Abstract
This study produced rGO/NiO nanohybrid through a simple hydrothermal strategy for photocatalytic degradation of noxious malachite green (MG) pollutants. Nanocomposites’ chemical structure was verified by X-ray diffraction (XRD), ultraviolet visible spectroscopy (UV–Vis. Spectroscopy), X-ray photoelectron spectroscopy (XPS) analysis and Raman spectra. Bandgap narrowing of nickel oxide was observed by UV–Visible spectroscopy after its integration with rGO. In terms of photocatalytic efficiency, rGO/NiO nanocomposites show remarkable efficiency (96.15%) in degrading malachite green dye than NiO (81.73%) under 120 min. rGO/NiO nanocomposites exhibit a 0.006 min−1 rate constant, which is 2% greater than bare nickel oxide. The scavenging analysis demonstrated that hydroxyl radicals and superoxide ion play a vital role in photodegradation. The photocatalyst’s repeatability was determined through a cyclic test. The remarkable photocatalytic efficiency was obtained due to the synergetic behavior of rGO and NiO. rGO provided a greater surface area exhibiting greater number of active zones responsible for the adsorption of electrolyte ions. This rGO/NiO nanohybrid has been employed as a photocatalyst in efficiently removing noxious pollutants from wastewater.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The growth of industry was quickened and productivity was enhanced by the industrial revolution. Because of this, severe problems have arisen all across the world, one of which is water contamination [1,2,3,4]. Malachite green (MG) is a widely used dye that has been shown to leach into waterways at alarming rates. It has adverse effects on human health and may have an impact on aquatic phytoplankton by reducing their ability to produce oxygen through photosynthesis [5,6,7]. The primary goal of the research was on the production of photocatalysts, as this is one of the most practical behaviors to eliminate environmental toxins and generate renewable energy [8, 9].
The physical, biological and chemical approaches to removing colors from wastewater are discussed in several published works. Coagulation [10], adsorption [11], activated sludge treatment [12] and electrochemical oxidation [13] and is only some of the standard approaches that have been explored. The photocatalytic degradation technique makes good use of catalysts. The hydroxyl radical (•OH) generated by the photoreaction enhances the transformation of effluents into innocuous species like CO2 and H2O [14, 15]. For widespread use, wastewater treatment must be both economical and feasible. Among the many potential benefits of wastewater treatment is the potential for large water savings that may be recycled across several industries and fields, including those involved in manufacturing and farming [16].
Scientists have attracted much attention to the degradation of organic contaminants using semiconductor photocatalysts during the past decade, thanks to their remarkable stability, low cost, environmental friendliness and non-toxicity [17, 18]. Transition metal oxide (TMOx) nanostructure or nanohybrids have been proposed as proficient photocatalysts for photomineralization of noxious dyes [19] and HER/OER electrocatalyst ZnO, NiF2O4 and CuS [20,21,22,23,24,25]. Among the several TMOx, nickel oxide (NiO) nanomaterials were selected as a photocatalyst due to their bandgap of 4.0–3.4 eV [26]. It is essential in several fields of research, including biology, the removal of hazardous dyes and other colors, environmental science and inorganic contaminants [27]. The high coagulation tendency low light transmittance and poor dispersibility of this material limit its use in a wide range of environmental contexts. Because of this shortcoming, new developments in this area are possible [26].
Nanohybrids including metal oxides and noble metals, graphene and metal oxides have garnered a lot of interest because of their remarkable photocatalytic activity [28, 29]. Graphene/MOx nanocomposites are the best candidate owing to large electron mobility, large specific surface area (SSA), and flexibility of their sheet resistance [30]. For photocatalytic uses, rGO, which has been exceptionally reduced, is a great option. In the pursuit of nanohybrid photocatalysis, reduced graphene oxide is often employed as the electron transport layer. The segregation and transmission effectiveness of photo-induced carriers are facilitated by rGO that reduces electron–hole (e- & h+) pair rejoining [31]. rGO’s band gap allows it to be employed as a photocatalyst, allowing scientists to venture into unexplored terrain. rGO may be able to prevent the spread of harmful bacteria, according to research [32, 33]. Therefore, it has the potential to be a beneficial material in a variety of biological settings. As a result, there has been extensive research into developing rGO/WO3 hybrids with the aim of boosting photocatalytic activity. Similarly, a recent study by K. Rahimi et al. study that a NiO nanohybrid with rGO has greater photocatalytic behavior towards methyl orange dye mineralization. In terms of photocatalytic activity, rGO/NiO composites synthesized using the hydrothermal strategy were shown to be superior to pure NiO [14]. rGO/NiO nanocomposites were also developed by Amer Al-Nafiey et al. utilizing a low-temperature solution to increase photocatalytic behavior and reduce nitrogen containing compounds when exposed to visible light [34]. Hydrothermally produced rGO/NiO composite apparently destroyed 99.9% of MO in around 50 min, conducted by V. Shanmugam et al. P. Vivek et al. investigate the photocatalytic features of rGO/NiO nanohybrid for decomposition of rhodamine B (RhB). The result suggests that the rGO/NiO nanohybrid exhibited a 95.4% photocatalytic efficiency than bare NiO (86%) [35]. In the light of above discussion, different scientist explores the photocatalytic behavior of rGO/NiO toward the photomineralization of MB, MO and RhB dye as model pollutant. Therefore, we are attractive to hydrothermally synthesized rGO/NiO photocatalytic properties for malachite green (MG) dye as model pollutant dye under UV light.
