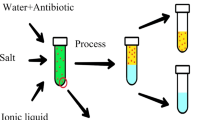
Abstract
Antibiotics discharged with medical waste have had a negative impact on humans as well as aquatic organisms. Because of the severity of its effects and the frequency with which it appears in medical effluent, extraction has become obligatory. Conducting mass transfer operations in microchannels is a promising technology that has several benefits over traditional methods. In this study, the potential of performing two-phase aqueous extraction of amoxicillin in a microchannel setup was investigated. To fully comprehend the process, it was necessary to study the system's hydrodynamics and consider the variables that had the greatest influence on the extraction in the microchannel. In the hydrodynamics part, the inlet junction showed an insignificant effect on the flow pattern type while the flow rate and volume fraction had a major effect. The plug flow zone was chosen for the microchannel extraction based on its high surface area and ease of separation. Aqueous two-phase system extraction (ATPS) was conducted in a microchannel to extract amoxicillin from the aqueous phase. Three process parameters were investigated, namely temperature, salt concentration, and volume fraction, which showed a direct proportionality influence on extraction efficiency. The optimum operation conditions obtained were found to be, a temperature of 44.3 °C, a salt concentration of 42.6 wt.%, and a volume fraction of 0.45. This was accomplished in 1.96 min as compared to the 540 min reported for the conventional ATPS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Water scarcity is a major issue that influences life on the planet. Therefore, governments and international organizations emphasize the need of providing clean water and to develop new technologies to clean wastewater. The treatment technology or method chosen is determined by the source of wastewater. Here, we consider medical wastewater which consists of a significant amount of antibiotics, which are antibacterial drugs used to inhibit the growth of bacteria. These materials are released into the wastewater via the disposal of unused and expired pharmaceuticals and excreta of humans and animals. For instance, antibiotics are present mostly in medical effluent. The amount of antibiotics used worldwide per year is estimated to be around 100,000 tons, with a consistent increase. Thus, there is widespread concern about the fate of these large quantities of antibiotics [12]. Even in trace amount, the presence of antibiotics in effluent-treated water has a significant impact. Antibiotic resistance is one of the consequences of their excessive use. In addition, drift to the aquatic ecosystem has an impact on organisms living in surface water and agriculture [3]. The antibiotics found in trace amounts make it extremely difficult to be extracted using conventional means of wastewater treatment. Therefore, a post-treatment stage is critical for reducing the environmental impact of antibiotics and drugs.
Extensive research activities on amoxicillin extraction from the aqueous phase have been conducted. These techniques include, but not limited to, amoxicillin ozonation [6], photocatalysis and ozonation combined [15], nanoparticles with enhanced oxidization [16], and classic liquid–liquid extraction [1]. As demonstrated in Table 1, each of the procedures stated above has advantages and limits.
The aqueous two-phase system (ATPS) is a mass transfer process between two liquids. It has gained special attention due to its enormous potential for purifying, separating, extracting, and enriching proteins, viruses, enzymes, and other biomolecules. However, complexity and difficulty in partition behavior are associated. Nevertheless, it has been successfully used for the detection of drug residues in foods and wastewater, sewage treatment, precious metals separation, and other applications. ATPS has considerable separation and purification advantages over traditional separation and purification procedures due to its high water volume. [11]. At the same time, ATPS is simpler and faster than the conventional process since the time of equilibrium is short, and consequently, the operation cost is low. This process can be easily scaled up and can be applied in multiple stages to increase the product purity. In addition, it is possible to control the volume of the phases by concentrating the extractant and the raffinate. Furthermore, by removing one of the phases, continuous extraction can be achieved [5]. Another important advantage is that the two phases have high water content, so the interfacial tension is low, which protects the biological molecules during the extraction process. The ionic strength and the pH can be regulated by adding salts or solutes. ATPS is highly efficient, relatively economical, and eco-friendly. It can be formed by mixing water with different components. There are different types of ATPSs, but the most commonly used types are polymer–polymer systems and polymer-salt system [8]. Microfluidics is the science and technology that deals with fluids with geometries ranging from sub-microns to a few millimeters [18]. It involves the chemical and physical characteristics of the process in addition to the production and engineering or design of structures in the micrometer scale. More and more research has been done by reducing the size of equipment to enhance the efficiency of processes. According to the definition given by the Institute fur Mikrotechnik Mainz (IMM), Germany, it refers to a process which is better in efficiency, less time-consuming, safer, less costly, and eco-friendly [14]. Due to reducing the size to the microscale, the mass and heat transfer improves at the macro-scale and, as a result, enhances the process performance [8]. There are many industrial applications of microtechnology. For instance, micro-devices in computer science, microscopic investigation in the biological field, and micro-contact or microfluidics in the process industry field [14]. In reactions, microfluidics was used to improve yield and selectivity as well as separate, purify, and filter valuable material. One such example of microfluidics is liquid–liquid extraction (solvent extraction), one of the most important processes in separation and purification in recent years. Microfluidics necessitates a deeper understanding of hydrodynamics and mass transfer to overcome the difficulties of the processes and enhance performance by looking at the flow patterns to make them more stable and lowering the pressure drop to save energy resources and cost.
