Abstract
Reducing carbon emissions has emerged as a critical challenge, and among various methods, solvent-based CO2 capture technology is the most widely employed. Amines, particularly effective solvents for CO2 capture, play a significant role in this process. To advance this technology, it is essential to understand the thermodynamic properties of the interactions between the constituent components. In this study, we examined the deviation in dynamic viscosity (\({\Delta} {\eta}\)), and the deviation in refractive index (\(\Delta n_{D}\)) calculated from the measured \(\eta\) and \(n_{D}\) data for n-butylamine (NBA) with alkanol systems. The temperature range for our study was 298.15–318.15 K. We utilized the Ab-initio approach for \(\Delta \eta\) data analysis. Our findings revealed that the depolymerization power of alkanol is dependent on the unlike interactions. Furthermore, we employed various correlations/mixing rules to predict the \(\eta\) as well as \(n_{D}\) values from the experimental pure components data. The standard deviation was utilized to express the predictive abilities of these correlations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There has been a growing emphasis on developing technologies that effectively capture and store CO2 emissions from various sources [1,2,3,4]. The scientific community and policymakers worldwide are increasingly concerned about the rise in global temperatures due to escalating CO2 [5, 6]. As a result, the development of efficient technologies for capturing and storing CO2 emitted from diverse sources has gained momentum. The fundamental concept of CCS involves capturing CO2 from industrial processes, transporting it to storage sites, and safely storing it underground [7, 8]. However, significant technical, economic, and environmental challenges hinder the development and deployment of CCS technologies [9].
The CO2 capture system is a critical component of CCS technologies, which entails isolating CO2 from flue gas streams emitted by industrial facilities or power plants. Solvent-based CO2 capture technologies have attracted significant interest due to their high efficiency in capturing CO2 and relatively low cost [10, 11]. Alkylamines are a class of organic compounds containing both amine and alcohol functional groups [12, 13]. They are widely used as solvents for CO2 capture, due to their high reactivity with CO2 and ability to form stable CO2-alkylamine complexes [14, 15]. n-Butylamine is one of the most effective alkylamines for CO2 capture, as it has a high CO2 absorption capacity and low energy requirement for solvent regeneration [16]. The capacity of amines and alcohols to form hydrogen bonds leads to the association of these components, and alcohol is chosen as the other component to enhance amine solubility.
To accurately interpret intermolecular interactions between the components, it is crucial to thoroughly understand their thermodynamic properties [17, 18]. This study examines the dynamic viscosity (\(\eta\)) and refractive index (\(n_{D}\)) of n-butylamine (NBA) with alkanol at 0.1 MPa pressure and temperatures ranging from 298.15 to 318.15 K. The deviation in viscosity (\(\Delta \eta\)) was calculated using the measured viscosity data. Additionally, an Ab-initio approach was employed to analyze the \(\Delta \eta\) data. The \(\Delta \eta\) data were analyzed using multiple correlations. The refractive index (\(n_{D}\)) reflects the speed of light, which is influenced by the medium density and specific interactions between binary mixture molecules [19, 20]. An increase in refractive index corresponds to a denser medium, resulting in slower light speed. By analyzing the \(n_{D}\) data, we investigate the intermolecular behavior in n-butylamine (NBA) + alkanol mixtures at temperatures between 298.15 and 318.15 K. The \(\Delta \eta\) and \(\Delta n_{D}\) data were fitted using the Redlich–Kister (RK) equation, and the \(\Delta n_{D}\) for these systems, based on pure component data, were evaluated using various mixing rules.
Experimental Section
Materials
NBA and alkanol were obtained from Sigma-Aldrich and Merck. Before use, they were stored in amber-colored bottles over molecular sieves (3 Å) (Sigma-Aldrich) for 72 h [21]. Table 1 presents the specification and CAS numbers of the chemicals, along with purity reported by the vendors and by analysis performed using gas chromatography. The purities of the compounds were verified by comparing the measured \(\rho\), \(\eta\) and \(n_{D}\) data with literature values, as shown in Table 2.
