, 17].
Thus, the efforts to address the limitations of PP as a battery separator have been substantial, driven by the aim to enhance battery performance, safety, and efficiency. In particular, to overcome the limited thermal stability of PP separators, much research has focused on developing separator materials with improved resistance to high temperatures by utilizing specific materials such as ceramic, inorganic composites, and polyimides as thermally resistant polymers. Furthermore, for the vulnerability of PP separators to puncture-induced short circuits, nanocomposite and multi-layered films have been proposed to enhance mechanical strength. By incorporating these materials with high mechanical property, the risk of internal short circuits by physical collapse can be minimized. For enhancement of ionic conductivity for battery, the research has focused on surface modifications of separator. When modified separator promote efficient ion movement between electrodes, the internal resistance can be diminished, and higher power output can be generated [18,19,20,21].
Recently, various nanomaterials have been researched to overcome the limitations of PP separators. For example, nanoporous polymers and nanofiber-based materials, offer both the increased surface area and enhancement of ion transport pathways. Furthermore, these materials have attracted much interest since they can improve both thermal and mechanical properties for stable operation of battery [28,29,30,31].
Furthermore, another approach such as functional coatings onto surface of PP separators has been researched for solving the drawbacks. The specific coatings with flame-retardant, thermal-resistant, and mechanically reinforcing properties have been developed with various organic, inorganic, and their hybrid materials [22, 23].
Very recently, as substitutes of PP for separator, cellulose materials have been much interested since it has thermally stable characteristics, chemical resistance, long-term stability, and low cost [16,17,18,19,20,21,22,23,24,25,26,27]. Thus, much research has been investigated for processing of cellulose materials to replace the PP for stable battery. For porous cellulose, processing is mainly dependent on phase separation method.
Phase separation is a well-known fundamental phenomenon that arises from both kinetic and thermodynamic behaviors. This phenomenon entails the spontaneous transformation of a homogeneous material into distinct phases with varying compositions and morphologies. Such behaviors are primarily observed in materials comprising multiple components when subjected to environmental changes. Notably, phase separation occurs due to the thermodynamic driving force. When a specific system deviates from its equilibrium state, the free energy for thermodynamic stability tends to be minimized. Materials containing components with differing affinities for each other are inherently unstable, as maintaining the homogeneous mixture requires additional energy. Consequently, the free energy of the materials decreases by segregating their components into separate phases, while each constituent exhibits a favorable interaction with others. As a result, the phase separation occurs to reduce interfacial energy between the segregated phases, potentially resulting in the formation of porous structures through precipitation with time [18,19,20,21,22,23].
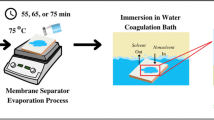
However, phase separation method has the disadvantage in viewpoint of pore structure since it only forms the random structure. For the fast lithium-ion transport, the structure close to a straight line is more desirable. To form the structure close to a straight line, our group have suggested new method based on water-pressure process for cellulose acetate with various additives [16,17,18,19,20,21,22,23,24,25,26,27]. In this review, the research results on additives are summarized and a strategy to further increase the porosity is suggested.
Results and Discussion
Hydrated Metal Nitrate as Additives
As additives to generate pores in cellulose acetate, hydrated metal nitrates were introduced as shown in Table 1.
For hydrated metal nitrates, the study to utilize the Ni(NO3)2·6H2O presents a novel approach to enhance lithium-ion battery separator materials through the creation of nanoporous cellulose acetate (CA) polymers [16]. It utilized an inorganic complex and water pressure treatment to generate interconnected nanopores within the CA polymer matrix. The resulting porous structure exhibited improved electrolyte uptake capacity and stability, making it a promising candidate for advanced battery separators. The fabrication process, characterization techniques, and electrochemical performance of porous CA polymer are extensively investigated. In detail, the water pressure treatment induced the formation of interconnected nanopores within the CA polymer matrix. The electrochemical performance of the porous CA polymer was evaluated, revealing enhanced stability and reduced resistance compared to conventional separators. The improved properties of porous CA polymer is generated from the plasticization effect induced by the incorporated Ni(NO3)2·6H2O and the subsequent water pressure treatment. The generated nanopores contribute to enhanced electrolyte uptake and stable lithium-ion transfer within the battery. As a result, the proposed approach of creating nanoporous cellulose acetate polymer through inorganic complex and water pressure treatment holds promise for advancing lithium-ion battery. The resulting porous structure with improved electrolyte uptake and stability makes it a potential candidate for high-performance battery separators [16].
