Abstract
To furnish high-quality poly-alpha-olefin oils, hydro-finishing under mild reaction conditions by a novel heterogeneous catalyst was accomplished. In this regard, Pd nanoparticles were immobilized on Boehmite. Then, the effect of hydrogenation variables, i.e., catalytic loading, reaction pressure, and temperature on the catalytic efficiency, was investigated with response surface method. According to the results, a hydrogenation efficiency of 85% was obtained at the optimum reaction conditions of T = 130 °C, P = 8 bar, and catalyst dosage = 5 wt%. Notably, the catalyst was recyclable for four runs with insignificant leaching of palladium nanoparticles. The comparison results suggested Pd/Boehmite catalyst as an appropriate alternative to the commercial Pd/Alumina and Pd/Silica. Hot filtration test also approved heterogeneous nature of catalysis.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nowadays, there is a huge concern to reduce the final cost of catalysts for industries. The heterogeneous catalysts in contrast, due to the recyclability and easily product separability, are acceptable and appropriate alternatives to homogeneous catalysts with practical difficulties [1,2,3].
In recent years, supported heterogeneous catalysts on nanoscale inorganic material have received a great deal of interest in organic reactions [4], [5]. Some kinds of nanostructure materials, such as silica, montmorillonite, and graphene are applied as support [6,7,8]. Among the various nanoparticles, aluminum oxyhydroxide (AlOOH) is known as a suitable support for the production of heterogeneous catalysts [9]. Aluminum oxyhydroxide has two polymorphs: Boehmite (γ-AlOOH) and diaspore, (α-AlOOH) [10, 11]. Boehmite is used as a precursor for the preparation of alumina and could provide great flame retardancy for polymer materials [12,13,14]. It can be prepared in various sizes and shapes by hydrolysis of alkoxides using different aluminum salts or in the other way by peptization of aluminum hydroxides [15,16,17,18]. Low thermal conductivity and proper mechanical stability are two of the numerous advantages of this material. Boehmite nanoparticles have surface hydroxyl groups and high specific surface area and can be considered as a potential support that can lead to high metal loading and high catalytic activity even which without surface treatment [19]. Additionally, according to the literature, Boehmite can also suppress metal nanoparticle leaching, and enhance final reaction efficiency. Boehmite properties can be modified by two methods. The first method is chemical treatment [20,21,22,23,24], such as functionalization with ligands [25,26,27]. The second method is physical treatment, such as magnetizing with Fe3O4 NPs [28].
There are certain reactions using heterogeneous catalysts on various supports [29]. One of the catalytic reactions that are highly attractive from industrial point of view is hydrogenation of polyalphaolefins (PAOs). This class of lubricants is obtained from oligomerization of higher α-olefins (C6 to C12) and benefits from some advantages, such as low pour point and high viscosity index (VI) [30]. The synthesized PAOs have unsaturated C=C double bonds in their backbones that diminishes their oxidation stability. To circumvent this issue, PAOs are hydrogenated using precious metal-based catalysts [31, 32]. To improve the stability and recyclability of the metallic catalysts and decrease the required loading of precious metals, they are mostly supported. To date, some clays and carbohydrates have been applied by our research group as supporting materials [33]. Mostly, we used halloysite clay that is a tubular alumina silicate for stabilization of Pd NPs and development of hydrogenation catalysts [34, 35]. In this regard, mostly halloysite nanotubes were chemically functionalized with ligands [31, 36,37,, 37], polymers [38], ionic liquids [39, 40], and surfactants [41]. In some cases also physical treatment of halloysite was used for modification of its properties [42]. Although halloysite-based catalysts showed excellent catalytic activity for PAO hydrogenation, multi-step functionalization processes were applied for their synthesis, which made their preparation time-consuming and tedious. On the other hand, compared to conventional clays, halloysite is relatively costly. Recently, we have shown excellent performance of Boehmite as a support for hydrogenation catalysts [43]. To the best of our knowledge, there is no report on the utility of Boehmite for developing catalysts for PAO hydrogenation. Hence, as this clay is very cheap and available, in this report, we investigate its performance as a support for the immobilization of Pd nanoparticles (NPs). Moreover, to make the synthetic process facile, cost-effective and environmentally benign, we decided not to modify this clay.