Methods
The \(\rho\), \(\eta\) and \(n_{D}\) data of the pure components and binary mixtures were measured by using a vibrating tube densimeter (DSA-5000, Anton Paar), a rolling-ball viscometer (LOVIS 2000 ME, Anton Paar) integrated with a densimeter, and a refractometer (Abbemat-200, Anton Paar) as presented in Table 3. The temperature has a precision of ± 0.01 K. The instrument was calibrated every day at 298.15 K using ultra-pure water and cleaned with warm water and ethanol after every sample [37,38,39,40]. Binary mixtures were prepared using an analytical balance (OHAUS, AR224CN) with a precision of ± 0.1 mg. The measurement uncertainties were 5 × 10–4 \({\text{g}}\;{\text{cm}}^{ - 3}\) for \(\rho\), 0.003 \({\text{mPa}}\;{\text{s}}\) for \(\eta\), 0.0001 for mole fraction, and 0.0005 for \(n_{D}\) [19].
Results and Discussion
Viscosity
Utilizing Eq. (1), the \(\Delta \eta\) values were computed from the measured \(\eta\) data of pure components and mixtures. The \(\Delta \eta\) of binary mixtures are shown in Fig. 1 and Table 3.
Here \(\eta_{{{\text{mix}}}} \;{\text{and}}\;\eta_{i}\) are the \(\eta\) data of binary mixtures and pure components.
To fit the \(\Delta \eta\) data, the RK equation [Eq. (2)] was employed.
where \(A^{(j)}\) symbolize adjustable parameters reported in Table 4 along with standard deviation \(\sigma (X)\) given by the equation.
Here m and n represent the number of experimental points and adjustable parameters (j − 1), respectively.
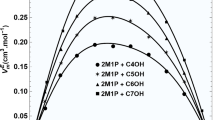
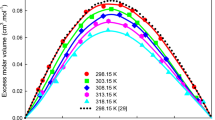
The \(\Delta \eta\) versus \(x_{1}\) values were found to be negative over the entire composition range for the present binary mixtures as represented in Fig. 1 and negative values of \(\Delta \eta\) follow the sequence: n-propanol > n-butanol > iso-butanol > sec-propanol > sec-butanol. The \(\Delta \eta\) values increase with an increase in temperature for all the NBA (1) + alkanol (2) systems (Fig. 2).
The viscosity of a mixture depends upon the structure of molecules and molecular interactions among, unlike molecules. If the deviation in viscosity between the binary liquid mixtures shows a positive contribution, it indicates strong interactions, whereas the negative contribution shows the mixtures without specific interactions [41]. Thus, in the present systems, the negative \(\Delta \eta\) of the mixtures cannot explain the strong interactions between the NBA and alcohol. This confirms that the strength of intermolecular hydrogen bonding is not the only factor affecting the \(\Delta \eta\) of these mixtures but the size and shape of the molecules (NBA and alkanol) play a vital role. The excess free energy of activation (ΔG*E) is also considered a reliable criterion to detect or exclude the presence of interactions between the binary liquid mixtures. The positive ΔG*E values indicate specific interactions, while negative values indicate the dominance of dispersion forces [42]. For this binary (A + B) system, ΔG*E is negative over the entire composition range (Fig. 3). This further confirms the dominance of dispersion forces in these binary systems. The increase in temperature weakens the intermolecular association due to the rise in the kinetic energy of molecules, thus resulting in decreased viscosity [43].
Ab Initio Approach
In this paper, we utilized an Ab initio approach that not only characterizes the \(\Delta \eta\) of binary mixtures containing associated components but also provides insights into intermolecular interactions. Since both components (1 and 2) of binary mixtures are self-associated through hydrogen bonding, these components are assumed to exist in polymeric form and are expressed as 1n and 2n.
The utilized approach assumes that the \(\Delta \eta\) values for the present NBA (1) + alkanol (2) mixtures are the viscosity change caused by the following three processes (a), (b), and (c):
The formation of (1n)–(2n) interactions between the NBA (1) and alkanol (2) exist as self-associated (1n and 2n), with an interactional parameter of \(\chi_{12}^{\dag }\) per mole.
