Abstract
Purpose
Regular primary care may be important to prevent ambulatory care-sensitive hospitalizations among older individuals living with breast cancer. The current study aimed to examine the relationship between preventable hospitalizations and primary care among Medicare beneficiaries diagnosed with breast cancer.
Methods
We used SEER-Medicare to identify 61,673 patients with incident stage 0-III breast cancer diagnosed between January 1, 2008, and December 31, 2015. Potentially preventable hospitalizations, defined using the Agency for Healthcare Research and Quality, were captured from diagnosis until death, second malignancy, or December 31, 2016. Primary care and non-oncology specialist visits were identified by distinct utilization groups (low utilizers vs. high utilizers). Incidence rate ratios [IRR]) were estimated for preventable hospitalizations. Multivariable Cox regression models estimated the association of primary care with 5-year overall survival.
Results
Median age at diagnosis was 74 years (range 66–101), median follow-up was 46 months (12–60), and 5-year survival was 82%. Over half of patients (59%) received primary care ≥ 1 time per year, and 7.8% had no primary care. Among low utilizers, no primary care was associated with a 55% increase in the rate of preventable hospitalizations (p < 0.001). Primary care was not associated with preventable hospitalizations among high utilizers. Factors associated with hospitalization for both high and low utilizers included higher stage at diagnosis, older age, and having a preventable hospitalization prior to cancer. Not receiving any primary care was associated with higher risk of 5-year mortality for low utilizers (HR 1.66, p < 0.001; HR 1.46, p < 0.001).
Conclusions
Among the group with less utilization, not engaging annually with a primary care provider was associated with a significantly increased rate of preventable hospitalizations.
Implications for Cancer Survivors
Consistent engagement with primary care may provide an opportunity for care coordination and management and should be considered critical in the context of long-term survivorship.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Five-year mortality rates for older (≥ 65 years) women with breast cancer have declined from 99.5 per 100,000 in 2010 to 91.2 per 100,000 in 2017 [1]. Declining mortality rates, coupled with the high incidence among those over the age of 65 (427.8 per 100,000), has resulted in a growing population of breast cancer survivors. As of January 2017, there were over 2.2 million older individuals living with a history breast cancer [1]. This population will continue to live years beyond their cancer diagnosis and will have healthcare needs associated with aging [2, 3] as well as those due to breast cancer or its treatment [4,5,6]. Recent data suggest accelerated aging in cancer survivors, underscoring the need for management of co-occurring chronic conditions in the post-treatment phase of cancer care [7, 8].
After cancer diagnosis, there is often a shift towards oncology-focused and cancer surveillance-focused care. Survivors of breast cancer may be followed simultaneously by medical oncologists, other cancer specialists, and primary care providers (PCPs) [9], to ensure comprehensive care [10]. Whether or not the survivor is cared for primarily by the PCP or by the oncology provider may be attributable to stage of disease, ongoing cancer treatment, or comorbidities [11]. PCPs may be better positioned to conduct surveillance for and manage non-oncologic chronic health conditions (e.g., diabetes, hypertension)[12]. Further, breast cancer survivors followed by a PCP are more likely to receive preventive care [9]. Regular primary care is supported by the Center for Medicare and Medicaid Services (CMS) and Center for Disease Control and Prevention[13] and is recognized as being instrumental for meeting the recommendations of the US Preventive Services Task Force [14]. These visits offer the opportunity for a comprehensive review of current conditions, medical history, medication use, and any additional intervention needs [15].
There is emerging evidence that not receiving primary care may increase the risk for preventable hospitalizations [16]. The concept of potentially preventable or ambulatory care sensitive hospitalizations in older adults has been widely studied by the Agency for Health Care Research and Quality (AHRQ) [17]. The few studies that have examined hospitalizations among cancer patients have focused on cancer treatment-related hospitalizations (e.g., chemotherapy toxicity) [16, 18]. A 2008 study [16] of Medicare beneficiaries diagnosed with breast cancer between 1992 and 1999 demonstrated that 13.3% had an ambulatory care-sensitive hospitalization after cancer diagnosis over an average follow-up of 47 months (range 0–96).