Other approach presents a novel approach for enhancing the porosity of CA polymer matrices by incorporating Zn(NO3)2·6H2O and applying external water pressure [17]. This study is to enable controlled pore size and distribution in the polymer structure. The experiment involves introducing Zn(NO3)2·6H2O to an acetone/water solvent, resulting in an observable increase in porosity and pore size on the CA/Zn(NO3)2·6H2O polymer matrix surface. This enhancement is attributed to molecular interactions between Zn ions, nitrate ions, and water molecules, causing modified evaporation dynamics and subsequent pore formation. Water flux measurements at various ratios of Zn(NO3)2·6H2O indicate the effects of the added compound on water flow through the polymer matrix.
The experiment demonstrates that the introduction of Zn(NO3)2·6H2O leads to enhanced porosity and pore size on the polymer matrix. This enhancement is attributed to interactions between Zn ions, nitrate ions, and water molecules. Thus, it is thought that the study successfully demonstrates the feasibility of enhancing porosity in cellulose acetate polymer matrices through the introduction of Zn(NO3)2·6H2O and external water pressure. This novel method offers controllable pore size and distribution, with potential applications in diverse industries [17].
Another approach is to utilize the Cd(NO3)2·4H2O for porous CA battery with water pressure as an external physical force to investigate the effect of ionic radius as shown in Table 2. When the CA was complexed with Cd(NO3)2·4H2O and exposed to external water pressure, the water flux through the CA was, surprisingly, observed to be above 250 LMH (L/m2h), which is higher flux than those of CA/other metal nitrate salts (Ni(NO3)2 and Mg(NO3)2) complexes as shown in Table 3 [18]. The higher value of Cd(NO3)2·4H2O indicated that the abundant pores were generated in cellulose polymers, when compared at the same water pressure. From these results, it is concluded that the Cd(NO3)2·4H2O played a role as effective plasticizer than Ni(NO3)2 and Mg(NO3)2. In detail, ionic radius of the Cd among the three elements was the largest, resulting in that the relatively larger Cd ions can easily be dissociated into cations and counteranions. As a result, the free nitrate ions can be easily hydrated with water molecules than those of Ni(NO3)2 and Mg(NO3)2, and the plasticization on cellulose acetate is more effective. Therefore, it can be concluded that it is desirable to select the hydrated metal salts to have relatively larger ions for abundant pores in cellulose acetate. Thus, if the relatively large ions are utilized, the porosity of cellulose acetate will be increased.
Organic Compounds as Additives
As additives to generate pores in cellulose acetate, organic additives such as glycerin, propylene glycol, and lactic acid were introduced as shown in Tables 4 and 5.
For the glycerin as organic additives, the research presents a novel approach to creating high porosity nano-porous cellulose acetate membranes [20]. The study with glycerin aimed to develop a cost-effective and environmentally friendly process for generating pores in cellulose acetate without the use of inorganic salts. Glycerin can be well dispersed within the cellulose acetate matrix, leading to plasticization of the polymer chains and subsequent pore formation upon water-pressure treatment. The hydration effect of glycerin, attributed to its hydroxyl groups, facilitated the generation of flexible regions in the cellulose acetate matrix. Consequently, water-pressure forces tore out the weakened glycerin-plasticized regions, resulting in abundant nano-sized pores with high porosity. Through various analyses, it was confirmed that glycerin interacts with cellulose-polymer chains, enhancing the thermal stability of the resultant membranes. As a result, porosity measurements indicated a high porosity of 78.3% with an average pore diameter of 630 nm. Water flux data demonstrated the dependence of flux on the cellulose-polymer/glycerin ratio, with an optimal ratio of 1:0.5 yielding the highest flux of 7.4 LMH [20].
On the other hand, propylene glycol was introduced into cellulose acetate for the development and properties of nanoporous polymer membranes [21]. As a result, the study confirms that water pressure treatment significantly enhances the flexibility and porosity of CA membranes. The presence of propylene glycol further increases membrane flexibility. Comparative analysis demonstrates that nanoporous membranes with propylene glycol exhibit improved flexibility and compactness compared to glycerin-containing membranes. Therefore, it is concluded that the water pressure treatment method enhances membrane flexibility and porosity, and the interaction with propylene glycol further contributes to improved properties [21]. The average pore diameter, and porosity of the CA/propylene glycol film were 300 nm, and 69.7%, respectively. Compared to glycerin as an additive, the average pore size is smaller, and the pores are well distributed. Since propylene glycol has fewer hydrophilic groups per molecule than glycerin, smaller hydration area was formed when it was dispersed between polymer chains [21]. Thus, as the water pressure was applied to composite film with smaller hydrated region, the average pore diameter became diminished. Furthermore, the porosity was reduced compared to those in the previous studies with glycerin.