Experimental
Reagents
To prepare the hydrogenation catalyst, the following raw materials were provided from Sigma-Aldrich (Germany): Boehmite, palladium acetate (Pd(OAc)2), sodium borohydride (NaBH4, purity ≥ 96%), toluene and methanol (MeOH, purity ≥ 99.8%). PAO oil was also prepared by using 1-decene, aluminum chloride (AlCl3), and sodium hydroxide (NaOH > 98%), all purchased from Merck (Germany).
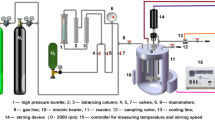
Apparatus
To characterize the as-prepared hydrogenation catalyst, transmission electron microscopy (TEM, Philips CM30300Kv instrument), inductively coupled plasma (ICP, Vista-pro apparatus), X-ray diffraction (XRD, Siemens, D5000 with a Cu Kα radiation), Fourier transform infrared (FTIR, BRUKER, EQUINOX 55 tool, using KBr pellets), SEM/EDS and elemental mapping analysis (TESCAN device), and thermogravimetric analysis (TGA, METTLER TOLEDO device, under O2 and heating rate of 10 °C min−1) were applied.
Characterization of the hydrogenized PAO and calculation of the yield of the reaction were conducted via 1HNMR spectroscopy (Bruker DRX400MHz NMR spectrometer in CDCl3 at ambient temperature).
Synthesis of PAO
PAO applied for this research was prepared in our laboratory according to our previously reported protocol [33]. Typically, a stainless steel reactor was heated up to 80 °C under N2 gas for 1 h to remove the humidity from the reaction media. Subsequently, both monomer (1-decene, 30 g) and the catalyst (AlCl3 (1.5 g, equal to 5 wt% of the monomer)) were charged and the reaction temperature was elevated to 80 °C. The reaction was kept under stirring condition and after 1 h, the reactor was cooled and the oily product was first collected and washed with a solution of NaOH (5 wt%). Finally, the obtained mixture was heated under − 0.8 bar vacuum up to 180 °C to furnish the desired PAO. The yield of this process was 86%.
Preparation of Hydrogenation Catalyst: Pd/Boehmite
Palladation of Boehmite was conducted using facile and known wet-impregnation procedure [31]. More precisely, Boehmite (2 g) was suspended in toluene (35 mL) and homogenized via ultrasonic irradiation (power 100 W) for 10 min. Meanwhile, a solution of Pd(OAc)2 (0.03 g in 5 mL toluene) was prepared and slowly introduced to Boehmite suspension at ambient temperature. Stirring was kept for 4 h under Ar atmosphere, and then, a fresh solution of NaBH4 (0.13 g in 20 mL MeOH) was prepared and gently dropped in the previously mentioned suspension to reduce Pd (II) to Pd (0), which was resulted in a blackish precipitate. After stirring for 2 h, the reaction was stopped and the solid, Pd/Boehmite, was filtered, rinsed with MeOH, and dried at room temperature overnight.
PAO Hydrogenation Using Pd/Boehmite
Hydrogenation of PAO was conducted in a stainless steel reactor under hydrogen pressure of 8 bar at 130 °C. In more detail, the reactor was first purged with nitrogen gas at 100 °C and after that charged with PAO (20 g), Pd/Boehmite (5 wt%), and H2 gas at desire pressure. The reactor content was mixed thoroughly for 8 h at the aforementioned condition. At the end, both circulator and hydrogen flow were shut down and the oily product was collected. Then, separation of Pd/Boehmite was achieved via centrifugation and to recover the separated catalyst, it was washed repeatedly with hexane and dried at 50 °C overnight. To find out the yield of hydrogenation, the oily product was then subjected to 1HNMR spectroscopy.
