Abstract
Naphthenic acid (NA) widely exists in acidic crude oil. To reveal the mechanism of electrocoalescence of acidic crude oil emulsion, the influence of NA on the electrocoalescence of W/O emulsion is studied by high-frequency pulsed electrocoalescence technology and alkali washing method by changing electric field parameters and physical parameters. The results showed that the desalination (dehydration) rate of W/O emulsion increases with the increase of electric field strength, frequency, duty ratio, initial water contents and divalent salts. With the increase of monovalent salts, naphthenic acid and alkali injections, the desalination effect decreases. The sodium naphthenate intensified the emulsification effect. The presence of NA reduces the desalination rate. By increasing the concentration of CaCl2, the desalination effect of acidic crude oil emulsion is improved. The desalination rate is up to 99.63%, the water content is less than 0.5%, the salt concentration is less than 3 mg L−1, and the acid value is less than 0.03 mgKOH g−1. Our studies provide a theoretical basis for high-frequency pulsed electrocoalescence technology to improve the electrocoalescence and the multiple removal mechanism of inorganic salts and organic acids of acidic crude oil.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Crude oil pretreatment is an essential part of petrochemical industry [1]. With the development and utilization of heavy and inferior crude oil [2], the composition of crude oil has become increasingly complicated, and the content of impurities such as inorganic salts and organic acids increases, which will increase the energy consumption of equipment, aggravate the corrosion of equipment, cause the subsequent equipment pipeline scaling and even blockage, and seriously affect the long-term safe operation of equipment [3,4,5,6]. It is well known that electrocoalescence technology is an effective method of crude oil dehydration [7]. In the 1980s, Bailes [8] first presented pulsed electrocoalescence technique, which used a DC pulsed electric field to remove water from crude oil. Through decades of development, the form of electric field has developed to high frequency pulsed electric field [9]. The high frequency pulse coalescence technology not only has good dehydration effect and stable operation compared with traditional electrocoalescence technology, but also can suit the high moisture content conditions [10, 11]. Naphthenic acid (NA) is an important component of the oil containing acid. It has been a primary problem in the oil industry since it was first observed in the 1920s when refineries were damaged by corrosion [12]. NA has surface and interface activity, leading to the formation of stable colloidal structures by adsorption at the oil–water interface [13]. These structures hinder the coalescence [14], which is one of the main reasons for the formation of highly stable emulsions. Consequently, the study of the influence mechanism of NA on the electrocoalescence of emulsion under electric field conditions provides a theoretical basis for the development of pulsed electrocoalescence technology for acidic crude oil, which is beneficial to improve the electrocoalescence efficiency of acid-containing oil.
NA is a naturally occurring compound in most petroleum resources. Numerous studies have shown that NA can stabilize emulsion [13, 15, 16]. Zhu et al. [17] found by molecular dynamics simulation that the emulsion stability was enhanced due to the interaction between metal ions and NA in the emulsion. The decrease in interfacial tension and the formulation of viscoelastic interfacial films are two mechanisms by which NA improve the stability of emulsions [18]. Li et al. [19] investigated the effects of NA and asphaltenoid molecules in the oil-water interfacial tension (IFT). NA and their anions in crude oil could significantly reduce the crude oil-water IFT by competing with asphaltenes and adsorb at the crude oil-water interface. Wang et al. [20] have discovered that NA and their anions in crude oil can significantly reduce the crude oil-water IFT by competing with asphaltenes and adsorb at the crude oil-water interface. He further validated this finding: NA was found to bind tightly to the asphaltene molecules at the interface, resulting in a more swollen and flexible film. The decrease in interfacial film hardness was proportional to the content of stearic acid in the system. Garcia-Olvera et al. [21] investigated the impact of asphaltenes and organic acids on the viscoelasticity of the crude oil-brine interface. The study found that the interfacial viscoelasticity increases with increasing asphaltene concentration and decreases with increasing acid concentration. The above studies mainly focused on the effect of NA on stability of emulsion and uncovered the emulsification of acidic crude oil mechanism. Nevertheless, whether the emulsion containing NA can continue to be stable under the action of electric fields needs to be further investigated.
As the water content of crude oil recovery fluid increases, many scholars have intensively studied the dehydration of crude oil. Anand et al. [22] analyzed the stability as well as destabilization of the water in oil (W/O) emulsion system, and proposed the optimal dehydration parameters. Hadidi et al. [23] studied the impact of non-homogeneous electric field on dehydration effect, and optimized the electrode structure. Guha et al. [24] presented a system which combined high K dielectrics with surfactant bilayers, which achieved fast electrocoalescence of droplets at low voltage. Lu et al. [25] proposed a device that uses fiber media to capture droplets to improve dehydration efficiency. Scholars have studied the impact of inorganic salts on the stability of emulsion. Silva et al. [5, 26, 27] systematically studied the influence of salinity on the stability of water-in-oil emulsion, and found that salt made the emulsions unstable when the salt concentration was 4–30 wt%. Olesen et al. [19, 28, 29] researched the influence of inorganic salt type on the IFT of droplets, and found that multivalent salt was more effective in demulsification than monovalent salt solutions. The above scholars studied the dehydration of oil-water emulsions through experiments and numerical simulations, discussed the best dehydration conditions and improved the efficiency of dehydration. However, there is a lack of research on electrocoalescence of acidic crude oil.