In this research, we developed rGO/NiO nanohybrid using a quick hydrothermal technique. The characteristics of the fabricated nanohybrid were determined with different instrumental techniques. The photocatalytic behavior for the breakdown of malachite green (MG) under UV irradiation was improved by the rGO/NiO nanohybrid. As a result, rGO/NiO nanocomposite shows that MG dye was degraded with 96.15% more efficiency than bare NiO. Our results show that the improved carrier separation efficacy of the rGO/NiO nanocomposites was the main cause of the higher photocatalytic activity.
Experimental Segment
Fabrication of NiO
In the typical synthesis of NiO, 0.1 M nickel nitrate (Ni(NO3)20.6 H2O, Sigma Aldrich, 99%) was dissolved in the 100 mL of deionized (DI) H2O under constant stirring. The 3.0 M of sodium hydroxide (NaOH, Merck, 98%) was inserted into the nickel nitrate aqueous solution to maintain the pH of 11 and agitated for 30 min. The resulting mixture was transferred into the hydrothermal reactor (HR) and thermally treated at 443.15 K for 6 h. After cooling the HR at ambient conditions, the produced precipitates were rinsed several times with DI H2O and ethanol (C2H5OH, Sigma Aldrich, 99%). The rinsed product was dehydrated at 323.15 K for overnights and calcined at 723.15 K for 5 h to remove loosely attached water species.
Synthesis of rGO/NiO Nanohybrid
The rGO/NiO was synthesized with the hydrothermal method and rGO were synthesized by following the same procedure as reported earlier [36]. The prepared 0.5 g nickel oxide and 0.5 g rGO (1:1 molar ratio) was combined in 50 mL deionized water (DI H2O) under the vigorous stirring. The combined mixture was then sonicated for 20 min and shifted into HR and placed into a muffle furnace for 5 h at 443.15 K. The obtained precipitates were separated with centrifugation and then rinsed with ethanol and DI H2O. The rinsed product was dehydrated with 323.15 K overnight and saved it for further characterizations.
Physio-Chemical Analysis
The purity and crystalline phase of the fabricated products were measured using CuKα radiation (1.54 Å) with a Rigaku D/Max-2200 X-ray diffraction spectrometer (40 kV). The surface functionality of the materials was investigated with fourier transform infrared spectroscopy (FT-IR, JASCO 6800). Absorption spectra of the synthesize products were recorded using a UV-spectrometer (JASCO, UV-VIS V740). Raman spectroscopy was used to characterize the chemical makeup of the synthesized material with an NVIA spectrometer at room temperature. A scanning electron spectroscopy (SEM) was utilized to investigate the surface morphology of newly synthesized materials (0.1–30 kV) with Qunata 200 FEG. X-ray photoelectron spectroscopy (XPS) was employed to determine the binding energy and electronic state of rGO/NiO nanocomposites (Versa probe III) using Al Kα (1486.6 eV).
Photocatalytic Performance
Photocatalytic tests evaluated the material’s ability to degrade antimicrobial pollutants like malachite green (MG), when exposed to ultraviolet light (450 W, Mercury lamp). The initial concentration was established by creating a 10 ppm water solution of malachite green (MG) and scanning it with a UV–Visible instrument at 616 nm. For this experiment, 0.005 g of fabricated product was diluted into a 10 ppm aqueous dye solution in a 50 mL volume. The reaction solution was stirred in the dark for 1 h to establish adsorption/desorption equilibrium between the dye molecules and produced product. The absorbance of a dye was measured in the UV–Visible range so that adsorption capabilities could be evaluated. Aliquots were taken at regular intervals of 20 min during the reaction, then centrifugated at 5000 rpm for 5 min to remove the catalyst and purify the dye mixture. Finally, a UV–Vis spectrophotometer was employed to investigate the photomineralization of MG pollutant with the following expression 1 [37].
Herein, Ct and Co expressed the amount of MG dye at any time and the concentration of dye at the initial time, respectively.