Results and Discussion
Pd/Boehmite Characterization
In the following, Pd/Boehmite was characterized. To appraise the effect of Pd NPs immobilization on Boehmite structure and crystalline phase, XRD patterns of Boehmite and Pd/Boehmite were compared, Fig. 1. XRD pattern of Boehmite is in good accordance with the literature [44] and consists of sharp characteristic peaks at 2θ = 14.61°, 28.91°, 39.10°, 49.16°, 55.31°, 65.25°, and 72.38°. Pd/Boehmite XRD pattern is identical to that of pristine Boehmite and showed the same characteristic peaks at exactly the same 2θ values with lower intensity. This finding is indicative of Boehmite stability upon wet-impregnation process. Although it was expected that characteristic peaks of Pd NPs would be observable in the XRD pattern of Pd/Boehmite, they were not detectable [43] due to the fine particle size and high dispersion of Pd NPs [45]. The non-shifting of the characteristic peaks during synthesis is in good accordance with previous study [43].
Structure of Pd/Boehmite was also studied using FTIR spectroscopy. In Fig. 2, comparison of FTIR spectra of both Boehmite and Pd/Boehmite indicates that the two compounds have similar FTIR spectra and in both of them the characteristic absorbance bands at 3080 and 3380 cm−1 are observed that are related to the symmetric and asymmetric vibrations of the O–H functionalities of Boehmite [46, 47]. Moreover, the sharp absorbance bands at 1070 and 1161 cm−1 are due to the vibrations of hydrogen bonds of hydroxyl groups [48]. Also, the absorbance bands at 480, 605, and 735 cm−1 are ascribed to Al-O bands absorption [49].
Further characterization of Pd/Boehmite was accomplished by performing EDS and elemental mapping analyses. As depicted in Fig. 3, EDS analysis of the catalyst showed Al, O, and Pd atoms, among which, Al and O atoms are representative of Boehmite structure. The presence of Pd atoms also is indicative of successful impregnation of Pd salt and formation of Pd NPs on Boehmite. Elemental mapping analysis of Pd/Boehmite also confirms high dispersion of Pd NPs.
TEM images of Pd/Boehmite were also recorded to elucidate dispersion of Pd NPs on Boehmite surface. In Fig. 4, a TEM image of Pd/Boehmite is presented. In this image, the dark spots are representative of Pd NPs and their average particle size was estimated to be 4.8 ± 0.2 nm.
Thermal stability of Pd/Boehmite was also investigated and compared with that of Boehmite, Fig. 5. As shown, both Boehmite and Pd/Boehmite thermograms had two weight losses, one below 110 °C (removal of the absorbed water) and another one, at 450 °C that is due to Boehmite decomposition.
Optimization of the Reaction Variables for PAO Hydrogenation
As known, regression models are one of the best statistical processes for estimating the relationships between independent variables. Response surface method (RSM), as one of these models, was used to optimize the reaction conditions [50,51,52,53]. To this purpose, three effective variables on the yield of PAO hydrogenation, i.e., the catalyst amount, hydrogen pressure, and reaction temperature, were investigated and optimized. The limitation areas of selected variables after initial tests are as follows:
Catalyst amount (A) = 3–5 g.
Reaction temperature (B) = 110–130 °C.
Hydrogen pressure (C) = 6–8 bar.
Accordingly, the ANOVA results (analysis of variance) for the reaction variables are represented in Table 1.
The quadratic model for the yield of PAO hydrogenation (Y) is presented in Eq. 1:
As illustrated, the signs of A, C, AB, AC, BC, and B2 are positive in this equation, detecting that these factors have significant effect on the response (yield of PAO hydrogenation). Nevertheless, the signs of B, A2, and C2 are negative, which declared the antagonistic effect of these factors. Particularly, the significance of the factors is as follow:
Notably, the accuracy of the model was assessed by the correlation coefficient value, R2, to be 0.97, which approves a good relationship between theoretical and experimental responses. Furthermore, the values for adjusted R2 and predicted R2 were 0.94 and 0.79, respectively.