The investigation of flow patterns is very important because the type of flow affects the process efficiency. Researchers have defined several types of flow patterns. Some of the common types of flow patterns are drop, plug, parallel, annular, and deformed interface [2, 9]. The main parameters that affected the flow pattern based on the previous studies are the flow rate of the two phases, phase volume fraction, geometry, production materials of the micro-channel, and fluids physical properties (density, viscosity, interfacial, and contact angle).
The most significant parameter affecting the flow patterns is the micro-channel material. Commonly, most micro-channels are made of glass due to its mechanical stability. Other materials like polytetrafluoroethylene (PTFE), poly-methyl-methacrylate (PMMA), polydimethylsiloxane (PDMS), or quartz [9] can be used. The second affecting parameter is the fluid inlet configuration and the entrance depth. For example, the Y-junction forms parallel flow whereas the T-junction forms slug flow. On the other hand, many studies reported that the type of junction has minor influence in developed flow. The hydraulic diameter of the inlet has the greatest impact on the flow. This was noticed by Kashid and Agar when they studied the impact of the diameter of the Y-junction. They observed that when the diameter of the junction decreases, the slug becomes narrower [2]. The third affecting parameter is the micro-channel geometry and hydraulic diameter of the channel. Kashid et al. investigated the effect of the micro-channel geometries, and they used different channel geometries with same depth. They noticed that the channel geometries with same hydraulic diameter have insignificant effect while the hydraulic diameter of the micro-channel has the major influence [10].
The treatment methods used in medical wastewater treatment suffer from many drawbacks. For example, the ozonation process is one of the high-cost operational processes. The photocatalytic degradation process is one of the complex processes. The extraction-based microchannel, on the other hand, is simple, safe, low cost, extremely efficient, with minimal contact time and no by-products. The purpose of this study is to demonstrate the effective removal of amoxicillin from wastewater using micro-extraction technology while overcoming the drawbacks of traditional two-phase aqueous extraction.
Despite the important role of antibiotics in treating various types of infections that can harm or end the lives of humans and animals, there are numerous side effects associated with antibiotic use. Every year, hundreds of thousands of tons of antibiotics are used. Only a small portion is consumed by humans, with the remainder being transferred to the water. Antibiotics in water cause serious problems for the environment and other creatures. Most areas of the world employ traditional wastewater treatment facilities, which are unable to provide the needed level of treatment efficiency. As a result, some antibiotics are discharged with treated water and then transferred to the ecosystem, causing a negative impact on human life. Because of these reasons, the scientific community continues to strive for an efficient and cost-effective method of removing antibiotics from treated water. The techniques utilized to remediate medical wastewater have numerous drawbacks.. For example, the ozonation process is one of the high-cost operation processes while photocatalytic degradation-based technologies are rather complicated. The extraction-based microchannel, on the other hand, is a simple, safe, low-cost, low-processing time, with no-byproducts, and highly efficient process. This work introduces the use of micro-extraction technology to efficiently remove amoxicillin from wastewater while overcoming the challenges associated with traditional two-phase aqueous extraction.
Experimental Methodology
Chemicals
The chemicals used in this work are the same as the chemicals used in our previous work [4]. For the organic phase, polyethylene glycol (6000) with a purity of 99.5% from Merck KGaA (Germany) was used. The aqueous phase consists of Amoxicillin Trihydrate, which was donated by the National Pharmaceutical Industries (NPI) Co. (SAOC) and Potassium dibasic phosphate (99%), purchased from Vinipul inorganic chemical company (India). For the analyzing step, methanol (99%) in isocratic grade from VWR chemicals (USA) was used as a mobile phase.
The micro-extraction of amoxicillin was conducted in a 10 cm long glass (borosilicate) micro-channel with a 1 mm diameter. The aqueous and the organic phases were injected using two syringe pumps (NE-300, USA). The aqueous phase amoxicillin concentration was measured by HPLC.
Note: The physicochemical properties of the system with HPLC specification and calibration curve are mentioned in previous work [4].
Experimental Design of Selected System
The response surface methodology (RSM) is one of the most widely used experimental designs for optimization. RMS is a very effective statistical method for investigating the relationship between variables and one or more responses in order to provide meaning to the used process. Furthermore, it is critical to demonstrate which variables have a greater, lesser, or no relationship. It is a useful method because it allows for the evaluation of the effects of multiple factors and their interactions on one or more response variables. Amoxicillin extraction efficiency was optimized using response surface methodology (RSM) with a central composite design (CCD). In response surface methodology, the Box-Behnken design (BBD) and the central composite design (CCD) are the two most commonly used designs. Despite having fewer runs, CCD outperforms BBD in terms of having two axial points. In contrast to the BBD, which focuses on the central area of the factor's range, CCD shows the effect of all factors on all of the studied range areas. The Design-Expert statistical software was used to design, analyze, and optimize the experiment. The three independent factors studied were temperature (23 °C–50 °C), salt concentration (15 wt.%–50 wt.%), and volume fraction (0.1–0.6). Five levels with a factor range were used to obtain the model. When investigating three parameters with five levels, a central composite design suggests a set of 20 runs (with six central points). P and F values were used to check if the model and independent factors were significant or not. A quadratic equation model was used to describe the effect of three factors and the extraction efficiency.
where Y is the predicted extraction efficiency, Xi, Xj are the actual values of independent variables and Bi, Bii, Bij are the coefficients of linear, second order and binary interactions on the response. Data analysis results are then presented in terms of charts and graphs.