Results and Discussion
Physical Analysis
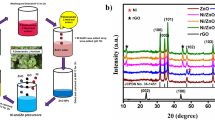
The XRD diffraction analysis of both NiO and rGO/NiO is represented in Fig. 1a and used to investigate the crystal structure and other crystallographic features of generated products in the range of 20–90°. The XRD spike of nickel oxide observed at 37.65°, 43.65°, 63.26°, 75.66° and 79.67° belong to (111), (200), (200), (311) and (222), respectively, and well matched with JCPDs No. of 00-001-1239. The NiO displayed the cubic crystal system with crystal feature of α = β = γ = 90° and a = b = c = 4.17 Å. The XRD diffractogram of rGO/NiO displays the all-diffraction spike of NiO and the distinctive diffraction peak of rGO at 23.84° for 002 indicating the successful fabrication of rGO based nanohybrid. The shifting of the diffraction angles toward the lower angles also due to the incorporation of rGO in NiO, indicate the expansion of the crystal system [38]. Scherrer equation was used to compute the crystal size of NiO and rGO/NiO are 52 nm and 26 nm, respectively, with expression 2 [16].
Herein, λ = Wavelength of X-rays for Cu as the radiant source, K = 0.9 shape factor (Scherer constant), θ = Bragg’s angle and β = full width half maxima (FWHM), respectively. The small crystallite size was plays crucial role in the enhancement of the photocatalytic process [39]. Figure 1b represents the surface functional moieties present on the interface of fabricated product investigate with Fourier transform infrared spectroscopy (FT-IR) in the range of 4000–400 cm−1. The board spectrum band present in the range of 3300 to 3700 represents the OH adsorbed on the interface of the photocatalyst. The distinctive band that appeared below 1000 cm−1 is responsible for the Zn-O stretching band in the FT-IR of NiO. The FT-IR of rGO/NiO display the characteristics reflection peak at 1668.12 and 1389.87 cm−1 are due to C=C and C=O are appeared due to the successful incorporation of rGO and the other two bands located below 1000 cm−1 region responsible in NiO nanostructure. However, the existence of NiO and rGO reflection speaks in the rGO/NiO indicates the successful fabrication of nanohybrid.
Figure 1c displays the Raman spectrum of nickel oxide and rGO/NiO nanohybrid. It is believed that the optical mode of a first-order longitudinal vibration is responsible for the noticeable peak at 539 cm−1 (LO). The nanocomposites showed a little alteration in the phonon modes, pointing to an electron transfer among the nickel oxide nanoparticle and rGO nanosheet. By lowering contact resistance, this transfer accelerates the rate of electron transport. Moreover, the rGO/NiO nanocomposites’ maxima at 1355 cm−1 and 1617 cm−1 are D and G of rGO, respectively. The D band appeared due to structural defects in the graphene lattice such as sp3 hybridized carbon atoms or vacancy defects. and G band is related to the in-plane stretching vibration of the sp2 hybridized carbon atoms in the graphene lattice [40]. The nickel oxide and rGO sheets appear to interact well, and the existence of both NiO and rGO is confirmed by the Raman spectra of the NiO/rGO nanocomposite.
The weight decomposition of rGO/NiO nanocomposite and NiO was determined using thermogravimetric analysis (TGA) on all samples. The observed mass loss below 450 °C in rGO/NiO samples due to the presence of adsorbed H2O and residual oxygen functional groups on rGO. The second decomposition in rGO/NiO nanocomposite upto 650 °C is due to the destruction of carbon skeleton in rGO. Then, there was no weight loss was observed upto 800 °C indicating that material exhibited great thermal stability. However, the NiO displays a small change in the weight loss than the nanocomposite suggesting the absence of rGO contents and purity of the materials.
X-Ray Photoelectron Spectroscopy (XPS) was used to probe the valence states and interaction types in NiO and rGO/NiO. The full width spectra represent in the Fig. 2a show the existence of nickel, oxygen and carbon elements. Figure 2b depicts the three deconvoluted band of graphenic carbon 1s located at 283.95, 285.21 and 287.88 eV belonging to C-C, C-O and O=C-O, respectively. Figure 2c represents the two deconvoluted curve of oxygen 1s at 528.12 and 530.98 eV belonging to nickel-oxygen and O-C=O, respectively. Figure 2 (d) represents the four deconvoluted band appeared at 853.12 and 872.81 eV are belonging to Ni 3p3/2 and Ni 3p1/2 and other two bands at 860.20 & 879.76 eV are corresponding to satellite peaks, respectively. The existence of a carbon-based peak in rGO/NiO confirms the successful fabrication of nanohybrid.
The optical property of both fabricated materials was investigated with a UV–Visible spectrophotometer in the 200 to 800 nm range. The optical results show that the NiO displays absorption maxima in the UV region, however, the incorporation of rGO into NiO is more toward the visible region as represented in Fig. 3a. The bandgap of the fabricated product is calculated using the fundamental absorption, which is correlated to the movement from the valence band (V.B) to the conduction band (C.B). The bandgap property of the both materials was estimated with Tauc’s plot with the following expression 3 [41].