The high-frequency pulsed electric field dehydration method is widely used in petroleum industry. Li et al. [9] researched the impact of applied electric field parameters on the formation of water chains in W/O emulsion, and acquired the optimal electric field parameters to inhibit water chains formation and water droplets coalescence. The deformation of a droplet under a pulsed electric field was simulated by a linear model of droplet dynamics by Vivacqua et al. [30]. The effects of electric field frequency and waveform on the droplet motion pattern were predicted. During the electrocoalescence process, some adverse phenomena, such as the formation of secondary droplets can occur. Mousavi et al. [31] studied the factors influencing the formation of secondary droplets in a pulsed electric field, and obtained the best waveform and frequency to inhibit the formation of secondary droplets. Zhang et al. [32] demonstrated the impacts of operating parameters on demulsification in oil-water emulsions under high-frequency pulsed direct-current (DC) electric field (above 1 kHz). The variation pattern of dehydration effectiveness with frequency and pulse time was obtained. The above scholars studied the best parameters of crude oil dehydration under high-frequency pulsed electric field. However, there was no indication of the most appropriate electric field parameters for acid crude oil to optimize process efficiency.
Under the background of high salt and acid concentration in domestic crude oil, the removal process of inorganic salts and organic acids in crude oil was long and the removal efficiency was low. Therefore, the organic acids removal process of crude oil can be combined with the electrocoalescence process, and the electrocoalescence device can be used to treat the deacidified crude oil, which can not only improve the emulsification problem caused by the deacidification process and remove the residual water and inorganic salts in the crude oil, but also shorten the process flow and reduce the operation cost. High-frequency pulse electric dehydration is a dehydration method with low energy consumption, high efficiency and good stability [33]. Thus, it is necessary to investigate the impact of deacidification on the electrocoalescence of crude oil emulsion under high-frequency pulsed electric field. To further explore the applicability of electrocoalescence technology in the treatment of high acid crude oil, based on high-voltage and high-frequency pulsed electrocoalescence technology [34, 35], combined with alkali washing method [36, 37], this paper studied the desalination and deacidification laws of salts and acids containing W/O emulsion from two aspects of electric field parameters and emulsions physical parameters, so as to provide guidance for the coupling of electrocoalescence process and deacidification process of high acid crude oil.
Experiment
Experimental setup
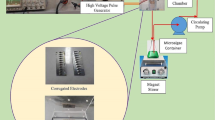
As shown in Fig. 1, the experimental setup consists of a rectangular wave pulse supply, transformer, and mini-sized electrostatic coalescence cell. These were designed by China University of Petroleum (East China). The supply voltage, frequency and duty ratio can be adjusted according to the experimental requirements [9]. The voltage output waveform of rectangular wave pulse power supply is shown in Fig. 2. When considering the effect of electric field strength, the range of Umax is 0.5–1.5 kV cm−1. In other cases, Umax is a fixed value of 1.5 kV cm−1.
After drawing the primary voltage from the rectangular wave pulse power supply, the voltage is stepped up through by a step-up transformer with a ratio of 100:1 and connected to a closed electrostatic demulsification device for electrostatic demulsification experiments. The electric field of the electrostatic demulsification has an effective action space of 40 mm × 30 mm × 220 mm, as shown in Fig. 3. In order to prevent the electric field short circuit due to the droplet chains, the electrostatic demulsification device is set to be insulated by Perspex. l indicates the distance between electrode plates is 30 mm. d refers to the thickness of Perspex, which is 2.5 mm.
The analysis of electric field acting on W/O emulsion between plate electrodes is as follows [9]:
According to Eq. (1), if the voltage U on the electrostatic coalescence cell, the structural parameters of the electrostatic coalescence cell (insulation material thickness d and electrode plate spacing l) and the physical parameters of the emulsions and insulation material (dielectric constant of the emulsions \(\varepsilon e\) and insulation material \(\varepsilon i\)) are known, the electric field acting on the oil-water emulsions can be obtained, so as to facilitate the analysis of the impact of the electric field parameters on the deformation and coalescence of water droplets.
Materials
Due to the complex composition of crude oil, in order to better analyze the influence law of various factors, this paper used QC320 heat transfer oil (College of New Energy, China University of Petroleum (East China)) as the simulation oil sample, and studied the feasibility of QC320 heat transfer oil replacing crude oil. The important physical properties at 25 ℃ are shown in Table 1.