Here \(S_{2}\) represents the surface fraction of alkanol in (1n)–(2n) interaction [44, 45].
These (1n)–(2n) interactions lead to the rupturing/depolymerization of (i) intermolecular association (H-bonding) in pure NBA and alkanol molecules to produce their monomers with interactional parameter as \(\chi_{11}\) and \(\chi_{22}\) per mole.
Here \(S_{2}^{\prime }\) is referred to as the alkanol surface fraction that causes the NBA changes.
The monomers of NBA and alkanol would then interact with each other to form (1)–(2) interactions with interactional energy parameters as \(\chi_{12}\) per mole.
Therefore, the total change in \(\eta\) (\(\Delta \eta\)) including all three processes (a), (b), and (c) is estimated as
Since both the components of the current mixtures are self-associated through hydrogen bonding, it is reasonable to assume that.
\(\chi_{12}^{\dag } = K\chi_{12} = \chi_{12}^{*}\) and \(K\chi_{11} = K\chi_{22} = \chi^{*}\) so Eq. (10) reduces to
The experimental values at two compositions (\(x_{1} \approx 0.5{\text{ and }}0.6\)) were used to compute parameters \(\chi_{12}^{*} {\text{ and }}\chi^{*}\) from Eq. (11) and represented in Table 6. Using these \(\chi_{12}^{*} \;{\text{and}}\;\chi^{*}\) parameters, the \(\Delta \eta\) values were calculated at other mole fractions and are shown in Fig. 4. The prediction capability of this approach is expressed as standard deviations in Table 5. Since \(\chi^{*}\) is a measure of the extent of depolymerization of NBA or alkanol upon mixing, the magnitude of \(\chi^{*}\), as shown in Table 6, indicates that maximum depolymerization occurs with n-propanol. Moreover, the magnitude of \(\chi_{12}^{*}\), which is a measure of unlike interactions between NBA and alkanol, reveals that n-propanol and n-butanol interacts strongly with NBA compared to other alkanol and weakly with sec-butanol. This may be due to the two –CH2 groups attached to the C atom involved with hydroxyl oxygen which offers steric hindrance and restrict the proper orientation of –OH required for the interaction with the amine group.
Correlations for Viscosity
The viscosity of the binary mixtures was predicted by following four correlations employing the experimental pure components viscosity (\(\eta\)) data and results are demonstrated in Fig. 5 [13, 46,47,48,49] and prediction ability of these correlations were expressed in terms of \(\sigma (\eta )\), as presented in Table 5.
Grunberg and Nissan [50]
Here, \(G_{12}\) is Grunberg-Nissan interactional parameter, with the results shown in Table 6. Positive \(G_{12}\) values imply stronger specific interactions in butanol mixtures, while a negative \(G_{12}\) value indicates relatively weaker interactions in propanol mixtures.
Tamura and Kurata [51]
where \(T_{12}\) is TK interactional parameter as given in Table 6 and its value is positive for all the binary mixtures as shown in Fig. 5.
Hind, McLaughlin, and Ubbelohde [52]
where \(H_{12}\) is the Hind interactional parameter shown in Table 6 and all of the binary systems studied show positive values, indicating favorable interactions as presented in Fig. 5.
Katti and Chaudhari [53, 54]
The interactional energy parameter value of Wvis/RT is given in Table 6 and correlated data results are shown in Fig. 5.
The \(\eta\) values were predicted using various correlations and analyzed using standard deviation. The values of \(\sigma (\eta )\) for these correlations [Eqs. (11), (13)–(15)] were found to be in the range of 0.006–0.088, whereas for Grunberg and Nissan correlation, it varies in the range of 0.096- 0.565 for the present NBA + alkanol systems.
Refractive Index
The (\(\Delta n_{D}\)) values were calculated as follows:
The measured \(n_{D}\) and \(\Delta n_{D}\) data of NBA (1) + alkanol (2) systems are presented in Table 3 and depicted as \(\Delta n_{D}\) versus \(x_{1}\) in Fig. 6. The adjustable parameters \(A^{(j)}\) along with standard deviations \(\sigma (\Delta n_{D} )\) were calculated by using RK equation (Table 4).