The qualifying diagnoses for “ambulatory care sensitive hospitalizations” have changed since this 2008 study; there are no studies in cancer patients using the current AHRQ definitions. Further, there is limited information regarding the impact of primary care on such hospitalizations; such information could inform survivorship care recommendations for older patients with breast cancer. Additionally, identifying high-risk populations that may be less likely to receive primary care and/or more likely to experience preventable hospitalizations could guide targeted interventions to improve access to and/or the use of primary care. This study proposes to examine ambulatory care-sensitive hospitalizations among Medicare beneficiaries diagnosed with breast cancer and to determine the association between primary care visits and rates of such hospitalizations.
Methods
Data source
For the current study, Surveillance, Epidemiology, and End Results (SEER) data were linked to Medicare administrative claims data, providing billing information for diagnosis codes from inpatient, outpatient, and non-institutional settings [19]. The SEER data are comprised of geographically diverse cancer registries designed to represent US cancer incidence and mortality. The Institutional Review Board of the University of Alabama at Birmingham deemed this study exempt.
Population
We included females with incident stage 0–III breast cancer diagnosed between January 1, 2008, and December 31, 2015, at 66 years of age or older and enrolled in Medicare. We captured Medicare claims 1 year prior to diagnosis (to capture pre-existing comorbidities and pre-cancer preventable hospitalizations) and up to the date of second malignancy, death, or December 31, 2016 (study end date), whichever occurred first.
There were 309,666 breast cancer patients diagnosed between 2008 and 2015. We excluded (1) those with a history of previous cancer or those where breast cancer was not listed as the primary diagnosis (n = 27,192), (2) those diagnosed before the age of 66 (n = 115,312), (3) those diagnosed at death or autopsy (n = 1467), and (4) males with breast cancer (n = 1482). Among the remaining 164,213 patients, we excluded those who did not have Medicare enrollment data after 1999 (n = 12,021), those who did not have Part A/B coverage or were covered by an HMO during follow-up (n = 28,099), and those patients who did not have one year of continuous coverage prior to diagnosis (n = 38,286). Among the remaining 78,046, we excluded those patients with completely missing SEER information (n = 12,021). We further excluded patients with no evidence of treatment in the claims data (n = 2657) and those with Stage IV or unknown stage disease (n = 1874). Additionally, we excluded patents where the follow-up was < 12 months (n = 14,650). Finally, we excluded patients likely residing in a nursing home identified through the Minimum Data Set file [20] (n = 17,064). The final analytic sample includes 61,673 patients (Supplementary Fig. 1). Supplementary Table 1 describes the differences between the analytic sample and those excluded from the SEER population.
Primary care
We identified evaluation and management (E&M) visits conducted by primary care providers in outpatient and office settings [21] each year after diagnosis until diagnosis of recurrence/second malignancy, death, loss of coverage, or end of study (December 31, 2016). Healthcare Common Procedure Coding System (HCPCS) codes used to identify the visits included 99,201–99,205, 99,211–99,215 [21]. Physician specialty was defined using the Medicare-provided specialty codes. Primary care includes family medicine, general internal medicine, geriatric medicine, obstetrics-gynecology, and physicians in multispecialty group practices. We created a four-category variable to describe a patient’s regular use of primary care based on the number of years of follow-up for each patient and whether or not the individual had PCP E&M office visit: no PCP (0%), visits on up to half the years of follow-up (1–50%), visits on more than half of the years of follow-up, but not all years (51–99%), visits every year of follow-up (100%).
Non-oncology specialist care and oncology care
To characterize the overall utilization of each patient, the same evaluation and management (E&M) codes in outpatient and office visits setting were identified for non-oncology specialists and oncology specialists. Oncology specialists included hematology, hematology/oncology, medical oncology, surgical oncology, and radiation oncology. Non-oncology specialists included all specialists not categorized as oncology or primary care (e.g., gastroenterology, neurology, etc.).