Here, \(\alpha\) = absorption coefficient, hv = photon energy, A is optical constant, n is indirect transitions and Eg is bandgap energy. The estimated bandgap value of nickel oxide and rGO/NiO are 3.45 and 3.27 eV, respectively. The results show that the incorporation of the rGO into NiO reduces the bandgap high energy to the lower energy region toward the visible region.
Photoluminescence is a useful technique for determining the nature of intrinsic point defects in thin films, such as nickel vacancies, interstitial oxygen, and interstitial nickel, oxygen vacancies. Photoluminescence (PL) spectra of newly produced and annealed NiO thin films are shown in Fig. 3c. When the excitation energy in NiO is greater than the bandgap energy, an electron is propelled from the V.B to the C.B, creating holes (h+) in the V.B in the process. These h+ movement from the V.B to the deeper levels, where they combine with donor electrons and conduction electrons (e−) from the upper energy levels. The PL results suggests that the incorporation of rGO into NiO reduced the PL intensity of the rGO/NiO indicating lower the electron–hole (e−/h+) recombination.
The SEM analysis shows that the NiO exhibited agglomerated nanoparticles and some of them are highly diffused into each other as represented in Fig. 4a. The rGO/NiO nanocomposite displays the diverse morphology having nanoparticles anchored on the rGO nanosheet which reduces the aggregation of nanoparticles display a large interfacial area as depicted in Fig. 4b. The elemental confirmation was confirmed with EDX analysis indicating the existence of Ni, O and C and the existence of no other substance confirms the purity of the nanomaterials.
Photocatalytic Evaluation
The rate of photomineralization of malachite green (MG) dye under UV irradiation mimic was employed to investigate the photocatalytic behavior of nano-powders produced in this study. The time-dependent UV–Vis spectrum of MG dye was examined in the existence of different photocatalysts, with NiO and rGO/NiO as represented in Fig. 5a, b. The surface reactivity of photocatalysts towards the adsorption of dye molecules is crucial during photocatalysis. The absorption capability of various photocatalysts in the dark was demonstrated by the spectra obtained at 0 min. After 120 min of irradiation, bare NiO showed low photodegradation with removal rates of only 81.73% of the MG dye, respectively, due to photo-induced electron–hole pairs that quickly recombine, making ultraviolet energy harvesting impractical. Furthermore, the development of semiconductor- carbon (NiO-rGO) heterojunctions, improved visible light response or suitable optical band gap, and effective separation of photo-exciton species caused by rGO led to a synergistic improvement in the photocatalytic performance of rGO/NiO (96.15%) measured with Eq. 1. The comparison between the reported research and fabricated material is given in Table 1. Additionally, the high interfacial area of reduced graphene oxide facilitated the transport of MG molecules over the photocatalytic surface by enabling counterbalanced face-to-face stacking between MG molecules and rGO. Adsorbed molecules on the photocatalyst surface have a shorter half-life than those in solution. The comparison between the photocatalytic degradation efficiency of nickel oxide and rGO/NiO is displayed in Fig. 5c. The reduction in concentration of MG dye with respect to time is represented in Fig. 5d. The results show that the rGO/NiO shows faster degradation of MG than the NiO nanoarray.
According to the pseudo first-order Langmuir-Hinshelwood model, the photocatalytic effects of pure nickel oxide and rGO/NiO composite on the rate of MG degradation may be explained with expression 4 [47].
The concentration of dye in MB (measured in parts per million or ppm) at a given time t is represented by Ct. MG (ppm) denotes the concentration after adsorption equilibrium has been achieved, but before irradiation. The reaction rate constant (k) is measured in units of time (minutes or min). Figure 6a shows the plot with time on the x-axis and ln(C/Ct) on the y-axis. This was done after studying the kinetics of MG dye degradation for different times (min). The resulting graph shows straight lines, indicating a first-order response mechanism. By calculating the slope of these lines, the estimated rate constant values can be determined. The estimated rate constant of nickel oxide and rGO/NiO are 0.02 and 0.06 min−1. The highest value of the rate constant indicates the faster degradation rate of MG for rGO/NiO with respect to the time than bare NiO. Five identical tests were carried out using the reaction conditions illustrated in Fig. 6b to evaluate the reusability of the rGO/NiO nanocomposite. The findings indicate that the composite reduced a photocatalytic efficiency of 6% even after undergoing five cycles, which highlights its exceptional stability and reusability. The existence of MG intermediates adsorbed on the catalyst’s interface may cause a slight decrease in its photocatalytic efficiency. The scavenger test is a method utilized to gain insight into the involvement of photogenerated reactive species (superoxide radical, hydroxyl radical, electron and holes) in photocatalysis. When rGO/NiO was present with IPA, Na2C2O4 and BQ acted as scavengers, removing superoxide anion radicals (O2), photogenerated holes (h+), and hydroxyl radicals (OH.). Figure 6c illustrates the contribution of reactive intermediate species in the photomineralization of MG dye. The results shows that hydroxyl radicals are primarily responsible for the mineralization of MG when exposed to a UV–Visible radiant source. In addition, IPA, Na2C2O4 and BQ show scavenger degradation percentages of 46, 58 and 36%, respectively, with rGO/NiO nanohybrid.