Figures 6, 7, and 8 show the 3-D graphs of yield of PAO hydrogenation versus reaction variables (reaction temperature, reactor H2 pressure, and Pd/Bo amount). As demonstrated in Fig. 6, the yield of hydrogenated PAO increased with enhancement of the hydrogen pressure to 8 bar and temperature to 130 °C. Figure 7 also indicates that maximum amount of the yield of hydrogenated PAO (85%) was obtained upon elevating of the temperature to 130 °C and catalyst amount to 5 wt. %. 3-D surface plot of interaction between the amount of catalyst and hydrogen pressure, Fig. 8, approved that increase of the catalyst amount to 5 wt% and hydrogen pressure to 8 bar raised the yield of PAO hydrogenation.
As a result, the optimum condition for PAO hydrogenation reaction was specified as using 5 wt% of catalyst under 8 bar hydrogenation pressure at 130 °C.
In Fig. 9, the 1HNMR spectra of the as-synthesized and hydrogenated PAO are depicted. As shown and according to the quantitative calculation, upon hydrogenation, the intensity of peaks related to unsaturated –C=C bonds (δ = 1.99 ppm) reduced, affirming successful saturation of –C=C double bonds.
Investigation of the Effect of Support
Considering the importance of the support in the performance of heterogeneous catalysts, the efficiency of Pd/Boehmite with two other control catalysts, i.e., Pd/Alumina and Pd/Silica, was compared. The control catalysts were prepared through a same procedure used for the synthesis of the catalyst, except γ-alumina and silica were applied as support, respectively. Comparison of the efficiency of the two control catalysts with that of Pd/Boehmite under the optimized conditions, Table 2, showed that Pd/Boehmite has superior activity compared to the other catalysts. To find out the origin of this observation, the three catalysts have been analyzed via ICP. In fact, as Pd NPs are the dominant catalytic species, it was investigated whether different supports can affect the loading of Pd NPs. As tabulated, upon changing the support, the loading of Pd NPs differs according to the following order: Pd/Boehmite > Pd/Silica > Pd/Alumina. This result approved the role of support on the anchoring of Pd NPs. According to ICP results, higher activity of Pd/Boehmite was attributed to the higher content of Pd NPs in this sample.
Recovery and Recyclability
As mentioned in the experimental section, Pd/Boehmite could be readily collected from the reaction media and recovered. The recovered Pd/Boehmite was then reused for four successive runs of PAO hydrogenation to elucidate whether it is a recyclable catalyst. The obtained yields of hydrogenated PAO for all five runs (fresh and reused catalysts) are depicted in Fig. 10. As presented, reuse of Pd/Boehmite led to slight decrement of its activity upon each run. This issue can stem from leaching of Pd NPs that are the main catalytic species in the hydrogenation reaction. This issue was verified by ICP analysis of the reused Pd/Boehmite (obtained from the last run of hydrogenation reaction). This analysis indicated that Pd leaching was ~ 0.9 wt% of initial Pd loading.
Study of the Nature of the Catalysis
A known method for investigation of the true nature of catalysis in heterogeneous catalysts is hot filtration test [54]. Using this test, it is possible to verify whether Pd/Boehmite catalysis is true heterogeneous and Pd NPs are remained stabilized on the support in the hydrogenation reaction. To this purpose, Pd/Boehmite was removed from the reactor after 3 h and the yield of hydrogenation reaction was measured via 1HNMR spectroscopy. Then, the reactor was sealed and the reaction was continued in the absence of Pd/Boehmite and the reaction yield was traced each hour. As displayed in Fig. 11, upon separation of Pd/Boehmite, the hydrogenation reaction was no longer proceeded, indicating that the catalysis was heterogeneous.