Utilization groups
For all three provider groups, we examined the total number of visits and generated a visits/per year variable (PCP E&M, oncology E&M, and non-oncology specialist E&M). Examining the rate of visits per year revealed a very distinct pattern of low utilizers and high utilizers within the population. Low utilizers had a rate of visits at or below the 75th percentile for both PCP (≤ 4.8 visits per year) and non-oncology specialist visits (≤ 7.3 visits per year). High utilizers included those with greater than the 75th percentile of visits for PCP (> 4.8 visits per year) or non-oncology specialists (> 7.3 visits per year). The rate of oncology visits did not vary between the high and low utilizers.
Potentially preventable hospitalizations
The primary outcome was hospitalizations for ambulatory care-sensitive conditions and considered potentially preventable [17]. We identified these potentially preventable hospitalizations using the Prevention Quality Indicators, defined by AHRQ [17]. We included hospitalizations secondary to chronic conditions (e.g., diabetes [short-term complication, long-term complications, uncontrolled diabetes, lower extremity amputation secondary to diabetes], chronic obstructive pulmonary disease [COPD], asthma, hypertension, and congestive heart failure), and those attributable to an acute condition (urinary tract infection [UTI], community-acquired pneumonia). Total preventable hospitalizations were counted from breast cancer diagnosis to recurrence/second malignancy, death, 5 years, or end of study (December 31, 2016).
Covariates
Based on their age at breast cancer diagnosis, we placed patients into the following categories: 66–70 years, 71–75 years, 76–80 years, and > 80 years. Stage at diagnosis was determined using the 6th American Joint Committee on Cancer staging. Race/ethnicity was categorized as non-Hispanic white (NHW), non-Hispanic Black (NHB), Hispanic, and Asian American/Pacific Islander (AA/PI). We measured pre-existing comorbidity using Elixhauser Comorbidity Index [22] in the 12 months prior to breast cancer diagnosis. Chemotherapy, hormone therapy, and radiation details were drawn from outpatient claims, physician carrier claims, inpatient claims, durable medical equipment Medicare claims, and from Part D data. Patients were classified as having received chemotherapy (yes/no), hormone therapy (yes/no), and radiation (yes/no). Surgical interventions were identified from inpatient claims and SEER file, and patients were classified as having received local excision, partial mastectomy, or total mastectomy. We included a covariate for “total other hospitalizations” (total hospitalizations minus preventable hospitalizations) to our model to control for overall health care utilization.
Geographic region at diagnosis was defined using the reporting SEER registry (Northeast, South, Midwest, West). Rural vs. urban residence was defined using the Rural/Urban Continuum Codes [23]. Using census-tract information from the US Census Bureau, area-level low-income status and area-level low education status variables were defined as below median income and above the median proportion of the population with less than high school education.
Statistical analysis
We examined the relative rate of preventable hospitalizations after cancer diagnosis per person-time. The primary exposure was the variable indicating the percent of years receiving primary care (0, 1 to 50%, 51–99%, 100%). The interaction between receipt of primary care (0, 1–50, 51–99, 100%) and level of utilization (low-utilizers, high-utilizers) suggested a need to examine the association between PCP care and preventable hospitalizations separately within each of the utilization groups. All models were adjusted for race/ethnicity, marital status, urban/rural designation at diagnosis, Elixhauser comorbidity index, age at breast cancer diagnosis, stage at diagnosis, treatment received (surgery type, chemotherapy, hormone therapy, radiation), prior preventable hospitalizations, total other hospitalizations, region, rate of non-oncology specialist use, and census tract education and income. Multivariable zero-inflated negative binomial models with an offset of the log of patient time (months) were used to estimate the incident rate ratios for preventable hospitalizations.