The structural stability of the rGO/NiO nanohybrid was determined with XRD stability analysis. The results revealed that the material showed slight variation in the XRD diffractogram after 5 reusability tests as represented in Fig. 6d. The minor decline in the stability was attributed to the destruction of the nanostructure or may be the blockage of active sites (see Fig. 7).
Photodegradation Mechanism
Conclusion
In this study, we present the production process of a novel rGO/NiO nanocomposite using a hydrothermal method. By conducting various spectral investigations such as SEM, XRD, Raman spectrometry, XPS, and UV–Visible light, we were able to examine the, morphological structural and optical features of the nanocomposites. The incorporation of rGO enabled us to create rGO/NiO nanocomposites, which showed improved photocatalytic activity when compared to bare NiO under UV light exposure. The synthesized nanocomposites were effective in the degradation of MG dye (96.1%) as demonstrated by the scavenger test, a considerable improvement over pure NiO (81%). Additionally, we observed the breakdown of MG dye in part by OH radicals. The photostability and reusability of rGO/NiO nanocomposites were also outstanding in five successive cycles. Thus, these nanocomposites have the potential to be utilized as photocatalysts in the elimination of different noxious pollutants from wastewater and further modification in the structural and morphological features can also help to resolve the other environmental-related issues.
References
M. Jothibas, E. Paulson, A. Mathivanan, S. Srinivasan, K.S. Kannan, Biomolecules influences on the physiochemical characteristics of ZnO nanoparticles and its enhanced photocatalysis under solar irradiation. Nanotechnol Environ. Eng. 1, 1–23 (2023). https://doi.org/10.1007/S41204-023-00310-3/FIGURES/14
G. Kaur, M. Kaur, A. Thakur, A. Kumar, FeS2 pyrite nanostructures: an efficient performer in Photocatalysis 2020:55–71. https://doi.org/10.1007/978-3-030-16427-0_3
S. Khan, Q. Guan, Q. Liu, Z. Qin, B. Rasheed, X. Liang et al., Synthesis, modifications and applications of MILs Metal-organic frameworks for environmental remediation: the cutting-edge review. Sci. Total Environ. 810, 152279 (2022). https://doi.org/10.1016/J.SCITOTENV.2021.152279
A. Jose, S.D.K.R. Pai, D. Pinheiro, K. Kasinathan, Visible light photodegradation of organic dyes using electrochemically synthesized MoO3/ZnO. Environ. Sci. Pollut Res. 28, 52202–52215 (2021). https://doi.org/10.1007/S11356-021-14311-9/FIGURES/9
F. Bessaha, K. Marouf-Khelifa, I. Batonneau-Gener, A. Khelifa, Characterization and application of heat-treated and acid-leached halloysites in the removal of malachite green: adsorption, desorption, and regeneration studies. New. Pub Balaban. 57, 14609–14621 (2015). https://doi.org/10.1080/19443994.2015.1063090
I.D. Mall, V.C. Srivastava, N.K. Agarwal, I.M. Mishra, Adsorptive removal of malachite green dye from aqueous solution by bagasse fly ash and activated carbon-kinetic study and equilibrium isotherm analyses. Colloids Surf. Physicochem Eng Asp. 264, 17–28 (2005). https://doi.org/10.1016/J.COLSURFA.2005.03.027
A. Iqbal, E. Cevik, A. Bozkurt, S.M.M. Asiri, O. Alagha, T.F. Qahtan et al., Ultrahigh adsorption by regenerable iron-cobalt core-shell nanospheres and their synergetic effect on nanohybrid membranes for removal of malachite green dye. J. Environ. Chem. Eng. 10, 107968 (2022). https://doi.org/10.1016/J.JECE.2022.107968
A. Demirbas, Potential applications of renewable energy sources, biomass combustion problems in boiler power systems and combustion related environmental issues. Prog Energy Combust Sci. 31, 171–192 (2005). https://doi.org/10.1016/J.PECS.2005.02.002