Comparative Study
As mentioned previously, our research group has focused on the development of PAO hydrogenation catalysts and so far we have reported several catalytic systems. To elucidate whether the as-synthesized Pd/Boehmite has a comparable performance with previously reported catalysts, the results of PAO hydrogenation under Pd/Boehmite catalysis were compared with some randomly selected catalysts that have been developed previously, Table 3. As the result indicated, PAO hydrogenation has been conducted in the presence of Pd NPs, which have been supported on functionalized chitosan, halloysite, and halloysite composites. Comparison of the reaction conditions also shows that use of those catalysts led to higher hydrogenation yields. In most cases, the reaction temperature was 130 °C; however, for some catalysts, lower hydrogen pressure was applied. According to these results, although the catalytic activity of Pd/Boehmite is high for PAO hydrogenation, its inferior compared to previous catalyst. However, the main advantage of Pd/Boehmite catalyst is its simple preparation method and low cost. In more detail, for the preparation of Pd/Boehmite, no chemical or physical modification of support was applied, leading to an environmentally benign and cost-effective protocol. On the other hand, Boehmite is an available clay, which is remarkably cheaper than halloysite. Hence, it can be concluded that despite relatively lower activity of Pd/Boehmite, its simplicity and cost-effectiveness can render it a potential catalyst for large-scale uses.
Conclusion
Boehmite as an efficient support was utilized for the immobilization of Pd nanoparticles to attain a new heterogeneous catalyst, Pd/Boehmite. Various analyses were investigated to characterize Pd/Boehmite catalyst. Pd/Boehmite was then applied as a heterogeneous catalyst for the hydrogenation of PAO under relatively mild reaction conditions. Moreover, the effects of reaction variables, i.e., temperature, hydrogen pressure, and catalyst amount, were optimized using RSM. The results showed that Pd/Boehmite catalyst has a good efficiency in the PAO hydrogenation reaction (85%) at the optimum reaction conditions (T = 130, P = 8 bar, and catalyst dosage = 5 wt%). Moreover, the catalyst can be reused for four runs with low Pd NPs leaching and decrement of the catalytic activity. It is worth noting that the efficiency of Pd/Boehmite was higher compared to Pd supported on conventional supports, such as alumina and silica due to the higher anchoring of Pd NPs. Hot filtration test also approves heterogeneous nature of catalysis. Notably, although the activity of Pd/Boehmite is slightly lower than our previously developed halloysite-based catalysts, use of cost-effective Boehmite instead of relatively expensive halloysite as well as avoiding any physical and chemical treatment of support can be considered as the merit of this support.
Data availability
The data used and/or analysed during the current study may be available on reasonable request.
References
Z-E Tang S Lim Y-L Pang H-C Ong K-T Lee 2018 Synthesis of biomass as heterogeneous catalyst for application in biodiesel production: state of the art and fundamental review Renew. Sust. Energ. Rev. 92 235
NM Julkapli S Bagheri 2016 Magnesium oxide as a heterogeneous catalyst support Rev. Inorg. Chem. 36 1
M Toda A Takagaki M Okamura JN Kondo S Hayashi K Domen M Hara 2005 Biodiesel made with sugar catalyst Nature 438 178
M Hajjami A Ghorbani-Choghamarani R Ghafouri-Nejad B Tahmasbi 2016 Efficient preparation of boehmite silica dopamine sulfamic acid as a novel nanostructured compound and its application as a catalyst in some organic reactions New J. Chem. 40 3066