Kaplan–Meier method was used to estimate 5-year survival by PCP care. The association between PCP care, and all-cause mortality was examined using multivariable Cox regression models. Covariates included those listed above.
We excluded patients with missing data that was needed to assess outcomes. We required continuous Medicare Part A and Part B enrollment prior to diagnosis, censored at loss to follow-up in claims, and excluded patients with key missing SEER data (age at diagnosis, stage, year at diagnosis); therefore we assumed that if patients did not have claims in the database after these exclusionary criteria, they did not utilize the services. We used SAS V9.4 for all analyses.
Results
Median age at breast cancer diagnosis was 74 years (range 66–101), and median follow-up was 46 months (12–60 months) (Table 1). Approximately 82% of the cohort was non-Hispanic white; 49% of the patients were diagnosed with Stage I disease; 70% were treated with local excision, 46% received radiation, 23% received chemotherapy, and 42% received hormone therapy. Overall, 21% had a pre-cancer diagnosis Elixhauser comorbidity index score of ≥ 2. Only 1.4% of the cohort had a preventable hospitalization in the year prior to breast cancer diagnosis.
Fifty-nine percent of the cohort received regular primary care (at least once per year of follow-up), while 7.8% had no visits to primary care. Compared with those with any primary care, those with no primary care were older (> 80 years), diagnosed with stage III disease, lived in rural and lower education areas, and had a lower burden of pre-existing comorbidity. All demographic characteristics by the regular primary care use variable are summarized in Table 1.
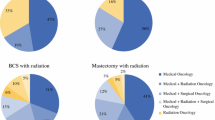
Figure 1 displays the distribution of utilization overall and provides rates by low and high utilization groups. The median number visits per year of follow-up by type of visits was as follows: PCP = 3 (range, 0–78; IQR 1.4–4.8), oncology = 2.5 (range, 0–70; IQR 1.2–4), and non-oncology specialist = 4.4 (range, 0–107; IQR 2.4–7.3). Supplemental Tables 2 and 3 describe characteristics associated with group membership. High users were diagnosed at an older age (39 vs. 33% diagnosed at 76 years of age or older, p < 0.001), more likely to be urban (13.1 vs 9.6%, p < 0.001), and more likely to have 1 more comorbidity (62 vs. 21.8%, p < 0.001).
Preventable hospitalizations
Approximately 7% of the sample had a preventable hospitalization during their follow-up time. In year one, 2.1% of patients had preventable hospitalization, 2% in year two, 2.2% in year three and year four, and 2.3% in year five. Among low utilizers, multivariable-adjusted models demonstrated that having no primary care (0%) was associated with a 55% increased rate of preventable hospitalization (IRR = 1.55, 95%CI 1.29–1.85) (Table 2), when compared with those who had a primary care visit at least once a year. Other factors with significant differences among the low utilizers included having a prior preventable hospitalization (IRR 4.6, p < 0.001), Stage III at diagnosis (IRR 3.22, p < 0.001), and > 80 years at diagnosis (IRR 2.92, p < 0.00). There was no association between receiving less regular primary care and preventable hospitalizations among those classified as high utilizers. The most frequent preventable hospitalizations were pneumonia (26% of all preventable hospitalizations) and heart failure (26%), followed by COPD/asthma (23%). Supplementary Table 4 provides the rate of hospitalizations for each individual diagnosis by the regular use of primary care.
Overall survival
The overall unadjusted 5-year survival was 82% (Stage 0: 87%, Stage I: 86%, Stage II: 77%, Stage III: 60%, p < 0.0001). For each stage, there was a survival advantage for those who received 100% regular primary care vs. no primary care (Stage 0: 86% vs. 80%, Stage I: 84 vs. 81%, p < 0.001; Stage II 75 vs. 68%, p < 0.001; Stage III: 57 vs. 37%, p < 0.001). Multivariable analysis (Table 3) demonstrated that among the low utilizers and high utilizers, those with 0% primary care had a significantly increased hazard of death (HR = 1.61, 95% 1.46–1.78; HR = 1.45 95% CI1.23–1.71, respectively), when compared with those with 100% primary care. Conversely, among both utilization groups, those with 51–99% primary care had a decreased hazard of death (HR = 0.66 95% CI 0.61–0.72, HR = 0.57, 95% CI 0.51–0.64).