The \(\Delta n_{D}\) values for binary systems are positive and follow the sequence: sec-propanol > n-propanol > iso-butanol > n-butanol > sec-butanol, as demonstrated in Fig. 6. The observed positive values of \(\Delta n_{D}\) values can be attributed to the formation of specific hydrogen bonding interaction between (–OH) of alkanols and NH2 group of the amine. The amine has a lone pair electron in p-orbitals, which readily interacts with the acidic hydrogen of the –OH group in alkanols [55,56,57]. As shown in Fig. 6, the \(\Delta n_{D}\) values decrease with an increase in the chain length of alkanol molecules [55]. This trend may be due to a decrease in the acidity of the –OH group resulting from the addition of an alkyl group, which ultimately leads to a reduction in the specific interaction between the component molecules. The \(\Delta n_{D}\) values for NBA (1) + sec-propanol (2) are higher as compared to NBA (1) + sec-butanol (2), which may be due to the larger size of the butanol molecule providing more steric hindrance and forming intermolecular associations. In contrast, the smaller size of the propanol allows it to interact more easily with NBA molecules, resulting in higher excess refractive index values. In this study, we also examined the effect of temperature on \(n_{D}\); as the temperature increases, the \(\Delta n_{D}\) data for NBA (1) + alkanol (2) mixtures decrease, as demonstrated in Fig. 7, indicating a weakening of intermolecular interactions between the components of the mixture.
The \(n_{D}\) data were calculated using the following mixing rules [19] and then compared using standard deviation (σ) at all temperatures as shown in Table 5:
In these equations,\(n_{D}\), \(n_{D1}\) and \(n_{D2}\) represent the refractive index of mixture and pure compounds, respectively.
The values of \(n_{D}\) for these binary mixtures predicted from above Eqs. (17)-(23) are shown in Fig. 8. All the correlations predict similar values of refractive index (Fig. 8) of mixtures as these plots are overlapping and indistinguishable. This is because the present mixtures are highly non-ideal due to like (i–i) and unlike (i–j) intermolecular interaction (hydrogen bonding) and therefore, these correlations are not very impressive to correlate such types of associated mixtures. The difference in experimental and predictive values are shown in terms of standard deviations [calculated from Eq. (3)] in Table 5. Similar behavior was also reported in the literature for associated binary mixtures [19, 20, 58, 59].
Conclusions
In conclusion, we investigated viscosity and refractive index properties in NBA + alkanol mixtures over a temperature range T = (298.15–318.15 K) and at pressure 0.1 MPa over the entire composition range. The sequence of viscosity deviation \(\Delta \eta\) follows the trend: n-propanol > n-butanol > iso-butanol > sec-propanol > sec-butanol, which reveals the unlike interactions were stronger in the case of n-propanol, resulting in the maximum deviation, while sec-butanol exhibited the lowest deviation due to steric hindrance. Additionally, the Ab-initio approach was employed to determine the \(\Delta \eta\) values, and various correlations were used to predict the viscosity data at 298.15 K.
This study contributes to the parameters required for CO2 capture by blended absorbents, a critical technology for reducing carbon emissions. Amines, commonly used solvents for capturing CO2, were combined with alkanols as the second component to enhance amine solubility through hydrogen bonding. The refractive indices (\(n_{D}\)) of NBA with alkanol binary systems were studied and exhibited positive \(\Delta n_{D}\) values with the sequence: sec-propanol > n-propanol > iso-butanol > n-butanol > sec-butanol, indicating specific hydrogen bonding interactions between the hydroxyl (–OH) group of alkanol and –NH2 group of amines.
Data availability
Available upon requesting the corresponding author. yongjin.lee@inha.ac.kr.