J Karger-Kocsis L Lendvai 2018 Polymer/boehmite nanocomposites: a review J. Appl. Polym. Sci. 135 45573
S Shabbir S Lee M Lim H Lee H Ko Y Lee H Rhee 2017 Pd nanoparticles on reverse phase silica gel as recyclable catalyst for suzuki-miyaura cross coupling reaction and hydrogenation in water J. Organomet. Chem. 846 296
F Huang Y Deng Y Chen X Cai M Peng Z Jia P Ren D Xiao X Wen N Wang 2018 Atomically dispersed pd on nanodiamond/graphene hybrid for selective hydrogenation of acetylene J. Am. Chem. Soc. 140 13142
M Munir M Ahmad M Saeed A Waseem M Rehan A-S Nizami M Zafar M Arshad S Sultana 2019 Sustainable production of bioenergy from novel non-edible seed oil (prunus cerasoides) using bimetallic impregnated montmorillonite clay catalyst Renew. Sustain. Energy Rev. 109 321
M Mohammadi M Khodamorady B Tahmasbi K Bahrami A Ghorbani-Choghamarani 2021 Boehmite nanoparticles as versatile support for organic–inorganic hybrid materials: synthesis, functionalization, and applications in eco-friendly catalysis J. Ind. Eng. Chem. 97 1
P Pardo MA Kojdecki JM Calatayud JM Amigó J Alarcón 2017 Crystalline microstructure of boehmites studied by multi-peak analysis of powder x-ray diffraction patterns Powder Diffr. 32 S87
L Huang Z Yang Y He L Chai W Yang H Deng H Wang Y Chen J Crittenden 2020 Adsorption mechanism for removing different species of fluoride by designing of core-shell boehmite J. Hazard. Mater. 394 122555
T Sun Q Zhuo Y Chen Z Wu 2015 Synthesis of boehmite and its effect on flame retardancy of epoxy resin High Perform. Polym. 27 100
I Kozerozhets G Panasyuk E Semenov M Danchevskaya L Azarova N Simonenko 2020 Transformations of nanosized boehmite and γ-al 2 o 3 upon heat treatment Russ. J. Inorg. Chem. 65 587
X Zhang W Cui KL Page CI Pearce ME Bowden TR Graham Z Shen P Li Z Wang S Kerisit 2018 Size and morphology controlled synthesis of boehmite nanoplates and crystal growth mechanisms Cryst. Growth Des. 18 3596
V Jokanovic B Jokanovic B Markovic-Todorovic Z Markovic 2009 Synthesis and characterization of hydrothermally obtained colloidal pseudoboehmite/boehmite J. Optoelectron. Adv. Mater. J. 11 164
I Kozerozhets G Panasyuk E Semenov M Vasil’ev YD Ivakin M Danchevskaya 2020 How acid medium affects the hydrothermal synthesis of boehmite Russ. J. Inorg. Chem. 65 1529
H Alinezhad M Zabihi D Kahfroushan 2020 Design and fabrication the novel polymeric magnetic boehmite nanocomposite (boehmite@ fe3o4@ pla@ sio2) for the remarkable competitive adsorption of methylene blue and mercury ions J. Phys. Chem. Solids 144 109515
AA Al-Rashed R Ranjbarzadeh S Aghakhani M Soltanimehr M Afrand TK Nguyen 2019 Entropy generation of boehmite alumina nanofluid flow through a minichannel heat exchanger considering nanoparticle shape effect Phys. A Stat. Mech. 521 724
A Jabbari B Tahmasbi M Nikoorazm A Ghorbani-Choghamarani 2018 A new pd-schiff-base complex on boehmite nanoparticles: its application in suzuki reaction and synthesis of tetrazoles Appl. Organomet. Chem. 32 e4295
B Tahmasbi A Ghorbani-Choghamarani P Moradi 2020 Palladium fabricated on boehmite as an organic–inorganic hybrid nanocatalyst for c–c cross coupling and homoselective cycloaddition reactions New J. Chem. 44 3717
A Jabbari P Moradi M Hajjami B Tahmasbi 2022 Tetradentate copper complex supported on boehmite nanoparticles as an efficient and heterogeneous reusable nanocatalyst for the synthesis of diaryl ethers Sci. Rep. 12 11660
A Jabbari P Moradi B Tahmasbi 2023 Synthesis of tetrazoles catalyzed by a new and recoverable nanocatalyst of cobalt on modified boehmite nps with 1, 3-bis (pyridin-3-ylmethyl) thiourea RSC Adv. 13 8890