Discussion
Among the approximately 61,000 Medicare beneficiaries with breast cancer, approximately 7% experienced at least one AHRQ-defined preventable hospitalization after their cancer diagnosis. Fifty-nine percent of the received primary care at least once every year of follow-up. Distinct groups of users of primary care and non-oncology specialist care were identified, with large outliers of very high utilization of both primary care and non-oncology specialist care. Importantly, those who were in the lowest overall utilization group were those that were most at risk for preventable hospitalizations (55% increased rate). There was no association with very high utilizers, a group who may already be plugged into the healthcare system. This underscores the importance of regular primary care, especially for those that may not have an extensive history of needs. Among both groups, there was an increased risk of mortality if they did not receive any primary care.
The 2008 study by Schootman et al. [16] found that among 47,634 older Medicare beneficiaries diagnosed with breast cancer, 13.3% experienced an AHRQ-defined potentially preventable hospitalization, higher than the rate of 7% reported in the current study. However, the Schootman study included conditions that have since been removed from the AHRQ list of potentially preventable hospitalizations, such as dehydration, convulsions, gastroenteritis, cellulitis, and ear/nose/throat infection [16]. Schootman et al. also reported that office visits to primary care physicians (defined by specialty [e.g., internal medicine, family practitioner]) were associated with a reduced risk of preventable hospitalizations [16]. Another prior study using the Medicare Current Beneficiary Study (MCBS) found that older age, Black race, having less than a college education, and living in a rural county increased the odds of preventable hospitalizations [24]. Our study found similar factors to be statistically significant for low utilizers, but with incident rate ratios indicating limited clinical significance given the large sample size.
Previous studies examining preventable hospitalizations have either focused on non-cancer populations or, for studies in cancer populations, have focused on hospitalizations related to treatment toxicity [16, 25,26,27,28,29]. Estimates of preventable hospitalizations for unplanned acute care events during treatment range from 19 to 50% [18, 25, 27]. In the MCBS study [24], pneumonia accounted for the largest proportion of preventable hospitalizations, accounting for 19% of visits. In our study pneumonia and heart failure accounted for the largest proportion, accounting for 26% each. The risk estimates presented in this study are adjusted for the presence of prior disease, via the Elixhauser comorbidity index. Preventing avoidable admissions such as these requires aggressive and proactive symptom management through quality outpatient care or assisting patients in self-management.
We identified a significant increased hazard of 5-year mortality for those with no visits to primary care for intermediate and low utilizers. We did not observe this trend in high utilizers, even with decreasing regular use of primary care (1–50% or 51–99%). In fact, we observed counterintuitive findings across the two groups for those with primary care in over 50% of their years of follow-up, but not all years. This group demonstrated a decreased risk of mortality. It is possible that this is due to increased use of non-oncology and oncology specialists; the median number of visits per year was higher for these visits compared with those with no primary care. Findings of prior studies of patients with chronic lymphocytic leukemia, pancreatic cancer, and gastroesophageal cancer demonstrated a non-significant association of primary care follow-up with mortality, although studies trended in the direction of a protective effect [30,31,32].
It is important to contextualize the time frame of the data reported in this study (2008–2016), specifically in the context of survivorship guidelines and care plans. The 2006 Institute of Medicine (IOM) report on cancer survivorship was a landmark report highlighting quality health care for cancer survivors and identifying strategies to achieve it. Subsequently, major organizations began implementing guideline and recommendations for the delivery of quality survivorship care [33,34,35,36]. In 10 years after the IOM report through this study’s time period, several recommendations have remained consistent. Importantly, there has been a continued emphasis on primary care to engage in the care of patients with cancer [11, 37]. Handley and colleagues [38] suggested access to care and care coordination [29, 39] as ways to reduce unplanned acute events in the post-treatment survivorship phase. Suggestions for interventions included patient navigation, symptom monitoring, and nursing coordination to improve both access and care coordination.