According to Stokes formula Eq. (2), the settling velocity of water droplets in continuous phase (oil phase) is mainly related to the density difference between oil and water, the particle size of water droplets and the viscosity of oil phase. Through the comparison in Table 1, there is little difference in viscosity and density between QC320 heat transfer oil and the oil sample of an oil field in Xinjiang. Therefore, it is reasonable and feasible to use QC320 thermal oil to replace crude oil.
where ut is the settling velocity of water droplets (m s−1), dp is the droplet radius (m), ρw is the density of aqueous phase (kg m−3), ρo is the density of oil phase (kg m−3), g is the acceleration of gravity (9.8 m s−2), µo is the viscosity of oil phase (Pa s), and µw is the viscosity of water phase (Pa s).
In addition, the naphthenic acid used in the experiment is the national standard refined naphthenic acid with an acid value of 164 mgKOH g−1. Other chemical reagents used in the experiment, analysis and measurement are shown in Table S1.
As shown in Fig. 4, when the initial water content of the emulsion rises from 0 to 30%, the apparent viscosity of the emulsion rises from 56.6 to 139.2 mPa s. With the increase of initial water content, the number of water droplets in the oil phase increases, the size of water droplets increases, the probability of collision between water droplets increases, and the non-uniformity of the emulsion increases [38]. In addition, as the total area of oil-water interface increases, the free energy of the interface is higher, and the flow resistance in the fluid increases, which is reflected in the increase of apparent viscosity [39].
Experimental Procedure
Stable W/O emulsion were prepared before conducting the experiment. The preparation procedure of emulsion was as follows:
-
(1)
The salty water was made by dissolving inorganic salt in water.
-
(2)
The salty water and QC320 heat transfer oil were mixed with the volume ratio of 1:4 in a beaker.
-
(3)
The oil and water mixture was stirred by a high-speed homogenizer (FSH-2 A, Changzhou Yuexin Instrument Manufacturing Co., Ltd., China) for 5 min at 6000 r min−1.
-
(4)
The stirred solution was allowed to stand for 24 h to obtain the uniform and stable W/O emulsion. The percentage of water in stable emulsion was 20%.
Electrostatic demulsification of W/O emulsion under high-frequency pulsed electric field was conducted at room temperature (25 ℃). The electrostatic demulsification was carried out by adjusting electric field parameters (electric field strength, electric field frequency, electric field duty ratio) and emulsions physical parameters (water content, salts concentration and type, acid value and alkali injection amount). During the experiment, the electric field strength was 1.5 kV cm−1; the frequency was 5 kHz; the duty ratio was 50%; the electric field action time was 30 min; the initial water content was 20%; the concentration of inorganic salt (NaCl) was 100 mg L−1; the acid value was 1 mgKOH g−1; and NaOH solution equal to the mass of naphthenic acid substance was added.
After electrostatic demulsification, the upper oil sample was extracted with a sampler, and the sample was measured and analyzed.
Determination Method
-
(1)
Determination of water content.
The water content of crude oil referred to the mass fraction of water contained. In this experiment, XYHS-500 × 6 crude oil moisture rapid tester was used to determine the water content in oil samples. The range of the moisture receiver of XYHS-500 × 6 crude oil moisture rapid tester is 0–15 ml, the accuracy of 0–1 ml is ± 0.1 ml, and the accuracy of 1–15 ml is ± 0.2 ml. The principle was to use distillation method (GB/T 8929-2006), take an appropriate amount of oil samples according to the water content range of oil samples, add a certain amount of distillation solvent for full mixing, and then transfer them to the moisture rapid tester for heating and reflux. Until the amount of water in the water separator did not increase, read the quality of distilled water, and its water content can be calculated by Eq. (3):
$$W{\text{=}}\frac{{{m_w}}}{{{m_0}}} \times 100{\text{\% }}$$(3)where W is the mass fraction of water in crude oil (%), mW is the quality of distilled water (g), and m0 is the sampling mass (g).
-
(2)
Determination of inorganic salt concentration.
The concentration of inorganic salts in crude oil was determined by electric quantity method (SY/T 0536–2008), and the reaction principle was shown in Eq. (4). When measuring, take about 1 g of oil sample, add 1.5 ml of xylene and 2 ml of ethanol-water solution, mix well, and then centrifuge. Take an appropriate amount of lower clear liquid according to the salt concentration range, and use WC-200 inorganic salt concentration tester to measure the salt concentration. In order to reduce the experimental error, usually measure it for 2–3 times, and take the average value as the measured value. The WC-200 inorganic salt concentration tester used for the measurement had a range of 0.2–10,000 mg L−1 and a sensitivity of 0.1 mg L−1.
$${\text{A}}{{\text{g}}^+}+{\text{C}}{{\text{l}}^ - }={\text{AgC}}{{\text{l}}^ - }$$(4) -
(3)
Determination of acid value.