After the model was obtained, ANOVA was used to validate the model, which is a powerful statistical approach. The regression coefficients (R2) of the predicted and adjusted P value, F value, and lack-of-fit were used to determine the significance of each factor and the model.
Experimental Setup and Procedure
Hydrodynamics
The hydrodynamics of the selected liquid–liquid two-phase system was studied. The fluids were flowing horizontally in a 1 mm diameter channel made of borosilicate. The effect of different inlet junctions on the flow pattern map was investigated at different flow rates. After that, the general flow patterns map for different geometries was drawn.
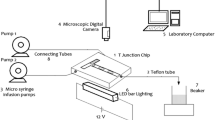
A schematic diagram of the experimental setup for different inlet micro-channels is depicted in Fig. 1. It consists of two high-precision syringe pumps to feed two immiscible liquids to the inlet junction. The syringe pumps NE-300, USA, can deliver up to 0.001 ml/ min. The inlet junction made of acrylic material was fabricated by a milling machine process. A hole was made in the acrylic plates with the same internal diameter (ID) of the channel tube (1 mm) to design inlet junctions (T-180°, T-90°, and cross junction) as shown in Fig. 1. The glass micro-channel tube was directly attached to the outlet of the junctions. The pressure drop across the micro-channel tube was measured by adding another acrylic T-180° junction at the end of the micro-channel with a 1 mm internal diameter (ID). The pressure drop was measured using a C9555 pressure meter (UK) with a range of (0–30 psi).The photographic system is installed at a length of 5 cm downstream from the inlet junction. It consists of a MIC-209 Microscope LED with 144 LEDs, an 8-inch TFT monitor (model: 1208), and a high-speed, high-resolution video camera.
Experiments were conducted for different inlet junction’s shapes having 1 mm diameter, namely, T-180° junction, T-90° junction, and cross junction (3 perpendicular inlets). Amoxicillin solution was prepared by dissolving amoxicillin powder in deionized water using an ultrasonic bath. The aqueous phase was made up of amoxicillin solution, and potassium dibasic phosphate, while the organic phase was made up of polyethylene glycol 6000 (PEG6000) solution. The aqueous phase (amoxicillin + K2HPO4) and the organic phase (PEG6000 solution) were introduced into the 1 mm diameter micro-channel simultaneously. The observed flow patterns were analyzed based on the total volumetric flowrates (Q) and organic volume fraction (εorg), which are calculated as follows:
where Qorg is the volumetric flowrate of the continuous phase and Qaq is the volumetric flow rate of the dispersed phase.
The physical properties of the liquids used in this work are listed in Table 2. The two liquids were introduced into the inlet junction using syringe pumps at the specified volumetric flowrates. The experiments were conducted with equal and unequal flowrates of each phase in the range of (0.01–1) mL/min for all inlet junctions. After allowing the pressure drop across the channel to stabilize, the pressure drop across the channel was measured for each experiment. Five different readings were taken, and the average value was computed. At a distance of 50 mm from the channel inlet, ten snapshots were taken for each flow condition to make sure that the photo of the flow pattern is clear and can be analyzed easily. All the experiments were repeated twice for accurate results. The length of the plug was measured with graph paper and placed just below the micro-channel tube. Three different plug length readings were taken to calculate the average length.
Microchannel Extraction
Since the hydrodynamics of an aqueous two-phase system are critical in determining the best operating conditions for the extraction process, they should be studied first. From the hydrodynamics study, specifically, flow pattern map and pressure data, the best zone for the microchannel extraction was chosen based on the results. Volume fraction, salt concentration, and temperature are the studied parameters within the specified ranges. To obtain 1000 ppm of amoxicillin solution, 0.5 g of amoxicillin was dissolved in 500 ml of deionized water and then diluted to 40 ppm. Different amounts of K2HPO4 were dissolved in 40 ppm amoxicillin solution to form an aqueous phase with a weight percentage of (15–50) wt.%. To make the organic phase, 40 g of solid PEG6000 were dissolved in 100 g of deionized water (weight percentage used in all solutions). Based on the previously mentioned volume fraction (0.1–0.6), the aqueous phase was introduced into the microchannel through one inlet, while the organic phase was introduced through the second. After some time, particularly when the flow in the microchannel is stable, a sample was taken from the top phase based on hydrodynamic conditions and studied parameters. HPLC was used to analyze a 1 mL sample of the aqueous phase, and the extraction efficiency was calculated using the following formula:
where Caq, in, Caq, out are the amoxicillin concentration in the aqueous phase before and after extraction, respectively.