As other approach, utilizing lactic acid-induced plasticization to enhance the porosity and performance of CA polymers by water treatment method is reported [22]. The cost-effectiveness and environmental friendliness of the proposed approach make it a viable solution to address contemporary water treatment challenges. The incorporation of lactic acid induces plasticization in the CA matrix, leading to the formation of micro-sized pores within the membrane structure. SEM images reveal the presence of sponge-shaped pores on the membrane surface and cross section [22]. TGA data demonstrate improved thermostability of the CA polymer following lactic acid-induced plasticization. Water flux measurements show that the 1:0.07 mol ratio of CA to lactic acid offers the highest water flux, indicating optimal porosity (65.3%) and pore size (380 nm) [22]. As a result, this study successfully demonstrates the potential of lactic acid-induced plasticization for the fabrication of highly porous cellulose acetate membranes suitable for battery separator applications.
From these results, it could be concluded as (1) hydroxyl group is more effective than carboxyl groups for high porosity and (2) as the number of hydroxyl groups increases, porosity also increases when organic compounds are utilized for CA separators due to the increased solvation effect of remined solvents. Thus, when CA separators are prepared with organic compounds, it can be expected that the porosity can be controlled by the number of hydroxyl groups per molecule.
Metal Compounds as Additives
As additives to generate pores in cellulose acetate, metal compound as additives such as calcium chloride, calcium oxide and sodium nitrate were introduced as shown in Table 6.
Among metal compounds, the use of calcium chloride as an additive appears to be a research focused on the fabrication and characterization of porous CA separator [24]. The study investigates the effects of CaCl2 on the structure, porosity, and performance of the porous CA as separator under different conditions, particularly with varying water pressures. As a result, SEM images showed the development of irregular reticulated channels and plasticization-induced pores in the CA polymers. Furthermore, a high porosity of 70.3% and significant water flux improvement (up to 316 flux(LMH) at 8 bar) were achieved using the CaCl2 additive [24]. In particular, FT-IR spectra revealed interactions between Ca2+ ions and carboxyl and hydroxyl groups in the CA polymer chain. The additive was found to enhance the thermal and mechanical stability of the separators, confirmed by thermogravimetric analysis (TGA).
Other approach to utilize the metal compound is to use of calcium oxide (CaO) for porous CA separator [25]. This study proposes the incorporation of calcium oxide (CaO) particles into the CA matrix to enhance thermal stability and porosity. The research aims to develop a composite material with improved properties for battery separators [25]. The CA/CaO composite material exhibited a high porosity of 73.1% and a remarkable water flux of 95.25 L/m² h at 8 bar. Furthermore, the addition of CaO improved thermal stability, allowing the composite material to withstand higher temperatures [25]. As a result, the study’s findings suggest that the CA/CaO composite material could serve as an improved separator for lithium-ion batteries. The composite offers both enhanced porosity and improved thermal stability compared to traditional porous CA separators.
Another approach to utilize the metal compound is to use sodium nitrate (NaNO3) for CA separator [26]. The study reveals that hydrated NaNO3 plasticizes the CA polymer chains, resulting in the formation of pores under the influence of external forces exerted by water pressure. The optimal CA:NaNO3 molar ratio is identified as 1:0.01, yielding a remarkable water flux of 8.6 LMH and a porosity of 78.15%. The thermal stability of CA remains intact even with the incorporation of NaNO3, suggesting that the plasticization primarily increases the interchain distance rather than directly interacting with CA chains [26]. This research presents an environmentally friendly and cost-effective method to manufacture porous CA polymer using sodium salts. The findings offer valuable insights into the potential of salt composition in tailoring material properties.
However, it is observed that even though metal compounds as additives are very effective for preparation of porous CA polymer as battery separator, the pore size is relatively bigger than those of organic compounds, possibly due to the high dissociation energy. Thus, these high dissociation energy causes the hydration effect to be diminished by the aggregation of particles and the decrease in dispersion of particles. Therefore, it is important to consider these effects when metal compounds are utilized for porous CA separators.