The acid value of crude oil referred to the KOH mass required for 1 g crude oil to be completely neutralized, with the unit of mgKOH·g−1. The color indicator method (GB/T 4945-2002) was used to detect the acid value in the experiment. When measuring the acid value, dissolve the sample in the mixed solvent of water, toluene and isopropanol, add the color indicator, use the magnetic stirring device to make it uniform, titrate with the known concentration of potassium hydroxide isopropanol solution at room temperature, and judge the titration end point according to the change of the color of the mixed solution. For oil samples with darker colors such as crude oil, potentiometric titration (GB/T 18609-2011) can be used. The titration method was similar to the color indicator method, but the method to judge the titration end point was different. Potentiometric titration was to titrate with potassium hydroxide isopropanol standard solution using a potentiometric titrator with glass electrode and Ag/AgCl reference electrode, plot the volume of standard solution consumed by potential reading, and take the sudden jump point of the curve as the titration end point. No matter which method was used, after determining the titration end point, calculate its acid value through Eq. (5):
$$\omega {\text{=}}\frac{{\left( {{V_1} - {V_0}} \right) \times C \times 56.1}}{m}$$(5)where ω is the measured acid value (mgKOH g−1), V1 is the volume of potassium hydroxide isopropanol standard solution that is consumed when the experimental specimen is titrated to the end point (mL), V0 is the volume of potassium hydroxide isopropanol standard solution that is consumed when the blank specimen is titrated to the end point (mL), C is the concentration of potassium hydroxide isopropanol standard solution (mol L−1), m is the mass of oil sample (g), and 56.1 is the molar mass of KOH (g mol−1).
Results and Discussion
The inorganic salt was uniformly dissolved in water. It was found through experiments that the inorganic salt was removed together in the dehydration process, and the desalination rate curve was consistent with the dehydration rate curve [40].
Effect of Electric Field Strength
a Desalination rate of emulsion without NA at different electric field strength; b desalination rate and acid value after deacidification of emulsion with NA at different electric field strength (the dotted line is the minimum standard of salt concentration after desalination, and the solid line is the minimum salt concentration measured in the experiment)
The effective electric field strength of the emulsion was calculated by Eq. (1). A line graph of the effect of electric field strength on removal efficiency of W/O emulsion were given in Fig. 5. With the increase of electric field strength, the desalination rate showed an upward trend. This is because the electric field causes the water molecules to polarize [41]. Under the action of electric field, the polarized droplets move towards the vicinity of the electrode, increasing the possibility of coalescence. When the spacing of water droplets was small, the dipole coalescence force played a major role, which was directly proportional to the square of the applied field strength [42]. As the electric field strength increased, the electric field energy between droplets increased, and the collision and coalescence rate increased.
As illustrated in Fig. 5a, the desalination rate can reach 98.37%. The water content had decreased to 0.76%. The salt concentration had decreased to less than 3 mg L−1 when the electric field strength was 1.25 kV cm−1. When the emulsion with naphthenic acid (NA), as shown in Eq. (6), NA reacted with NaOH to produce a large amount of sodium naphthenate (C10H17NaO2), which was a strong emulsifier [17]. It was known that sodium salt naphthenic acid stabilized water-in-oil emulsion due to its adsorption at the oil-water interface. The resulting interface became more elastic due to generation of interfacial tension gradient when water drop was deformed by the electric field. The oil-water interface was more stable, and the difficulty of oil-water separation increased. In addition, as displayed in Fig. 5b, when the electric field strength reached 1 kV cm−1, the increase of the electric field strength had a certain limit on the improvement of the desalination rate of the emulsions, and the desalination effect had not changed significantly, which indicated that the emulsification of sodium naphthenate weakened the promoting effect of high electric field strength on the electrocoalescence effect. So that the maximum desalination rate was reduced to 82.55%, the water content was more than 5.2% and the salt concentration was more than 17 mg L−1. Under different electric field strength, the acid value after deacidification was lower than 0.03 mgKOH g−1.
Effect of Electric Field Frequency
The effects of electric field frequency on removal efficiency of W/O emulsion are shown in Fig. 6. The results showed that when the electric field frequency increased from 2 to 6 kHz, the desalting rate first increased and then decreased. The desalting rate is the highest when the electric field frequency is 5 kHz. In addition, when the frequency was high, the intensity of electric field acting on the emulsion was also high enough and provide enough energy to promote the water droplets in the oil to drain away from the oil film and coalescence. At the same time, as shown in Fig. 6- (a) when the electric field frequency was high, the time was shorter, and the droplets cannot catch up with the electric field transition, which increased the polarization deformation of the droplets and promoted the droplets collision and coalescence. Because the crude oil emulsion is a capacitive load [9, 43], under the experimental conditions, when the electric field frequency is too low, the capacitance of the oil-water emulsion is large, and the voltage acting on it is small, so its water droplet polarization is low. Therefore, with the increase of electric field frequency, the water droplet polarization force increases, and the removal efficiency increases. When the frequency is further increased, the driving frequency of water droplets under the electric field is much higher than its own frequency, the polarization deformation effect of water droplets is suppressed, and the coalescence effect between water droplets is weakened [44]. Therefore, there is an optimal value for the frequency of the electric field. At this time, the self-owned frequency of the water droplet is close to the driving frequency of the electric field.