To calculate the overall mass transfer coefficient, the following equation was used:
where Caq, eq, Caq, in and Caq, out are the amoxicillin concentrations at equilibrium, inlet and outlet respectively.
Results and Discussion
Flow Hydrodynamics Study
Flow Behavior
Process hydrodynamics should be studied before the extraction study, as was already indicated. According to the collected data, the organic phase (PEG6000 solution) is the continuous phase with a surface tension of 55.34 mN/m, while the aqueous phase (amoxicillin + K2HPO4) is the dispersed phase with a surface tension of 71.89 mN/m. Four different flow patterns were observed in the micro-channel. These are drop flow, plug flow, deformed interface flow, and annular flow, as depicted in Fig. 2. The direction of the flow in the image is from left to right.
Drop Flow
In this type of flow pattern, the aqueous phase flows as a dispersed phase and the organic phase flows as a continuous phase. The diameter of the droplet is less than the diameter of the micro-channel. From the data and snapshots, this type of flow occurs due to the low aqueous (amoxicillin + K2HPO4) to organic (PEG6000) volume fraction. As shown in Fig. 2a, the drop can have a spherical or bullet shape with a diameter less than the micro-channel diameter.
Plug Flow
This flow regime is similar to drop flow but has larger diameter than the micro-channel diameter. The dispersed phase forms cylindrical plugs and sometimes bullet shapes, as shown in Fig. 2b. This flow pattern occurs when flow rates of both phases are. The dominant force in this flow pattern is interfacial tension, which dominates more than viscous and inertial forces. The plug is very stable in size when controlled by interfacial tension. However, the viscous and inertia forces become more important when the flow rates increase, and the plug shape deforms. As the flow rates increased, the length of plugs also increased, as seen in Fig. 4. The length was found to increase until a new flow pattern was established.
Deformed Flow
This type of flow occurs at relatively high flow rates and consists of different flow types mixing, like droplet with slug with annular. The annular distance increases as the flow rate increases. This unstable regime acts as a transition region between plug flow and annular flow. Figure 2c shows how a deformed interface forms when transitioning from plug to annular.
Annular Flow
At high flow rates, annular flow was observed in a micro-channel as shown in Fig. 2d. In this flow regime, the organic phase flows as a film on the micro-channel wall while the aqueous phase flows in the center of the micro-channel. At the beginning of the flow, the shape of the flow patterns is not smooth like the throat. As the flow rate increases further, the interface of annular flow becomes stable and smooth.
The path of flow pattern transition investigated is consistent with the work reported by Kashid and Agar [9] and Salim et al. [17]. Kashid and Agar studied the flow patterns in different junction types with different diameters. The flow patterns presented in their work were drop, slug (plug), and deformed interface. Similarly, Salim et al. group found in their system (oil–water) that the flow pattern path started by drop followed by plug and stratified (annular).
Flow Pattern Map
From the obtained results, it was found that the type of inlet junction does not significantly affect the flow pattern map. Accordingly, only the T- inlet junction map will be addressed. The volume fraction with total flow rate was used to show the influence of the significant factors that affect the flow patterns. In addition, the multiplication of the Weber number and Ohnesorge number of each phase was used to obtain the universal flow patterns for different inlet junctions.
The inlet diameter of all the junctions is 1 mm, which is similar to the main channel. From Fig. 3, the type of inlet junction did not significantly influence the flow type and the region of the flow. This can be attributed to the fact that all junctions are affected by the same influencing forces due to the same flow conditions. There is a limited influence on the size of the flow region, whereas the sequence and the location of the flow patterns in the flow pattern maps were the same. For instance, the drop flow region in the T-180° is smaller than that for other junctions. In addition, the plug flow regions start at the same PEG6000 volume fraction and end at 0.98 PEG6000 volume fraction for T-180°, while in the other two junctions they end at 0.9 PEG6000 volume fraction. The plug regions have different flow rate starting points of 0.25, 0.2, and 0.4 mL/min for the T-180°, T-90°, and the cross-inlet junctions, respectively. All junctions have the same plug region end flow rate of 1.4 mL/min.
Flow pattern map for the three-inlet junction. The flow pattern map for T-90° is represented with dotted lines, the cross-inlet channel represented with dash-dot lines while the T-180° map is given as symbols: (filled square) drop flow, (filled triangle) plug flow, (filled diamond) deformed interface flow and (filled circle) annular flow
(A) Effect of Total Flowrate and Organic Phase Volume Fraction
Unlike the effect of the inlet junction, the volumetric flow rate and the phase volume fraction significantly affect the flow pattern map. As shown in Fig. 3 for the T-inlet junction, the plug flow and annular flow are the main flow patterns observed in the flow pattern map. The plug flow is observed at a low flow rate (< 1 mL/min) and a PEG6000 volume fraction (< 0.95). The length of plugs increases as the total flow rate increases. For instance, at 0.02 mL/min, the plug average length was 2.15 mm, and at 0.11 mL/min it was 3.24 mm, while at 0.31 mL/min it was 4.4 mm. The plug length was measured using a graph paper inserted under the micro-channel and the average length was estimated.