M.U. Nisa, S. Manzoor, A.G. Abid, N. Tamam, M. Abdullah, M. Najam-Ul-Haq et al., CdSe supported SnO2 nanocomposite with strongly hydrophilic surface for enhanced overall water splitting. Fuel. 321, 124086 (2022). https://doi.org/10.1016/J.FUEL.2022.124086
T. Saitoh, K. Shibata, M. Hiraide, Rapid removal and photodegradation of tetracycline in water by surfactant-assisted coagulation–sedimentation method. J. Environ. Chem. Eng. 2, 1852–1858 (2014). https://doi.org/10.1016/J.JECE.2014.08.005
K.K. Chenab, B. Sohrabi, A. Jafari, S. Ramakrishna, Water treatment: functional nanomaterials and applications from adsorption to photodegradation. Mater. Today Chem. 16, 100262 (2020). https://doi.org/10.1016/J.MTCHEM.2020.100262
C. Cai, J. Liu, Z. Zhang, Y. Zheng, H. Zhang, Visible light enhanced heterogeneous photo-degradation of Orange II by zinc ferrite (ZnFe2O4) catalyst with the assistance of persulfate. Sep. Purif. Technol. 165, 42–52 (2016). https://doi.org/10.1016/J.SEPPUR.2016.03.026
Z. Zainal, C.Y. Lee, M.Z. Hussein, A. Kassim, N.A. Yusof, Electrochemical-assisted photodegradation of mixed dye and textile effluents using TiO2 thin films. J. Hazard. Mater. 146, 73–80 (2007). https://doi.org/10.1016/J.JHAZMAT.2006.11.055
S. Noor, A. Ashar, M.B. Taj, Z.A. Bhutta, Advanced oxidation processes for remediation of persistent organic pollutants. Adv. Oxid. Process. Wastewater Treat. (2022). https://doi.org/10.1201/9781003165958-17
H. Liu, C. Wang, G. Wang, Photocatalytic Advanced Oxidation Processes for Water Treatment: recent advances and perspective. Chem. – An. Asian J. 15, 3239–3253 (2020). https://doi.org/10.1002/ASIA.202000895
M. Abdullah, P. John, Z. Ahmad, M.N. Ashiq, S. Manzoor, M.I. Ghori et al., Visible-light-driven ZnO/ZnS/MnO2 ternary nanocomposite catalyst: synthesis, characterization and photocatalytic degradation of methylene blue. Appl. Nanosci. 11, 2361–2370 (2021). https://doi.org/10.1007/S13204-021-02008-X/TABLES/1
G. Vanthana Sree, P. Nagaraaj, K. Kalanidhi, C.A. Aswathy, P. Rajasekaran, Calcium oxide a sustainable photocatalyst derived from eggshell for efficient photo-degradation of organic pollutants. J. Clean. Prod. 270, 122294 (2020). https://doi.org/10.1016/J.JCLEPRO.2020.122294
F.M. Sanakousar, C. Vidyasagar, V.M. Jiménez-Pérez, K. Prakash, Recent progress on visible-light-driven metal and non-metal doped ZnO nanostructures for photocatalytic degradation of organic pollutants. Mater. Sci. Semicond. Process. 140, 106390 (2022). https://doi.org/10.1016/J.MSSP.2021.106390
A. Singh, H.P. Gogoi, P. Barman, Synthesis of metal oxide nanoparticles by facile thermal decomposition of new Co(II), ni(II), and zn(II) Schiff base complexes- optical properties and photocatalytic degradation of methylene blue dye. Inorganica Chim. Acta. 546, 121292 (2023). https://doi.org/10.1016/J.ICA.2022.121292
M.A. Almessiere, A.V. Trukhanov, Y. Slimani, K.Y. You, S.V. Trukhanov, E.L. Trukhanova et al., Correlation between composition and electrodynamics properties in nanocomposites based on hard/soft ferrimagnetics with strong exchange coupling. Nanomater 9, 202 (2019). https://doi.org/10.3390/NANO9020202
A.V. Trukhanov, V.G. Kostishyn, L.V. Panina, V.V. Korovushkin, V.A. Turchenko, P. Thakur et al., Control of electromagnetic properties in substituted M-type hexagonal ferrites. J. Alloys Compd. 754, 247–256 (2018). https://doi.org/10.1016/J.JALLCOM.2018.04.150
M. Hassan, Y. Slimani, M.A. Gondal, M.J.S. Mohamed, S. Güner, M.A. Almessiere et al., Structural parameters, energy states and magnetic properties of the novel Se-doped NiFe2O4 ferrites as highly efficient electrocatalysts for HER. Ceram. Int. 48, 24866–24876 (2022). https://doi.org/10.1016/J.CERAMINT.2022.05.140
A.V. Trukhanov, V.O. Turchenko, I.A. Bobrikov, S.V. Trukhanov, I.S. Kazakevich, A.M. Balagurov, Crystal structure and magnetic properties of the BaFe12 – xAlxO19 (x = 0.1–1.2) solid solutions. J. Magn. Magn. Mater. 393, 253–259 (2015). https://doi.org/10.1016/J.JMMM.2015.05.076