Additionally, the research on survivorship care over the 15 years has suggested that a risk-based model of care may provide more appropriate and more efficient care to patients [37, 38, 40]. This approach was initially seen in cancer survivorship clinics but is now also included in other models of survivorship care. The risk-based approach facilitates determining the type (e.g., oncology, primary care) and frequency of care needed for each patient. The current study provides evidence for future studies that could employ risk-factor monitoring and predictive analytic methods to identify high-risk subpopulations who would most benefit from a targeted intervention to facilitate receiving primary care. Risk models should consider social, economic, and service environment (access) factors when determining groups who would benefit from close monitoring or additional support via navigation to primary care, for example.
There are several changes that occurred at the end of the study period that should be considered when interpreting the rates of primary care and preventable hospitalizations in the study period compared to the current era (2017–2021). The Commission on Cancer has established requirements on delivery of survivorship care for hospital-based cancer programs to receive accreditation since 2015. Currently, hospital-based cancer programs need to deliver survivorship care plans in the setting of a structured survivorship program [41]. Patients diagnosed in the last 5y could potentially have greater access and/or more structured follow-up increasing the likelihood of greater adherence to primary care as part of the survivorship care. Second, changes involving value-based care models such as the Center for Medicare and Medicaid Services Oncology Care model may increase the likelihood of receiving care, as one of the required elements to receive payment for the episode of care is to provide survivorship care planning [42, 43]. Finally, the expanded use of electronic medical records to create survivorship care plans should theoretically increase the ease with which plans are developed and shared with patients’ other provider, particularly primary care—although its impact is not fully understood [44, 45]. This study does not fully explore the impact of shared care between primary care and oncologists. Future studies will need to examine the potential differential effect of shared care between primary care, non-oncology specialists, and oncologists.
Finding from the current study should be interpreted in the context of limitations. Administrative data retains strengths of a population-based approach but is designed for billing purposes. To mitigate this misclassification of hospitalization type, we used validated algorithms from AHRQ to identify preventable hospitalizations [17]. The classification of conditions as potentially preventable (ambulatory care sensitive) has changed over time, making it difficult to make comparisons between studies. Further, despite limitations in identifying recurrence or second malignancies in SEER-Medicare, we used previously recommended methods to identify these events [46]. This study does not include patients enrolled in Medicare Advantage, a group that may demonstrate different utilization patterns than Fee-for-service. In order to adequately assess prior comorbidities, we had to restrict our population to those with at least 12 months of continuous healthcare coverage prior to diagnosis. Compared with the final analytic sample, the population that was excluded due to loss of coverage had an over-representation of minorities and those who were younger at diagnosis and those diagnosed with stages II and III. We likely underestimate hormone therapy use, identifying 42% of the sample receiving hormone therapy, a low rate relative to the number of patients in this sample with a positive hormone receptor status (68%). This is likely due to the lack of Part D coverage; 30% of the total sample did not have coverage and 35% of those with hormone receptor positive status did not have complete Part D coverage. We likely underestimate the protective of hormone therapy on mortality. We also restrict our population to a community dwelling population; nursing home populations likely have very different patterns of use. Additionally, our results apply to older adults with early breast cancer; future studies should examine the differences in primary care adherence and preventable hospitalizations in other cancer types. Finally, it is possible that not all relevant confounders were captured in these analyses.