(B) Plug Flow Analysis
As previously demonstrated, the plug flow was obtained at relatively equal low flow rates for both phases, and the size of the plug (which was chosen as the best flow pattern type for micro-channel extraction) increased as the flow rate increased. In this section, the quantitative analysis of plug flow size in terms of flow rate and pressure drop is discussed. To measure the size of the plug, a piece of graph paper was placed under the micro-channel 5 cm from the inlet. Figure 4 presents the effect of the flow rates of both phases and pressure drop on plug size. At a constant aqueous flow rate, when the organic flow rate increases, the size of the plug decreases. The plug size is 2.15 mm at an aqueous flow rate of 0.03 mL/min and an organic phase flow rate of 0.01 mL/min, and it decreases to 2.09 mm at a flow rate of 0.05 mL/min. At a constant organic flow rate, the plug size increases by increasing the aqueous flow rate. This is expected because the aqueous phase is the dispersed phase. For example, fixing the organic phase flow rate at 0.05 mL/min, and at an aqueous flow rate of 0.01, 0.03, 0.05 and 0.1 mL/min, the plug size was recorded as 1.51, 2.15, 2.67 and 3.91 mm, respectively. There is no significant effect of pressure drop on plug size. When the plug size increases at a constant organic flow rate or decreases at a constant aqueous flow rate, the pressure drop keeps increasing due to the increase of the total flow rate.
(C) Universal Flow Pattern Map
As stated earlier, the influence of different parameters has been investigated, but the most influential parameters are the flow rate and phase volume fraction. The comparison between the three developed flow maps of the three inlet junctions (T-inlet, Y-inlet, and cross inlet) shows some similarities. Dimensionless numbers were used to plot the universal flow pattern map as mentioned in chapter two. However, not all these dimensionless numbers fit the experimental data well. To plot the universal flow pattern map, the Weber number multiplied by the Ohnesorge number [19] was used. The (We × Oh) shows the effect of different forces, inertia, and viscous and interfacial tensions. The universal flow pattern map was plotted in terms of the most common flow pattern in the three inlet junctions. The universal flow pattern map for the three developed maps of the different junctions is shown in Fig. 5. The flow pattern zones have some overlap of different flow patterns, but it achieves the separate zones.
The results achieved by the current study on the PEG6000-Amoxicillin were compared with two different studies that used different systems. Kashid and Agar [9] used a water–cyclohexane system, and they found that the drop flow pattern formed in low aqueous phase flow and high organic phase flow. Also, they observed the plug flow at medium flow rates of both phases. In Salim et al. work, they found that the drop flow pattern was observed at low water flow rates (less dense) and high oil flow rates (high dense), unlike the stratified flow patterns, which appeared at high water flow rates, while the slug flow pattern occurred at medium flow rates of both phases.
Extraction in the Microchannel
This part investigates the effects of temperature, salt concentration, and volume fraction. The DoE was utilized to determine the impact of each parameter and to create the system's regression model. This method relies on a hydrodynamics analysis to determine the ideal operating conditions, such as the best flow rate zone in the flow pattern map. The plug zone (0.01 mL/min to 0.1 mL/min organic and aqueous phase flow rate) was chosen based on the hydrodynamics study because it has the best mass transfer rate.
Response Surface Method Analysis
The experiments were repeated three times, and the amoxicillin removal efficiency was calculated. The measured removal efficiencies were fed to the DoE as the process response. The model for forecasting amoxicillin removal efficiency within the evaluated factors limits in terms of coded parameters was proposed by the DoE as given by Eq. (6).
where Y is the extraction efficiency in percent, and A, B and C are temperatures, salt concentration, and volume fraction, respectively. To check the reliability of the model, the predicted data must be analyzed by statistical software like ANOVA. Table 3 shows the ANOVA statistical data. The model was able to perfectly predict the system's behavior if the lack was insignificant, which means that the P value is greater than 0.05. From the result, the P value of the lack of fit is 0.1697, so the model can be used to predict the efficiency of amoxicillin removal. The P value of the model is found to be less than 0.0001, which confirms the significance of the terms. The coefficient of determination (R2) is used to check the goodness of the fit of the linear regression model, while the adjusted R2 is used to compare the goodness of the fit of the linear regression models that contain different variables. Predicted R2 is used to show how well the prediction is obtained from the regression model. All the values of R2 (0.9901), adjusted R2 (0.9812), predicted R2 (0.941) are close to 1, which indicates that the linear regression model obtained is significant and the prediction of the amoxicillin removal efficiency is of high quality. The P values of the majority of the investigated factors are significant and their P values are lower than 0.05, except for the interaction between B (salt concentration) and C (volume fraction) which is higher than 0.05. In most cases, the model must be reduced or modified to show the significance of all factors. However, this model is not modified because most of the parameters and their interactions are significant, and the influence of BC is very small on the mathematical model, as shown in Eq. (6). The F values show the importance of the studied parameters. The parameter with the highest F value is more important than the others. Hence, the order of importance of the parameters is as follows: salt concentration (406.61), volume fraction (142.9), and temperature (78.96). Adequate precision is used to compare the predicted output range at design points to the average predicted error. To obtain an adequate signal for the model, a value greater than 4 is required. This system's adequate precision value is (37.7211), indicating adequate signal.