As illustrated in Fig. 6a, the desalination rate of emulsion can reach 98.37%. The water content was higher than 0.76% as a whole, and the salt concentration had decreased to 3 mg L−1 at the frequency of 1 kHz. As displayed in Fig. 6b, affected by the sodium naphthenate, the maximum desalination rate of the W/O emulsion reduced to 79.29%, the overall water content was more than 4.9%, and the overall salt concentration was more than 20 mg L−1. When the frequency was higher than 2 kHz, the desalination rate hardly changed. The inorganic salts removal process had no significant effect on the removal of organic acids, but weakened the influence of electric field frequency. Under different electric field frequencies, the acid value after deacidification was lower than 0.03 mgKOH g−1.
Effect of Electric Field Duty Ratio
The images shown in Fig. 7a, with the increase of electric field duty ratio, the desalination rate of emulsion showed an upward trend. The reason for this was that the water droplets were polarized during the conduction time of the pulsed electric field, and returned to the sphere during the resting time due to the interfacial tension. The duty ratio determined the time to apply a positive voltage for a certain period of time. If the electric field duty ratio was comparatively low, the time to apply a positive voltage was small which will not conducive to the coalescence of droplets [45]. During the interruption, the water droplets turned from ellipsoidal to spherical, which was not favorable for the polarization deformation of W/O emulsion water droplets. On the contrary, if the duty ratio was higher, then the electric field force acted on the emulsion for a longer time, the electric field energy of dehydration increased, and the coalescence force between water droplets increased, which facilitated the coalescence of water droplets. As displayed in Fig. 7b, the desalination rate of emulsion first increased and then decreased with the increase of electric field duty ratio. Because sodium naphthenate reduced the interfacial tension between oil and water [19]. At high duty ratio of electric field, the water droplets will be broken because of excessive polarization, which was not favorable to the W/O emulsion demulsification.
As illustrated in Fig. 7a, the desalination rate was up to 98.76%. The salt concentration was 3 mg L−1 when the electric field duty ratio was 20%. When the emulsion with NA, sodium naphthenate increased the stability of emulsion, which led to poor desalination effect. As displayed in Fig. 7b, the maximum desalination rate was reduced to 89.28%, the overall water content was more than 4.8%, and the overall salt concentration was more than 10.5 mg L−1. The inorganic salts removal process had little impact on the removal of organic acids. Under the conditions of different pulse width ratio, the acid value after deacidification was lower than 0.03 mgKOH g−1.
Effect of Initial Water Content
The water content of crude oil could be reduced to less than 30% after pre-settlement treatment. Combined with the experimental background, the initial water content in this experiment was set as 5–30%. The impact of initial water content on the removal efficiency and the water content after dehydration of W/O emulsion was shown in Fig. 8. The desalination rate of emulsions increased with the increase of initial water content. When the water content of the emulsions was high, the number of water droplets as the dispersed phase was large and the spacing was small. According to Eq. (7), the dipole coalescence force of water droplets was inversely proportional to the fourth power of spacing [46], so the electrostatic attraction of water droplets under pulsed electric field was large and the coalescence effect was good. At the same time, the water droplets in W/O emulsion was rapidly polarized by the potential difference, which provided favorable conditions for drops-coalescing.
where Fdipole is the dipole coalescence force between two water droplets (N), β is the Clausius–Mossotti coefficient, εw is the dielectric constant of aqueous phase (F m−1), εo is the dielectric constant of oil phase (F m−1), E is the electric field strength (kV cm−1), r1 and r2 are the radius of two water droplets respectively (m), δ is the center distance between two water droplets (m), and K is a dimensionless constant.
As shown in Fig. 8a, the desalination rate could reach 96.85%, the water content could be reduced to 0.49%, and the salt concentration was generally higher than 3 mg L−1. However, as illustrated in Fig. 8b, c, when the emulsion with NA, the desalination effect was relatively poor. The water content after dehydration was higher, which was more obvious when the water content was low. On the one hand, the number of water droplets was small at low water content, the spacing was large, and the possibility of contact and coalescence was small. On the other hand, as an efficient emulsifier, sodium naphthenate hindered the coalescence of droplets. When the emulsion with NA, continue to increase the initial water content, the desalination rate would tend to be stable when the initial water content reached 25%. The removal effect would not change significantly. The salt concentration was generally higher than 13.3 mg L−1, and the acid value after deacidification was stable in the range of 0–0.03 mgKOH g−1. In the process of removal of water, inorganic salts and organic acids, although the deacidification effect met the standard, it would be difficult to meet the treatment requirements only by controlling the initial water content of W/O emulsion.