References
S. Gössling, S. Dolnicar, WIREs Clim. Change 14, e802 (2023)
N. Cahill, Significance 15, 24 (2018)
X. Song, F. Wang, D. Ma, M. Gao, Y. Zhang, Pet. Explor. Dev. 50, 229 (2023)
M. Meinshausen, N. Meinshausen, W. Hare, S.C.B. Raper, K. Frieler, R. Knutti, D.J. Frame, M.R. Allen, Nature 458, 1158 (2009)
M. Herrero, P. Gerber, T. Vellinga, T. Garnett, A. Leip, C. Opio, H.J. Westhoek, P.K. Thornton, J. Olesen, N. Hutchings, H. Montgomery, J.F. Soussana, H. Steinfeld, T.A. McAllister, Anim. Feed Sci. Technol. 166–167, 779 (2011)
R.A. Kerr, Science 312, 825 (2006)
D.Y.C. Leung, G. Caramanna, M.M. Maroto-Valer, Renew. Sustain. Energy Rev. 39, 426 (2014)
S. Anderson, R. Newell, Ann. Rev. Environ. Resour. 29, 109 (2004)
F. Bowen, Energy Policy 39, 2256 (2011)
E. Nessi, A.I. Papadopoulos, P. Seferlis, Int. J. Greenhouse Gas Control 111, 103474 (2021)
A. Afsharpour, S.H. Esmaeli-Faraj, Korean J. Chem. Eng. 39, 1576 (2022)
F.A. Perdomo, S.H. Khalit, E.J. Graham, F. Tzirakis, A.I. Papadopoulos, I. Tsivintzelis, P. Seferlis, C.S. Adjiman, G. Jackson, A. Galindo, Fluid Phase Equilib. 566, 113635 (2023)
S. Verma, P. Bhagat, S. Gahlyan, M. Rani, N. Kumar, R.K. Malik, Y. Lee, S. Maken, Korean J. Chem. Eng. (2023). https://doi.org/10.1007/s11814-023-1422-2
W.M. Budzianowski, Int. J. Global Warm. 7, 184 (2015)
P.D. Vaidya, E.Y. Kenig, Chem. Eng. Technol. 30, 1467 (2007)
M. Yiannourakou, X. Rozanska, B. Minisini, F. de Meyer, Fluid Phase Equilib. 560, 113478 (2022)
M. Rani, S. Maken, S.J. Park, Korean J. Chem. Eng. 36, 1401 (2019)
S. Gahlyan, P. Bhagat, R. Devi, S. Verma, M. Rani, S. Maken, J. Mol. Liq. 304, 112740 (2020)
P. Bhagat, S. Maken, J. Mol. Liq. 323, 114640 (2021)
S. Verma, S. Gahlyan, M. Rani, S. Maken, Korean Chem. Eng. Res. 56, 663 (2018)
J.A. Riddick, W.B. Bunger, T.K. Sakano, Organic Solvents. Physical Properties and Methods of Purification, 4th edn. (Wiley, New York, 1986)
M. Domínguez, I. Gascón, A. Valén, F.M. Royo, J.S. Urieta, J. Chem. Thermodyn. 32, 1551 (2000)
J.K. Shah, K.J. De Witt, C.E. Stoops, J. Chem. Eng. Data 14, 333 (1969)
M. Domínguez, J. Pardo, M.C. López, F.M. Royo, J.S. Urieta, Fluid Phase Equilib. 124, 147 (1996)
J.W. Bayles, T.M. Letcher, J. Chem. Eng. Data 16, 266 (1971)
H.A. Zarei, S. Asadi, H. Iloukhani, J. Mol. Liq. 141, 25 (2008)
C. Yang, H. Lai, Z. Liu, P. Ma, J. Chem. Eng. Data 51, 1345 (2006)
J. Ortega, J. Chem. Eng. Data 27, 312 (1982)
M. Rani, S. Maken, J. Ind. Eng. Chem. 18, 1694 (2012)
F.-M. Pang, C.-E. Seng, T.-T. Teng, M.H. Ibrahim, J. Mol. Liq. 136, 71 (2007)
C.-T. Yeh, C.-H. Tu, J. Chem. Eng. Data 52, 1760 (2007)