D.A. Vinnik, V.V. Kokovkin, V.V. Volchek, V.E. Zhivulin, P.A. Abramov, N.A. Cherkasova et al., Electrocatalytic activity of various hexagonal ferrites in OER process. Mater. Chem. Phys. 270, 124818 (2021). https://doi.org/10.1016/J.MATCHEMPHYS.2021.124818
S.V. Trukhanov, A.V. Trukhanov, V.A. Turchenko, A.V. Trukhanov, E.L. Trukhanova, D.I. Tishkevich et al., Polarization origin and iron positions in indium doped barium hexaferrites. Ceram. Int. 44, 290–300 (2018). https://doi.org/10.1016/J.CERAMINT.2017.09.172
M. Ismael, Facile synthesis of NiO-loaded g-C3N4 heterojunction photocatalyst for efficient photocatalytic degradation of 4-nitrophenol under visible light irradiation. J. Photochem. Photobiol A Chem. 439, 114576 (2023). https://doi.org/10.1016/J.JPHOTOCHEM.2023.114576
P.S. Sahu, R.P. Verma, C. Tewari, N.G. Sahoo, B. Saha, Facile fabrication and application of highly efficient reduced graphene oxide (rGO)-wrapped 3D foam for the removal of organic and inorganic water pollutants. Environ. Sci. Pollut Res. 2023. 1, 1–16 (2023). https://doi.org/10.1007/S11356-023-28976-X
C. Karthikeyan, P. Arunachalam, K. Ramachandran, A.M. Al-Mayouf, S. Karuppuchamy, Recent advances in semiconductor metal oxides with enhanced methods for solar photocatalytic applications. J. Alloys Compd. 828, 154281 (2020). https://doi.org/10.1016/J.JALLCOM.2020.154281
X. Liu, J. Iocozzia, Y. Wang, X. Cui, Y. Chen, S. Zhao et al., Noble metal–metal oxide nanohybrids with tailored nanostructures for efficient solar energy conversion, photocatalysis and environmental remediation. Energy Environ. Sci. 10, 402–434 (2017). https://doi.org/10.1039/C6EE02265K
X. Jin, T.-H. Gu, N. Hee Kwon, S.-J. Hwang, X. Jin, N.H. Kwon et al., Synergetic advantages of atomically coupled 2D inorganic and Graphene Nanosheets as versatile building blocks for diverse functional nanohybrids. Adv. Mater. 33, 2005922 (2021). https://doi.org/10.1002/ADMA.202005922
J. Yao, Z. Gao, Q. Meng, G. He, H. Chen, One-step synthesis of reduced graphene oxide based ceric dioxide modified with cadmium sulfide (CeO2/CdS/RGO) heterojunction with enhanced sunlight-driven photocatalytic activity. J. Colloid Interface Sci. 594, 621–634 (2021). https://doi.org/10.1016/J.JCIS.2021.03.034
Y. Jiang, J.F. Liao, H.Y. Chen, H.H. Zhang, J.Y. Li, X.D. Wang et al., All-solid-state Z-Scheme α-Fe2O3/Amine-RGO/CsPbBr3 hybrids for visible-light-driven photocatalytic CO2 reduction. Chem. 6, 766–780 (2020). https://doi.org/10.1016/J.CHEMPR.2020.01.005
X. Meng, Z. Li, Z. Zhang, Palladium nanoparticles and rGO co-modified BiVO4 with greatly improved visible light-induced photocatalytic activity. Chemosphere. 198, 1–12 (2018). https://doi.org/10.1016/J.CHEMOSPHERE.2018.01.070
A. Al-Nafiey, A. Kumar, M. Kumar, A. Addad, B. Sieber, S. Szunerits et al., Nickel oxide nanoparticles grafted on reduced graphene oxide (rGO/NiO) as efficient photocatalyst for reduction of nitroaromatics under visible light irradiation. J. Photochem. Photobiol A Chem. 336, 198–207 (2017). https://doi.org/10.1016/J.JPHOTOCHEM.2016.12.023
P. Vivek, R. Sivakumar, E. Selva Esakki, S. Deivanayaki, Fabrication of NiO/RGO nanocomposite for enhancing photocatalytic performance through degradation of RhB. J. Phys. Chem. Solids. 176, 111255 (2023). https://doi.org/10.1016/J.JPCS.2023.111255
H.M. Abo-Dief, S.M. El-Bahy, O.K. Hussein, Z.M. El-Bahy, M. Shahid, I. Shakir, Synthesis and characterization of rGO supported silver doped bimetallic ZnCo2O4 spinel oxides for enhanced photocatalytic degradation of industrial effluents. J. Alloys Compd. 913, 165164 (2022). https://doi.org/10.1016/J.JALLCOM.2022.165164
M. Abdullah, P. John, M.N. Ashiq, S. Manzoor, M.I. Ghori, M.U. Nisa et al., Development of CuO/CuS/MnO2 ternary nanocomposite for visible light-induced photocatalytic degradation of methylene blue. Nanotechnol Environ. Eng. 8, 63–73 (2022). https://doi.org/10.1007/S41204-022-00266-W/FIGURES/8