Conclusions
In this review, the fabrication and characteristics of porous cellulose acetate (CA) separators for lithium-ion batteries were comprehensively explored as shown in Scheme 1. The goal of tailoring separator properties to enhance battery performance was achieved through the incorporation of diverse additives and water-pressure treatments. Three distinct categories of additives, including hydrated metal nitrate, organic compounds, and metal compounds., were systematically examined for their impact on pore generation, and porosity. For hydrated metal nitrate Cd(NO3)2·4H2O played a role as effective plasticizer than Ni(NO3)2 and Mg(NO3)2 since ionic radius of the Cd among the three elements were largest, resulting in that the relatively larger Cd ions can easily be dissociated into cations and counteranions. As a result, the free nitrate ions can be easily hydrated with water molecules than those of Ni(NO3)2 and Mg(NO3)2, and the plasticization on cellulose acetate is more effective. For organic compounds, glycerin is more effective for generating abundant pores in CA polymers than propylene glycol and lactic acid. It is confirmed that hydroxyl group is more effective than carboxyl groups for high porosity and as the number of hydroxyl groups increases, porosity also increases when organic compounds are utilized for CA separators. For metal compounds, including calcium chloride, calcium oxide, and sodium nitrate, were introduced as additives to promote pore formation in CA separators. Even though metal compounds as additives are very effective for preparation of porous CA polymer as battery separator, the pore size is relatively bigger than those of organic compounds, possibly due to the high dissociation energy. Thus, these high dissociation energy causes the hydration effect to be diminished by the aggregation of particles and the decrease in dispersion of particles. Collectively, this review shows the feasibility of employing hydrated metal nitrate, organic compounds, and metal compounds to prepare porous cellulose acetate separators and it provides guidance for selecting the specific additive for the purpose. The resultant separators exhibit diverse porosity and characteristics, positioning them as strong candidates for enhancing the efficiency of lithium-ion batteries.
References
C. Song, C. Gao, Q. Peng, M.E. Gibril, X. Wang, S. Wang, F. Kong, A novel high-performance electrospun of polyimide/lignin nanofibers with unique electrochemical properties and its application as lithium-ion batteries separators. Int. J. Biol. Macromol. 246, 125668 (2023)
M. Makki, G. Ayoub, C.W. Lee, C. Bae, X. Colin, Effect of battery fast cyclic charging on the mechanical and fracture behavior of the lithium-ion battery separator. Polym. Degrad. Stab. 216, 110469 (2023)
H. Chen, B. Ren, Y. Wang, M. Liu, H. He, L. Chai, J. Jia, X. Yang, J. Chen, B. Li, The fabrication of high-performance α-Al2O3 coated PE separator for lithium-ion batteries based on multiple hydrogen bonds. Electrochim. Acta. 465, 142985 (2023)
M. Bhar, U. Bhattacharjee, K. Yalamanchili, S.K. Martha, Effective upcycling of waste separator and boosting the electrochemical performance of recycled graphite anode for lithium-ion batteries. J. Power Sources 580, 233403 (2023)
W. Miao, J. Wang, G. Li, S. Liu, X. Luo, Superior thermal stability of PVA/cellulose composite membranes for lithium-ion battery separators prepared by impregnation method with noncovalent cross-linking of intermolecular multiple hydrogen-bonds. J. Energy Storage 66, 107353 (2023)
R. Yang, G. Yu, Z. Wu, T. Lu, T. Hu, F. Liu, H. Zhao, Aging of lithium-ion battery separators during battery cycling. J. Energy Storage 63, 107107 (2023)
Y. Liu, S. Lv, M. Zhang, J. He, P. Ni, UV-photopolymerized cellulose acetate-acrylate membranes for lithium-ion battery separator. Colloids Surf. A 667, 131359 (2023)
Y. Sun, J. Wang, J.M. Prausnitz, Interfacial properties between ionic-liquid-based electrolytes and lithium-ion-battery separator. AIChE J. 67, e17208 (2021)
H. Guo, M. Li, F. Li, Q. Zhu, Y. Zhao, F. Wang, Z. Qin, Enhanced wettability of a PTFE porous membrane for a high-temperature stable lithium-ion battery separator. Chem. Eng. Technol. 45, 73 (2022)
J. Lee, J. Yoon, J. Jeon et al., Electrospun PVDF-HFP/PAN bicomponent nanofibers as separators in lithium-ion batteries with high thermal stability and electrolyte wettability. Korean J. Chem. Eng. 40, 1901 (2023)