Primary care provides an opportunity for care coordination and management among older adults with cancer, a much needed partnership when patients are receiving care from an oncologist. We demonstrate that while there are distinct patterns of utilization among breast cancer patients, those engaging the least were most negatively affected by not receiving annual primary care. Conversely, among those already engaging at the highest level in health care, regular primary care does not decrease the risk of preventable hospitalizations. Regardless of utilization, clinical characteristics (higher stage at diagnosis) and sociodemographic characteristics (minorities, rural, low-income areas, living in the south or midwest) trend towards increased risk of preventable hospitalizations. To use primary care as a method of reducing preventable hospitalizations, there must be adequate access and support for all patient groups to receive such care.
Data availability
Data is available to researchers for purchase from the National Cancer Institute (Surveillance Epidemiology and End Results (SEER) data linked with Medicare claims).
Code availability
Not applicable.
References
Surveillance Epidemiology, and End Results Program. SEER*Explorer Breast Cancer Incidence, Prevalence, Mortality Bethesda, MD: National Cancer Institute; 2020 [Available from: www.seer.cancer.gov/explorer. Accessed Feb 2021
Gironés R, Torregrosa D, Díaz-Beveridge R. Comorbidity, disability and geriatric syndromes in elderly breast cancer survivors. Results of a single-center experience. Crit Rev Oncol Hematol. 2010;73(3):236–45.
Williams GR, Mackenzie A, Magnuson A, Olin R, Chapman A, Mohile S, et al. Comorbidity in older adults with cancer. J Geriatr Oncol. 2016;7(4):249–57.
Blok EJ, Kroep JR, Meershoek-Klein Kranenbarg E, Duijm-de Carpentier M, Putter H, Liefers GJ, et al. Treatment decisions and the impact of adverse events before and during extended endocrine therapy in postmenopausal early breast cancer. Eur J Cancer. 2018;95:59–67.
Chou YH, Huang JY, Kornelius E, Chiou JY, Huang CN. Major adverse cardiovascular events after treatment in early-stage breast cancer patients receiving hormone therapy. Sci Rep. 2020;10(1):1408.
Hadji P, Kieback DG, Tams J, Hasenburg A, Ziller M. Correlation of treatment-emergent adverse events and clinical response to endocrine therapy in early breast cancer: a retrospective analysis of the German cohort of TEAM. Ann Oncol. 2012;23(10):2566–72.
Sanoff HK, Deal AM, Krishnamurthy J, Torrice C, Dillon P, Sorrentino J, et al. Effect of cytotoxic chemotherapy on markers of molecular age in patients with breast cancer. J Natl Cancer Inst. 2014;106(4):dju057.
Williams GR, Chen Y, Kenzik KM, McDonald A, Shachar SS, Klepin HD, et al. Assessment of sarcopenia measures, survival, and disability in older adults before and after diagnosis with cancer. JAMA Network Open. 2020;3(5):e204783-e.
Snyder CF, Frick KD, Kantsiper ME, Peairs KS, Herbert RJ, Blackford AL, et al. Prevention, screening, and surveillance care for breast cancer survivors compared with controls: changes from 1998 to 2002. J Clin Oncol. 2009;27(7):1054–61.
Snyder C, Frick K, Peairs K, Kantsiper M, Herbert R, Blackford A, et al. Comparing Care for Breast Cancer Survivors to Non-Cancer Controls: A Five-Year Longitudinal Study. J Gen Intern Med. 2009;24(4):469–74.
Nekhlyudov L, O’Malley DM, Hudson SV. Integrating primary care providers in the care of cancer survivors: gaps in evidence and future opportunities. Lancet Oncol. 2017;18(1):e30–8.
Wu AH, Kurian AW, Kwan ML, John EM, Lu Y, Keegan THM, et al. Diabetes and Other Comorbidities in Breast Cancer Survival by Race/Ethnicity: The California Breast Cancer Survivorship Consortium (CBCSC). Cancer Epidemiol Biomark Prev. 2015;24(2):361.
Centers for Disease Control and Prevention, Agency for Healthcare Research and Quality, and Centers for Medicare and Medicaid Services. Enhancing Use of Clinical Preventive Services Among Older Adults – Closing the Gap. Washington, DC: AARP; 2011.