P Moradi T Kikhavani Y Abbasi Tyula 2023 A new samarium complex of 1, 3-bis (pyridin-3-ylmethyl) thiourea on boehmite nanoparticles as a practical and recyclable nanocatalyst for the selective synthesis of tetrazoles Sci. Rep. 13 5902
A Ghorbani-Choghamarani Z Heidarnezhad B Tahmasbi 2019 New complex of copper on boehmite nanoparticles as highly efficient and reusable nanocatalyst for synthesis of sulfides and ethers ChemistrySelect 4 8860
A Ghorbani-Choghamarani Z Heidarnezhad B Tahmasbi G Azadi 2018 Tedeta@ bnps as a basic and metal free nanocatalyst for knoevenagel condensation and hantzsch reaction J. Iran. Chem. Soc. 15 2281
A Ghorbani-Choghamarani Z Seydyosefi B Tahmasbi 2018 Tribromide ion supported on boehmite nanoparticles as a reusable catalyst for organic reactions C. R. Chim. 21 1011
A Ghorbani-Choghamarani Z Seydyosefi B Tahmasbi 2018 Zirconium oxide complex anchored on boehmite nanoparticles as highly reusable organometallic catalyst for c–s and c–o coupling reactions Appl. Organomet. Chem. 32 e4396
P Moradi 2022 Investigation of fe 3 o 4@ boehmite nps as efficient and magnetically recoverable nanocatalyst in the homoselective synthesis of tetrazoles RSC Adv. 12 33459
M Flytzani-Stephanopoulos 2014 Gold atoms stabilized on various supports catalyze the water–gas shift reaction Acc. Chem. Res. 47 783
H Shao X Gu R Wang X Wang T Jiang X Guo 2020 Preparation of lubricant base stocks with high viscosity index through 1-decene oligomerization catalyzed by alkylaluminum chloride promoted by metal chloride Energy Fuel 34 2214
M Tabrizi S Sadjadi G Pareras M Nekoomanesh-Haghighi N Bahri-Laleh A Poater 2021 Efficient hydro-finishing of polyalfaolefin based lubricants under mild reaction condition using pd on ligands decorated halloysite J. Colloid Interface Sci. 581 939
T Bazanov L Petrov B Psikha S Psikha V Kharitonov 2008 Effect of hydrogenation on the oxidation resistance of decene oligomers Pet. Chem. 48 296
M Alleshagh S Sadjadi H Arabi N Bahri-Laleh E Monflier 2022 Pd on ligand-decorated chitosan as an efficient catalyst for hydrofinishing polyalphaolefins: experimental and computational studies J. Phys. Chem. Solids 164 110611
S Dehghani S Sadjadi N Bahri-Laleh M Nekoomanesh-Haghighi A Poater 2019 Study of the effect of the ligand structure on the catalytic activity of pd@ ligand decorated halloysite: combination of experimental and computational studies Appl. Organomet. Chem. 33 e4891
S Sadjadi F Koohestani 2022 Pd on imidazolium ionic liquid modified halloysite: a potent catalyst for the hydrogenation of nitro-compounds under mild reaction condition Inorg. Chem. Commun. 137 109205
Z Asadi S Sadjadi M Nekoomanesh-Haghighi S Posada-Pérez M Solà N Bahri-Laleh A Poater 2022 Lubricant hydrogenation over a functionalized clay-based pd catalyst: a combined computational and experimental study Appl. Organomet. Chem. 36 e6850
A Bayat S Sadjadi H Arabi N Bahri-Laleh 2022 Catalytic hydrofinishing of polyalphaolefins under mild condition using pd on amino acid-functionalized clay: study of the kinetic parameters Inorg. Chem. Commun. 144 109923
S Karimi N Bahri-Laleh G Pareras S Sadjadi M Nekoomanesh-Haghighi A Poater 2021 Pd on nitrogen rich polymer–halloysite nanocomposite as an environmentally benign and sustainable catalyst for hydrogenation of polyalfaolefin based lubricants J. Ind. Eng. Chem. 97 441
M Mehdizadeh S Sadjadi A Poater A Mansouri N Bahri-Laleh 2022 Molecular modelling aided catalyst design for pao oils hydrofinishing J. Mol. Liq. 352 118675
A Bayat S Sadjadi H Arabi N Bahri-Laleh 2022 Dual-task composite of halloysite and ionic liquid for the synthesis and hydrogenation of polyalphaolefins Res. Chem. Intermed. 48 3171