The predicted and actual process response plots demonstrate the model's accuracy. The high correlation between the model's predicted and actual process responses in Fig. 6a indicates that the model is accurate. The normal plot of residuals in Fig. 6b, c depicts a well-behaved residual-predicted relationship. The residuals versus run order plot is used to demonstrate the process grift. Figure 6d shows that the data obtained has no rise or fall, indicating that the error terms are not independent. The predicted and actual process response plot shows the accuracy of the model. Figure 6a shows the high correlation between the model's predicted and actual process responses, which means that the model is accurate. The normal plot of residuals plotted in Fig. 6b shows that the residual distributed on the straight line reflects the normal distribution of errors. The plot of residual versus predicted responses can be used to detect the process non-linearity. Figure 6c shows a well-behaved relationship between residual and predicted. The residuals versus run order plot is used to show the grift in the process. Figure 6d shows that there is no rise or reduction in the data obtained, indicating that the error terms are not independent. The model given by CCD passed all these statistical tests.
Effect of Salt Concentration in the Aqueous Phase
To separate the two miscible phases and produce ATPS, potassium dibasic phosphate (K2HPO4) is used as a separating agent in this work. (Figure 7a, c) In this work, potassium dibasic phosphate (K2HPO4) is used as a separating agent to separate the two miscible phases and produce ATPS. The effect of K2HPO4 on the separation efficiency of amoxicillin was investigated using different wt.% of the salt (15–50%) as shown in Fig. 7a, c. At 22.4 wt.% of K2HPO4, the amoxicillin extraction efficiency was 81% while it increased to 96% at 42.6 wt.%. These two values were obtained at a constant temperature and volume fraction of 28.7 °C and 0.5 respectively. Due to the salting out effect, when the salt concentration rises, the extraction effectiveness correspondingly rises. Higher than this range, the dispersion phenomenon will reduce the efficiency because of the increase in viscosity due to the salt concentration increase.
Effect of studied parameters on extraction efficiency for conventional extraction mode: a effect of salt concentration and temperature at constant volume fraction, b effect of temperature and volume fraction at constant salt concentration, c effect of salt concentration and volume fraction at a constant temperature
Effect of the Temperature
Temperature has a significant effect on the extraction efficiency of amoxicillin. The effect of temperature on the extraction is presented in Fig. 7a, b for the range of (23–50) °C. The graph clearly shows that as the temperature rises, amoxicillin extraction efficiency rises. For instance, at 28.7 °C, the first extraction efficiency was 81%. It rose to 93% when the temperature rose to 44.3 °C. This increase is attributed to the fact that the diffusion rate increases when the temperature increases due to decreasing system viscosity and as a result, the transfer of amoxicillin molecules to the organic phase improves [7]. In addition, the forces between the solute and water molecules are reduced at high temperatures, which leads to easier interaction of amoxicillin molecules with PEG6000 [13].
Effect of the Volume Fraction
Figure 7b, c The effect of volume fraction on micro-channel extraction efficiency was studied for the range between 0.1 and 0.6 as shown in Fig. 7b, c. At a volume fraction of 0.2, the efficiency is 90% at a constant temperature of 44.3 °C and a constant salt concentration of 42.6 wt.%. When the volume fraction was raised to 0.5, the extraction efficiency became 98%. Generally, maximum extraction efficiency was obtained at a higher volume fraction. This is because when the organic phase's volume fraction increased, the organic phase's ability to take additional amoxicillin molecules increased as well, until it reaches equilibrium, and no more molecules can be taken.
Mass Transfer in Micro-Channel Extraction
The overall volumetric mass transfer coefficient, calculated by Eq. (5), was investigated and studied at the plug flow pattern zone where the aqueous and organic phase flow rates were less than 0.1 mL/min. In particular, the effect of temperature, salt concentrations, and volume fractions on the overall volumetric mass transfer coefficient, as shown in Fig. 8, was examined.
The influence of temperature on overall volumetric mass transfer coefficient by micro-channel extraction was investigated at 32.5 wt.% of salt concentration and 0.35 volume fraction (Fig. 8a). The overall volumetric mass transfer coefficient was found to increase as the temperature increased. This is attributed to two main reasons. One is that the increase in temperature normally has a positive effect on increasing the rate of mass transfer. The second reason is that the viscosity of the mixture decreased due to the increase in temperature, which eased the movement of the molecules from the aqueous to the organic phase.