J. Shin, Y. Kim, J.M. Lee, Feature construction for on-board early prediction of electric vehicle battery cycle life. Korean J. Chem. Eng. 40, 1850 (2023)
S. Han, M. Park, S. Huh et al., The electrochemical performance of lotus-root shaped meso-/macroporous TiO2 anode for lithium-ion battery. Korean J. Chem. Eng. 40, 1234 (2023)
I. Choi, B. Gendensuren, J. Lee et al., Cyanoethyl-guar gum as an effective polymer binder for lithium titanate electrode of the lithium-ion battery. Korean J. Chem. Eng. 40, 802 (2023)
J.H. Choi, J. Hwang, T.J. Embleton et al., Selective outer surface modification of polycrystalline Ni-rich cathode for sulfide all-solid-state lithium-ion battery. Korean J. Chem. Eng. 40, 548 (2023)
M. Nazerian, N. Bahaloo-Horeh, S.M. Mousavi, Enhanced bioleaching of valuable metals from spent lithium-ion batteries using ultrasonic treatment. Korean J. Chem. Eng. 40, 584 (2023)
W.G. Lee, D.H. Kim, W.C. Jeon, S.K. Kwak, S.J. Kang, S.W. Kang, Facile control of nanoporosity in cellulose acetate using nickel(II) nitrate additive with water pressure treatment for highly efficient battery gel separators. Sci. Rep. 7, 1287 (2017)
W.G. Lee, S.W. Kang, Eco-friendly process for facile pore control in thermally stable cellulose acetate utilizing zinc(II) nitrate for water-treatment. J. Ind. Eng. Chem. 81, 88–92 (2020)
W.G. Lee, Y. Cho, S.W. Kang, Effect of ionic radius in metal nitrate on pore generation of cellulose acetate in polymer nanocomposite. Polymers 12, 981 (2020)
W.G. Lee, Y. Cho, S.W. Kang, Effect of ionic radius in metal nitrate on pore generation of cellulose acetate in polymer nanocomposite. Polymers 12, 981–989 (2020)
S.H. Hong, Y. Cho, S.W. Kang, Highly porous and thermally stable cellulose acetate to utilize hydrated glycerin. J. Ind. Eng. Chem. 91, 79–84 (2020)
S.H. Hong, Y. Cho, S.W. Kang, Formation of water-channel by propylene glycol into polymer for porous materials. Membranes 11, 881 (2021)
S.H. Kim, S.W. Kang, Preparation of highly stable cellulose separator by incorporation of lactic acid. Cellulose 28(15), 10055–10063 (2021)
S.H. Kim, S.W. Kang, Interconnected channels through polypropylene and cellulose acetate by utilizing lactic acid for stable separators. Chem. Commun. 57, 8965–8968 (2021)
H.J. Lee, Y. Cho, S.W. Kang, Development of low-cost process for pore generation in cellulose acetate by utilizing calcium salts. J. Ind. Eng. Chem. 94, 419–424 (2021)
H.J. Lee, S.W. Kang, Improvement of stability for cellulose polymer by calcium oxide for application to porous materials. Cellulose 29, 8319–8327 (2022)
H.J. Lee, Y. Cho, S.W. Kang, Low-cost process to utilize sodium salts for porous cellulose materials. J. Ind. Eng. Chem. 113, 368–372 (2022)
H.J. Lee, S.W. Kang, Cellulose acetate containing CaO coated on polypropylene for enhanced thermal stability of separator. Chem. Commun. 57, 4388–4391 (2021)
X. Xie, L. Sheng, C. Arbizzani, B. Gao, X. Gao, L. Yang, Y. Bai, H. Dong, G. Liu, T. Wang, X. Huang, J. He, Multi-functional groups decorated composite nanofiber separator with excellent chemical stability in ester-based electrolyte for enhancing the lithium-ion transport. J. Power Sources 555, 232431 (2023)
C. Cheng, R. Yang, Y. Wang, D. Fu, J. Sheng, X. Guo, A bacterial cellulose-based separator with tunable pore size for lithium-ion batteries. Carbohydr. Polym. 304, 120489 (2023)
Q. Zhao, L. Ma, Y. Xu, X. Wu, S. Jiang, Q. Zheng, G. Hong, B. He, C. Li, W. Cen, W. Zhou, Y. Meng, D. Xiao, An upgraded polymeric composite with interparticle chemical bonding microstructure toward lithium-ion battery separators with enhanced safety and electrochemical performances. J. Energy Chem. 84, 402–413 (2023)
J. Xing, W. Fan, J. Li, Z. Wang, Z. Wei, Y. Zhao, Orientation gradient architecture of nanofibrous separator towards mechanical enhancement and ion transport acceleration for lithium-ion batteries. Electrochim. Acta. 441, 141794 (2023)
Acknowledgements
This study was supported by the University Innovation Support Project through Sangmyung University in 2023.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kang, S.W., Cho, Y. Strategy to Increase the Efficiency of Battery Systems Equipped with Cellulose-Based Separators. Korean J. Chem. Eng. 41, 403–409 (2024). https://doi.org/10.1007/s11814-024-00098-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-024-00098-1