A Shams S Sadjadi J Duran S Simon A Poater N Bahri-Laleh 2022 Effect of support hydrophobicity of halloysite-based catalysts on the polyalphaolefin hydrofinishing performance Appl. Organomet. Chem. 36 e6719
Z Asadi S Sadjadi M Nekoomanesh-Haghighi N Bahri-Laleh 2022 Effects of acid-treatment of halloysite on the characteristics and catalytic performance of palladated halloysite in lubricants hydrogenation reaction Inorg. Chem. Commun. 140 109438
S Sadjadi N Abedian-Dehaghani MM Heravi 2022 Pd on cyclotriphosphazen-hexa imine decorated boehmite as an efficient catalyst for hydrogenation of nitro arenes under mild reaction condition Sci. Rep. 12 15040
A Ghorbani-Choghamarani H Aghavandi M Mohammadi 2020 Boehmite@sio2@ tris (hydroxymethyl)aminomethane-cu(i): a novel, highly efficient and reusable nanocatalyst for the c-c bond formation and the synthesis of 5-substituted 1h-tetrazoles in green media Appl. Organomet. Chem. 34 e5804
S Mallik SS Dash KM Parida BK Mohapatra 2006 Synthesis, characterization, and catalytic activity of phosphomolybdic acid supported on hydrous zirconia J. Colloid Interface Sci. 300 237
A Ghorbani-Choghamarani B Tahmasbi 2016 The first report on the preparation of boehmite silica sulfuric acid and its applications in some multicomponent organic reactions New J. Chem. 40 1205
K Bahrami MM Khodaei M Roostaei 2014 The preparation and characterization of boehmite nanoparticles-tapc: a tailored and reusable nanocatalyst for the synthesis of 12-aryl-8, 9, 10, 12-tetrahydrobenzo [a] xanthen-11-ones New J. Chem. 38 5515
L Rajabi A Derakhshan 2010 Room temperature synthesis of boehmite and crystallization of nanoparticles: effect of concentration and ultrasound Sci. Adv. Mater. 2 163
A Ghorbani-Choghamarani B Tahmasbi F Arghand S Faryadi 2015 Nickel schiff-base complexes immobilized on boehmite nanoparticles and their application in the oxidation of sulfides and oxidative coupling of thiols as novel and reusable nano organometal catalysts RSC Adv. 5 92174
T Rahimi D Kahrizi M Feyzi HR Ahmadvandi M Mostafaei 2021 Catalytic performance of mgo/fe2o3-sio2 core-shell magnetic nanocatalyst for biodiesel production of camelina sativa seed oil: optimization by rsm-ccd method Ind. Crops Prod. 159 113065
S Sadjadi MM Heravi M Malmir 2018 Bio-assisted synthesized ag (0) nanoparticles immobilized on sba-15/cyclodextrin nanosponge adduct: efficient heterogeneous catalyst for the ultrasonic-assisted synthesis of benzopyranopyrimidines Appl. Organomet. Chem. 32 e4286
H Soleimanzadeh A Niaei D Salari A Tarjomannejad S Penner M Grünbacher SA Hosseini SM Mousavi 2019 Modeling and optimization of v2o5/tio2 nanocatalysts for nh3-selective catalytic reduction (scr) of nox by rsm and ann techniques J. Environ. Manag. 238 360
TS Singh TN Verma 2019 Taguchi design approach for extraction of methyl ester from waste cooking oil using synthesized cao as heterogeneous catalyst: response surface methodology optimization Energy Convers. Manag. 182 383
S Shinde K Tarade G Mitra C Rode 2020 Integration of heterogeneous acid and base catalysis for clean synthesis of jet-fuel precursor from carbohydrates ChemistrySelect 5 392
M Alleshagh S Sadjadi H Arabi N Bahri-Laleh E Monflier 2022 Palladated chitosan-halloysite bead as an efficient catalyst for hydrogenation of lubricants Mater. Chem. Phys. 278 125506
Acknowledgements
The authors appreciate support of Iran Polymer and Petrochemical Institute.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Asadi, Z., Sadjadi, S., Bahri-Laleh, N. et al. Pd Nanoparticles Immobilized on Boehmite as an Efficient Heterogeneous Catalyst for Lubricant Hydrogenation. Korean J. Chem. Eng. 41, 933–943 (2024). https://doi.org/10.1007/s11814-024-00084-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-024-00084-7