As shown in Fig. 8b, the salt concentration is the second researched parameter that influences the mass transfer coefficient. At 36.5 °C and 0.35 volume fraction, the salt concentration was tested. The overall volumetric mass transfer coefficient is found to increase gradually with increasing salt concentration, and eventually the increase slows down around a concentration of 30 wt.%. This is because when the concentration of the salt increases, the mass transfer increases until it reaches a point where the viscosity of the mixture becomes high and, as a result, fewer molecules are transferred between the two phases.
The influence of volume fraction on overall volumetric mass transfer coefficient was conducted at a temperature and salt concentration of 36.5 °C and 32.5 wt.%, respectively (Fig. 8c). Generally, as the PEG6000 volume fraction increased, the overall mass transfer coefficient increased due to the increase in PEG6000 capacity to attract more molecules to its sites leading to an increase in molecules transfer to the PEG6000 phase. For example, at 0.1 PEG6000 volume fraction, KLa was 0.03 s−1 then increased to 0.09 s−1 at 0.6
Process Parameters Optimization
The optimization of the studied parameters (temperature, salt concentrations, and volume fraction) was done using the design expert software and the results are shown in Fig. 9. The optimum values of parameters were attained at a temperature of 44.3 C, a volume fraction of 0.45, and a salt concentration of 42.6 wt.%. At these conditions, the amoxicillin extraction efficiency predicted by the model was 98%. An experiment was conducted to verify the model at these optimum conditions. An efficiency of 97.57% was obtained experimentally at these conditions, which is very close to the value predicted by the model. This shows that the model can accurately predict and describe the system.
Optimum Micro-Channel Length
An experiment was conducted to obtain the ideal length of micro-channel at the aforementioned optimum conditions. The experiment was performed using 10 cm, 15 cm, 20 cm, 25 cm, and 30 cm micro-channel lengths, as demonstrated by the obtained results in Fig. 10. From the data, it is clear that extraction efficiency steadily rises with microchannel length until it approaches a plateau at 25 cm. Thus, it can be stated that 25 cm is the optimum micro-channel length required to get the maximum extraction efficiency.
Comparison between conventional extraction and micro-channel extraction
Table 4 presents the comparison's findings between the traditional extraction mode and the micro-channel extraction technique. The extraction efficiency and the overall mass transfer coefficient of both modalities t were compared for the same optimum conditions. The extraction efficiency results from Table 4 and Fig. 11 clearly reveal that micro-channel extraction is better than conventional extraction. Both methods can achieve 100% separation efficiency. However, the time needed for each process is considerably different. As shown in Fig. 11, only 1.96 min are needed to reach 100% separation efficiency for the microchannel-based extraction, while 540 min are needed for the conventional process. Furthermore, the overall mass transfer coefficient for micro-channel extraction (0.3078 s−1) is significantly higher than that for conventional extraction (0.0799 s−1). This is because the contact surface in micro-channels is higher than that in conventional contactors, which enhances both the extraction efficiency and transfer rate. Moreover, the amount of solvent used in conventional extraction is higher than that in micro-channel extraction, and this affects the cost of materials and, finally, the overall profit of the process. As a conclusion, the micro-technology presented by micro-channel is better than conventional extraction for the above-mentioned reasons.
Conclusion
The goal of this research was to use micro-technology in conjunction with an aqueous two-phase system to protect the aquatic environment from untreated antibiotics and pharmaceuticals. The antibiotic studied was amoxicillin, and the system used was a PEG6000-K2HPO4-amoxicillin solution. This study examined the hydrodynamics of micro-channels to identify the optimal flow rate zone based on high mass transfer rate and low pressure drop. The impact of inlet connections and both phase flow rates on flow patterns was investigated. This study used three different inlet connections: T-180°, T-90°, and cross-inlet junction. According to the findings, inlet connections have no substantial effect on flow patterns, except at a few sites where flow patterns were changed. The flow rates have a considerable impact on the form of flow patterns. Drop, plug, distorted interface, and annular flow patterns were the four main flow patterns found. At low aqueous flow rates and large organic flow rates, drop flow patterns were observed. The plug flow patterns were discovered at a relatively modest flow rate of both phases. At high flow rates of both phases, annular flow patterns were seen. A universal flow pattern map was created and compared to earlier work based on a similar flow pattern in a three-inlet junction.
After setting up the best hydrodynamic conditions, the extraction process in micro-channel was conducted in a 1 mm internal diameter and 10 cm long channel by considering the same parameters studied in the conventional extraction. The plug zone from 0.1 mL/min organic phase flow rate to 0.1 mL/min aqueous flow rate was chosen for the micro-channel extraction mode based on the hydrodynamics investigation. The experiment was carried out in the same way as a traditional extraction with the same parameter range. According to the results and data collected from DoE, the overall mass transfer coefficient and extraction efficiency improved as the temperature, salt concentration, and volume fraction of the organics increased. The optimal micro-channel extraction conditions were 44.3 °C, 42.6 wt.% salt, and a 0.5 volume fraction of PEG6000. From a quantitative and qualitative standpoint, micro-channel extraction outperforms traditional extraction. Micro-technology enables high extraction efficiency and mass transfer rates in less time, as well as a reduction in the amount of solvent required, all of which lower the operational expenses.