Effect of Inorganic Salts
a Desalination rate of emulsion without NA at different NaCl concentration; b desalination rate and acid value after deacidification of emulsion with NA at different NaCl concentration; c desalination rate and acid value after deacidification of emulsion with NA at different CaCl2 concentration; d salt concentration after desalination at different concentration and type of inorganic salt
The inorganic salts system in crude oil is relatively complex. In this paper, CaCl2 and NaCl were represented to study the influence of their concentration on the removal effect of W/O emulsion. Liang et al. [47] studied the salt content of crude oil in China, and the concentration of inorganic salts in the W/O emulsion was reduced by the process of water injection and salt washing, so the inorganic salts concentration was in the range of 200 mg L−1 ~1000 mg L−1. At the same time, we prepared 200 mg L−1, 400 mg L−1, 600 mg L−1, 800 mg L−1, 1000 mg L−1 NaCl solution and CaCl2 solution through on-site measurement of inorganic salt content. As shown in Fig. 9a, the desalination rate showed a declining trend with the increase of NaCl concentration. With the increase of inorganic salts concentration, its concentration in water droplets increased, which led to the increase of salts concentration in oil phase after dehydration, thus reducing the desalting rate. The highest desalination rate was 94.93%, and the water content was generally between 0.5 and 0.85%, but with the increase of NaCl concentration, the desalination rate decreased to 91.00%.
When the emulsion contained NA, the desalination rate of W/O emulsion decreased as the concentration of NaCl increased, and was increasing as the concentration of CaCl2increased. It could be seen from Fig. 9b, c that the removal efficiency of CaCl2 was much higher than that of NaCl, and showed an upward trend with the increase of initial concentration. The reason was shown in Eqs. (6) and (8). Due to the reaction between CaCl2 and sodium naphthenate, the density of sodium naphthenate continued to decrease. The emulsifying effect of sodium naphthenate was weakened, and water droplets were more likely to coalesce. However, with the addition of NaCl, the concentration of sodium naphthenate increased continuously, which hindered the coalescence of droplets. It was well-known that the presence of calcium and high pH reduced the partitioning of the acid. Also, the kinetics of partitioning and dissociation of naphthenic acid was much slower in the presence of sodium ions than calcium ions because divalent calcium ions complex twice as much naphthenate ion as the monovalent sodium ion, so the rate of calcium naphthenate formation was higher than that of sodium naphthenate. This could explain the improved desalination rate mentioned in the case of CaCl2. Under the influence of NaCl, the desalination rate decreased from 88.52 to 55.23%, the water content was higher than 4.8%, and the salt concentration increased from 23 to 447.7 mg L−1. Under the influence of CaCl2, the desalination rate was up to 99.63%, the water content was reduced to 0.6%, and the salt concentration was less than 10 mg L−1. After deacidification, the acid value was stable within 0–0.03 mgKOH g−1. Therefore, to achieve the removal effect, the selective addition of inorganic salts had a good auxiliary effect on desalination and dehydration.
Effect of Naphthenic Acid Value
Combined with the actual acid value of high acid crude oil, the acid value range was 2–10 mgKOH g−1. Figure 10 showed the effects of naphthenic acid value on the removal efficiency of W/O emulsion. It could be seen that the desalination rate of W/O emulsion decreased gradually with the increase of acid value. The increase of acid value contributed to the formation of sodium naphthenate, the emulsifying effect was enhanced. NAs were polar organic compounds found in crude-oil which were soluble in water and prone to partition from the oil phase to water phase especially as the pH increased. Hydrophilic and lipophilic groups were present in the NA molecules, where the carboxyl was a hydrophilic group and alkyl was a lipophilic group [48]. So the oil-water interfacial film was more stable, and the coalescence of water droplets was hindered. Moreover, sodium naphthenate seriously emulsified W/O emulsion, which greatly reduced the desalination rate of emulsions under high acid value. When the emulsion with alkali injection, the increase of acid value had a greater impact on the desalination effect. Because with the increase of acid value, the naphthenate generated in the emulsions increased, resulting in the increase of salts concentration in W/O emulsion. Compared with the emulsion without alkali injection, the desalination rate of W/O emulsion decreased greatly when the emulsion with alkali injection, which showed that the emulsification effect of naphthenic acid on W/O emulsion without alkali injection was much less than that of sodium naphthenate after alkali injection.
As illustrated in Fig. 10a, the desalination rate decreased from 96.8 to 91.51%. After the acid value reached 4 mgKOH g−1, the salt concentration began to exceed 3 mg L−1 after desalting. As shown in Fig. 10b, the desalination rate decreased from 82.41 to 16.17%, the water content was higher than 6.3%, the salt concentration was higher than 17.5 mg L−1, and the acid value after deacidification was stable in the range of 0–0.03 mgKOH g−1. Therefore, in the process of removal of inorganic salts and organic acids, when ensuring that the deacidification effect met the requirements, the acid value increased and the difficulty of desalination increased.
Effect of Alkali Injection
During the experiment, the range of alkali injection was 0–2 times the theoretical value, and desalination rate at different injected alkali content was obtained. As shown in Fig. 11, as the amount of alkali injection increased, the desalination rate of the emulsion gradually decreased. With the increase of alkali injection, the degree of positive reaction in Eq. (6) increased, which was conducive to the formation of sodium naphthenate and the improvement of emulsification effect. But it was not conducive to the coalescence of water droplets.