M. Domínguez, S. Rodríguez, M.C. López, F.M. Royo, J.S. Urieta, J. Chem. Eng. Data 41, 37 (1996)
S. Martı́nez, R. Garriga, P. Pérez, M. Gracia, Fluid Phase Equilib. 168, 267 (2000)
A.K. Nain, J. Chem. Eng. Data 53, 1208 (2008)
D.M. Majstorović, E.M. Živković, M.L. Kijevčanin, J. Chem. Eng. Data 62, 275 (2017)
T.M. Aminabhavi, B. Gopalkrishna, J. Chem. Eng. Data 39, 865 (1994)
S. Verma, S. Gahlyan, M. Rani, S. Maken, J. Mol. Liq. 265, 468 (2018)
S. Gahlyan, N. Verma, S. Verma, M. Rani, S.-J. Park, S. Maken, J. Mol. Liq. 298, 111946 (2020)
S. Verma, S. Gahlyan, J. Kaur, S. Maken, J. Mol. Liq. 292, 111359 (2019)
S. Gahlyan, S. Verma, M. Rani, S. Maken, J. Mol. Liq. 244, 233 (2017)
H. Vogel, A. Weiss, J. Phys. Chem. 86, 193 (1982)
G.P. Dubey, K. Kumar, J. Chem. Eng. Data 61, 1967 (2016)
G. Manukonda, V. Ponneri, S. Kasibhatta, S. Sakamuri, Korean J. Chem. Eng. 30, 1131 (2013)
M.L. Huggins, Polymer 12, 389 (1971)
M.L. Huggins, J. Phys. Chem. 74, 371 (1970)
S. Verma, S. Gahlyan, P. Bhagat, M. Rani, M. Bhagat, S. Rana, V.K. Rattan, Y. Lee, S. Maken, J. Mol. Liq. 386, 122498 (2023)
S. Verma, A. Sharma, S. Gahlyan, M. Rani, S. Maken, J. Mol. Liq. 387, 122709 (2023)
S. Verma, P. Bhagat, S. Gahlyan, M. Rani, N. Kumar, R.K. Malik, Y. Lee, S. Maken, J. Mol. Liq. 382, 121967 (2023)
S. Verma, M. Rani, Y. Lee, S. Maken, J. Mol. Liq. 387, 122663 (2023)
L. Grunberg, A.H. Nissan, Nature 164, 799 (1949)
M. Tamura, M. Kurata, Bull. Chem. Soc. Jpn 25, 32 (1952)
R.K. Hind, E. McLaughlin, A.R. Ubbelohde, Trans. Faraday Soc. 56, 328 (1960)
P.K. Katti, M.M. Chaudhri, J. Chem. Eng. Data 9, 442 (1964)
P.K. Katti, M.M. Chaudhri, O. Prakash, J. Chem. Eng. Data 11, 593 (1966)
M. Almasi, M. Shojabakhtiar, Thermochim. Acta 523, 105 (2011)
J.A. González, I.G. de la Fuentá, J.C. Cobos, Fluid Phase Equilib. 168, 31 (2000)
K.V. Zaitseva, M.A. Varfolomeev, V.B. Novikov, B.N. Solomonov, J. Chem. Thermodyn. 43, 1083 (2011)
S. Verma, S. Gahlyan, M. Rani, S. Maken, Arab. J. Sci. Eng. 43, 6087 (2018)
K. Kumari, S. Maken, J. Mol. Liq. 325, 115170 (2021)
Acknowledgements
This work was supported by Inha University Research Grant, and the National Research Foundation of Korea (NRF-2021R1A4A1022920 and 2022M3H4A1A04076372), Netaji Subhas University of Technology, New Delhi, India, and Deenbandhu Chhotu Ram University of Science and Technology, India.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Verma, S., Gahlyan, S., Bhagat, P. et al. Transport and Optical Properties of n-Butylamine + Alkanols (C3–C4) Mixtures for Enhanced CO2 Absorption. Korean J. Chem. Eng. 41, 715–727 (2024). https://doi.org/10.1007/s11814-024-00076-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-024-00076-7