References
A. Abbasi, A. Rahbar-Kelishami, Z. Seifollahi, M.J. Ghasemi, Intensified decontamination of amoxicillin drug wastewater assisted by liquid-phase micro extraction method. Environ. Technol. 43, 1551 (2022)
M. Al-Azzawi, F.S. Mjalli, A. Husain, M. Al-Dahhan, A review on the hydrodynamics of the liquid–liquid two-phase flow in the microchannels. Ind. Eng. Chem. Res. 60, 5049 (2021)
I.M. Al-Riyami, M. Ahmed, A. Al-Busaidi, B. Choudri, Antibiotics in wastewaters: a review with focus on Oman. Appl Water Sci 8, 1 (2018)
S. Al-Saidi, F.S. Mjalli, M. Al-Azzawi, B. Abutarboosh, M.A. AlSaadi, T. Al-Wahaibi, Amoxicillin removal from medical wastewater using an eco-friendly aqueous two-phase extraction system. Sep. Sci. Technol. 1, 34 (2022)
M. Alberto, Aqueous Two-Phase Systems: Properties, Functions and Advantages (Nova Science Publishers, Hauppauge, 2018)
R. Andreozzi, M. Canterino, R. Marotta, N. Paxeus, Antibiotic removal from wastewaters: the ozonation of amoxicillin. J. Hazard. Mater. 122, 243 (2005)
J.R. Dean, Extraction Methods for Environmental Analysis (Wiley, Chichester, 1998)
A.L. Grilo, M. Raquel Aires-Barros, A.M. Azevedo, Partitioning in aqueous two-phase systems: fundamentals, applications and trends. Sep. Purif. Rev. 45, 68 (2016)
M.N. Kashid, D.W. Agar, Hydrodynamics of liquid–liquid slug flow capillary microreactor: flow regimes, slug size and pressure drop. Chem. Eng. J. 131, 1 (2007)
M.N. Kashid, A. Renken, L. Kiwi-Minsker, Gas–liquid and liquid–liquid mass transfer in microstructured reactors. Chem. Eng. Sci. 66, 3876 (2011)
A.B. Kayitmazer, S.P. Strand, C. Tribet, W. Jaeger, P.L. Dubin, Effect of polyelectrolyte structure on protein−polyelectrolyte coacervates: coacervates of bovine serum albumin with poly (diallyldimethylammonium chloride) versus chitosan. Biomacromol 8, 3568 (2007)
A. Kumari, N.S. Maurya, B. Tiwari, Hospital wastewater treatment scenario around the globe. Current Developments in Biotechnology and Bioengineering (Elsevier, London, 2020), pp.549–570
Y. Liu, B. Shi, Hollow fiber supported liquid membrane for extraction of ethylbenzene and nitrobenzene from aqueous solution: a Hansen solubility parameter approach. Sep. Purif. Technol. 65, 233 (2009)
J. A. Moulijn, A. Stankiewicz, in Process intensification, eds. by V. Strezov, J. Zoeller, M. Abraham. Encyclopedia of Sustainable Technologies, vol 3. (Elsevier, London, 2017), pp. 509–518
E. Norabadi, A.H. Panahi, R. Ghanbari, A. Meshkinian, H. Kamani, S.D. Ashrafi, Optimizing the parameters of amoxicillin removal in a photocatalysis/ozonation process using box-behnken response surface methodology. Desalin. Water Treat. 192, 234 (2020)
S. Rahdar, S. Ahmadi, The removal of amoxicillin with zno nanoparticles in combination with us-H2O2 advanced oxidation processes from aqueous solutions. Iran. J. Health Sci. 7, 36 (2019)
A. Salim, M. Fourar, J. Pironon, J. Sausse, Oil-water two-phase flow in microchannels: flow patterns and pressure drop measurements. Can. J. Chem. Eng. 86, 978 (2008)
H. Santana, J. Silva, B. Aghel, J. Ortega-Casanova, Review on microfluidic device applications for fluids separation and water treatment processes. SN Appl. Sci. 2, 1 (2020)
A.A. Yagodnitsyna, A.V. Kovalev, A.V. Bilsky, Flow patterns of immiscible liquid-liquid flow in a rectangular microchannel with t-junction. Chem. Eng. J. 303, 547 (2016)
Funding
This study was funded by Sultan Qaboos University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Al-Saidi, S., Mjalli, F.S., Al-Azzawi, M. et al. Aqueous Two-Phase Extraction of Amoxicillin Using Miniaturization Technology. Korean J. Chem. Eng. 41, 1399–1413 (2024). https://doi.org/10.1007/s11814-024-00061-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-024-00061-0