Without alkali injection, the water content of W/O emulsion after dehydration was less than 0.5%, and the salt concentration after desalination was less than 3 mg L−1. After alkali injection, the desalination rate decreased from 97.25 to 72.90%, the water content and salt concentration increased greatly. The acid value after deacidification decreased with the increase of alkali injection. When the alkali injection exceeded the theoretical requirement by one time, the acid value was stable in the range of 0–0.1 mgKOH g−1. Therefore, in the process of removal of inorganic salts and organic acids, the requirement of deacidification could be realized by controlling the alkali injection amount to one time the theoretical requirement.
Conclusion
In this paper, the influence of naphthenic acid (NA) on the electrocoalescence of W/O emulsion was studied by high-frequency pulsed electrocoalescence technology and alkali washing method by changing electric field parameters and physical parameters.
(1) When the emulsion with NA, in the process of removal of inorganic salts and organic acids, due to the production of sodium naphthenate, the emulsification effect was enhanced. The effect of desalination was weaker. Compared to emulsion without NA, the salt concentration after desalination increased by 10–20 mg L−1.
(2) With the increase of electric field strength, electric field frequency, electric field duty ratio, initial water content and divalent inorganic salts concentration, the desalination (dehydration) effect of W/O emulsion after deacidification could be effectively improved. The water content and salt concentration could reach the requirements of 0.5% and 3 mg L−1 respectively.
(3) When the emulsion with NA, the desalination rate of W/O emulsion decreased with the increase of monovalent inorganic salts concentration, which was opposite to the change trend of divalent inorganic salts.
(4) Under the experimental conditions, when the electric field strength was 1.5 kV cm−1, the electric field frequency was 2 kHz, the electric field duty ratio was 50%, the initial water content was 30%, the CaCl2 concentration was 400 mg L−1, the naphthenic acid value is 1 mgKOH g−1, and the alkali injection exceeded the theoretical requirement by one time, the desalination effect was the best, and the acid value after desalination could also meet the requirements.
References
S. He, X. Tan, X. Hu, Y. Gao, Environ. Technol. 40, 1401 (2019)
R. Zolfaghari, A. Fakhru’l-Razi, L.C. Abdullah, S.S.E.H. Elnashaie, A. Pendashteh, Sep. Purif. Technol. 170, 377 (2016)
W.A. Derungs, Corrosion. 12, 41 (1956)
M. Meriem-Benziane, B. Bou-Saïd, N. Boudouani, J. Pet. Sci. Eng. 158, 672 (2017)
D. Subramanian, N. May, A. Firoozabadi, Energy Fuels. 31, 8967 (2017)
Z. Xiaofei, M. Tao, H. Xiaochun, Z. Jinde, W. Xiaoyi, R. Sixian, Eng Fail. Anal. 117, 104802 (2020)
S. Less, R. Vilagines, J. Pet. Sci. Eng. 81, 57 (2012)
P. Bailes, Trans. Inst. Chem. Eng. 59, 229 (1981)
B. Li, Z. Sun, Z. Wang, Y. Jin, Y. Fan, J. Electrostatics. 80, 22 (2016)
J. Drelich, G. Bryll, J. Kapczynski, J. Hupka, J.D. Miller, F.V. Hanson, Fuel Process. Technol. 31, 105 (1992)
L. Zhang, J. Chen, X. Cai, S. Huang, Y. Ji, Colloids Surf. Physicochem Eng. Aspects. 520, 246 (2017)
E. Slavcheva, B. Shone, A. Turnbull, Br. Corros. J. 34, 125 (1999)
M.H. Ese, P.K. Kilpatrick, J. Dispersion Sci. Technol. 25, 253 (2004)
T.E. Havre, J. Sjöblom, Colloids Surf. Physicochem Eng. Aspects. 228, 131 (2003)
R. Facanali, N.A. Porto, J. Crucello, R.M. Carvalho, B.G. Vaz, L.W. Hantao, Journal of Analytical Methods in Chemistry 2021, 6078084 (2021)
S. Simon, J. Sjöblom, D. Wei, J. Dispersion Sci. Technol. 37, 1751 (2016)