S.I. Sadovnikov, A.I. Gusev, A.V. Chukin, A.A. Rempel, High-temperature X-ray diffraction and thermal expansion of nanocrystalline and coarse-crystalline acanthite α-Ag2S and argentite β-Ag2S. Phys. Chem. Chem. Phys. 18, 4617–4626 (2016). https://doi.org/10.1039/C5CP07224G
D.S. Kim, S.J. Han, S.Y. Kwak, Synthesis and photocatalytic activity of mesoporous TiO2 with the surface area, crystallite size, and pore size. J. Colloid Interface Sci. 316, 85–91 (2007). https://doi.org/10.1016/J.JCIS.2007.07.037
X. Cai, X. Shen, L. Ma, Z. Ji, C. Xu, A. Yuan, Solvothermal synthesis of NiCo-layered double hydroxide nanosheets decorated on RGO sheets for high performance supercapacitor. Chem. Eng. J. 268, 251–259 (2015). https://doi.org/10.1016/J.CEJ.2015.01.072
A.K. Kaviti, S.R. Akkala, Influence of anodization time on Al2O3 nanoporous morphology and optical properties using energy band gap at room temperature. Results Eng. 17, 100816 (2023). https://doi.org/10.1016/J.RINENG.2022.100816
K. Rahimi, H. Zafarkish, A. Yazdani, Reduced graphene oxide can activate the sunlight-induced photocatalytic effect of NiO nanowires. Mater. Des. 144, 214–221 (2018). https://doi.org/10.1016/J.MATDES.2018.02.030
S. Mohan Botsa, M. Raju Imandi, K. Ravi, B. Sathish Mohan, G. Satya Sree, I. Manga Raju et al., ZnO/RGO nanocomposite via hydrothermal route for photocatalytic degradation of dyes in presence of visible light Environmental Impact Assessment of Anthropogenic Activities at Indian Polar Stations-A local and global perspective View project Poly 3-Thionic acid-(TiO2-Cu) nanohybrids: twin applications in effective photocatalysis and polymer sensitized solar cells View project ZnO/RGO nanocomposite via hydrothermal route for photocatalytic degradation of dyes in presence of visible light. Int. J. Chem. Stud. 144, 214 (2018)
S.H. Alwan, K.H. Salem, H.A. Alshamsi, Visible light-driven photocatalytic degradation of rhodamine B dye onto TiO2/rGO nanocomposites. Mater. Today Commun. 33, 104558 (2022). https://doi.org/10.1016/J.MTCOMM.2022.104558
P.B. Sreelekshmi, R.R. Pillai, B. Binish, A.P. Meera, Enhanced photocatalytic degradation of Malachite Green using highly efficient copper Oxide/Graphene oxide nanocomposites. Top. Catal. 65, 1885–1898 (2022). https://doi.org/10.1007/S11244-022-01693-4/FIGURES/15
S.D. Perera, R.G. Mariano, K. Vu, N. Nour, O. Seitz, Y. Chabal et al., Hydrothermal synthesis of graphene-TiO 2 nanotube composites with enhanced photocatalytic activity. ACS Catal. 2, 949–956 (2012). https://doi.org/10.1021/CS200621C/SUPPL_FILE/CS200621C_SI_001.PDF
I. Hasan, M.A. Albaeejan, A.A. Alshayiqi, W.S. Al-Nafaei, F.A. Alharthi, In situ hydrothermal synthesis of Ni1 – xMnxWO4 nanoheterostructure for enhanced photodegradation of Methyl Orange. Molecules. 28, 1140 (2023). https://doi.org/10.3390/MOLECULES28031140/S1
Acknowledgements
The researchers would like to acknowledge Deanship of Scientific Research, Taif University for funding this work.
Author information
Authors and Affiliations
Contributions
All the authors have contributed equally.
Corresponding author
Ethics declarations
Conflict of interest
The authors state that there is no conflict in their work.
Research data policy and data availability
On reasonable request, the author will make the datasets created during and/or analyzed during the current investigation.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fallatah, A.M., Aman, S. & Farid, H.M.T. Facile Hydrothermally Synthesized 2D-Based rGO/NiO Nanohybrid for Environmental Remediation of Malachite Green Pollutant. Korean J. Chem. Eng. 41, 503–513 (2024). https://doi.org/10.1007/s11814-024-00083-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-024-00083-8