H. Zhu, Q. Wang, Y. Yan, Y. Xu, S. Liu, S. Zhang, J. Xu, C. Yang, Energy Fuels. 36, 2561 (2022)
M. Moradi, E. Topchiy, T.E. Lehmann, V. Alvarado, Fuel. 112, 236 (2013)
H. Li, S.C. Mahavadi, A. Anton, S.I. Andersen, J. Dispersion Sci. Technol., 1 (2021)
X. Wang, E. Pensini, Y. Liang, Z. Xu, M.S. Chandra, S.I. Andersen, W. Abdallah, J.J. Buiting, Colloids Surf. Physicochem Eng. Aspects. 513, 168 (2017)
G. Garcia-Olvera, T.M. Reilly, T.E. Lehmann, V. Alvarado, Fuel. 185, 151 (2016)
V. Anand, R.M. Thaokar, Stability and Destabilization of water-in-crude oil Emulsion, Catalysis for Clean Energy and Environmental Sustainability: Petrochemicals and Refining Processes , vol. 2, ed. by K.K. Pant, S.K. Gupta, E. Ahmad (Springer International Publishing, Cham, 2021), pp. 707–728
H. Hadidi, R. Kamali, M.K.D. Manshadi, Eur. J. Mech. B-Fluid. 80, 206 (2020)
I.F. Guha, K.K. Varanasi, ACS Appl. Mater. Interfaces. 11, 34812 (2019)
H. Lu, S. Wu, Z. Miao, X. Xu, Y. Liu, Z. Wang, H. Wang, Q. Yang, Chem. Eng. Sci. 241, 116680 (2021)
M. da Silva, C.M.S. Sad, L.B. Pereira, R.R.B. Corona, J.F.P. Bassane, F.D. dos Santos, D.M.C. Neto, S.R.C. Silva, E.V.R. Castro, P.R. Filgueiras, Fuel. 226, 278 (2018)
N.N.A. Ling, A. Haber, B.F. Graham, Z.M. Aman, E.F. May, E.O. Fridjonsson, M.L. Johns, Energy Fuels. 32, 10042 (2018)
M. Lashkarbolooki, S. Ayatollahi, Chin. J. Chem. Eng. 25, 1820 (2017)
K.B. Olesen, L.T. Fogang, G. Palm-Henriksen, N. Alyafei, T.I. Sølling, J. Pet. Sci. Eng. 182, 106307 (2019)
V. Vivacqua, M. Ghadiri, A.M. Abdullah, A. Hassanpour, M.J. Al-Marri, B. Azzopardi, B. Hewakandamby, B. Kermani, Chem. Eng. Res. Des. 114, 162 (2016)
S.H. Mousavi, M. Ghadiri, M. Buckley, Chem. Eng. Sci. 120, 130 (2014)
Y. Zhang, Y. Liu, R. Ji, F. Wang, B. Cai, H. Li, Colloids Surf. Physicochem Eng. Aspects. 386, 185 (2011)
D. Yang, H. Wu, H. Sun, L. He, Y. Guo, Sep. Purif. Technol. 279, 119732 (2021)
N. Lemay, S. Mikhaylin, S. Mareev, N. Pismenskaya, V. Nikonenko, L. Bazinet, J. Membr. Sci. 603, 117878 (2020)
H. Xu, W. Jia, S. Ren, J. Wang, Carbon. 145, 229 (2019)
J. Li, X. Li, Y. Liu, J. Zhang, Chin. J. Chem. Eng. 25, 171 (2017)
C. Wu, A. De Visscher, I.D. Gates, Fuel. 253, 1229 (2019)
S. Mhatre, R. Thaokar, Chem. Eng. Process. 96, 28 (2015)
X. Zhang, J. Zhang, H. Liu, Powder Technol. 411, 117920 (2022)
M.A. Saad, M. Kamil, N.H. Abdurahman, R. M. Yunus and O. I. Awad. Processes 7 (2019)
B. Li, X. Dou, K. Yu, W. Zhang, H. Xu, Z. Sun, Z. Wang, J. Wang, Chem. Eng. Sci. 248 (2022)
Y. Zhou, B. Li, M. Zhang, Z. Sun, Z. Wang, J. Wang, Chem. Eng. Sci. 224, 115739 (2020)
B. Li, Y. Fan, Z. Sun, Z. Wang, L. Zhu, Powder Technol. 316, 338 (2017)
X. He, B. Zhang, S. Wang, Y. Wang, Y. Yang, X. Wang, D. Lee, J. Mol. Liq. 341, 117417 (2021)
D. Yang, M. Xu, L. He, X. Luo, Y. Lü, H. Yan, C. Tian, Chem. Eng. Sci. 138, 71 (2015)
J.S. Eow, M. Ghadiri, A.O. Sharif, T.J. Williams, Chem. Eng. J. 84, 173 (2001)
L. Zhi-Yong, Z. Jing, Pet. Process. Petrochem. 45, 28 (2014)
N. Li, Z. Sun, J. Sun, W. Liu, L. Wei, T. Li, B. Li, Z. Wang, Colloids Surf. Physicochem Eng. Aspects. 632, 127746 (2022)
Acknowledgements
This work was supported by the Natural Science Foundation of Shandong Province [grant number ZR2020MB137]; the Major Scientific and Technological Innovation Project of Shandong Province [grant number 2019JZZY010508].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sun, Z., Chen, Q., Liu, B. et al. Study in the Effect of Naphthenic Acid on the Electrocoalescence of Crude Oil Under High-Frequency Pulsed Electric Field. Korean J. Chem. Eng. 41, 461–472 (2024). https://doi.org/10.1007/s11814-024-00065-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-024-00065-w