US Preventive Services Task Force. Published Recommendations Rockville, MD2020 [updated 12/2019. Available from: https://www.uspreventiveservicestaskforce.org/BrowseRec/Index.
Simpson VL, Kovich M. Outcomes of primary care-based Medicare annual wellness visits with older adults: A scoping review. Geriatr Nurs. 2019;40(6):590–6.
Schootman M, Jeffe DB, Lian M, Deshpande AD, Gillanders WE, Aft R, et al. Area-level poverty is associated with greater risk of ambulatory-care-sensitive hospitalizations in older breast cancer survivors. J Am Geriatr Soc. 2008;56(12):2180–7.
Agency for Healthcare Research and Quality. Quality indicator user guide: prevention quality indicators (PQI) v2019. Rockville Department of Health and Human Services. https://www.qualityindicators.ahrq.gov/Modules/PQI_TechSpec_ICD10_v2019.aspx.
Brooks GA, Abrams TA, Meyerhardt JA, Enzinger PC, Sommer K, Dalby CK, et al. Identification of potentially avoidable hospitalizations in patients with GI cancer. J Clin Oncol. 2014;32(6):496–503.
Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40(8 Suppl):IV-3–18.
Koroukian SM, Xu F, Murray P. Ability of Medicare Claims Data to Identify Nursing Home Patients: A Validation Study. Med Care. 2008;46(11):1184–7.
Roetzheim RG, Ferrante JM, Lee J-H, Chen R, Love-Jackson KM, Gonzalez EC, et al. Influence of Primary Care on Breast Cancer Outcomes Among Medicare Beneficiaries. Ann Fam Med. 2012;10(5):401–11.
Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27.
Economic Research Service. Rural-Urban Continuum Codes: United State Departement of Agriculture; 2003. [updated 10/12/2016. Available from: https://www.ers.usda.gov/data-products/rural-urban-continuum-codes/.
Culler SD, Parchman ML, Przybylski M. Factors related to potentially preventable hospitalizations among the elderly. Med Care. 1998;36(6):804–17.
Brooks GA, Jacobson JO, Schrag D. Clinician perspectives on potentially avoidable hospitalizations in patients with cancer. JAMA Oncol. 2015;1(1):109–10.
Aprile G, Pisa FE, Follador A, Foltran L, De Pauli F, Mazzer M, et al. Unplanned presentations of cancer outpatients: a retrospective cohort study. Support Care Cancer. 2013;21(2):397–404.
Lash RS, Bell JF, Reed SC, Poghosyan H, Rodgers J, Kim KK, et al. A Systematic Review of Emergency Department Use Among Cancer Patients. Cancer Nurs. 2017;40(2):135–44.
Manzano JG, Luo R, Elting LS, George M, Suarez-Almazor ME. Patterns and predictors of unplanned hospitalization in a population-based cohort of elderly patients with GI cancer. J Clin Oncol. 2014;32(31):3527–33.
Montero AJ, Stevenson J, Guthrie AE, Best C, Goodman LM, Shrotriya S, et al. Reducing Unplanned Medical Oncology Readmissions by Improving Outpatient Care Transitions: A Process Improvement Project at the Cleveland Clinic. J Oncol Pract. 2016;12(5):e594-602.
Samawi HH, Yin Y, Lim HJ, Cheung WY. Primary Care Versus Oncology-Based Surveillance Following Adjuvant Chemotherapy in Resected Pancreatic Cancer. J Gastrointest Cancer. 2018;49(4):429–36.
Peixoto RD, Lim HJ, Kim H, Abdullah A, Cheung WY. Patterns of surveillance following curative intent therapy for gastroesophageal cancer. J Gastrointest Cancer. 2014;45(3):325–33.
Parry HM, Damery S, Mudondo NP, Hazlewood P, McSkeane T, Aung S, et al. Primary care management of early stage chronic lymphocytic leukaemia is safe and effective. QJM. 2015;108(10):789–94.
Mayer DK, Nekhlyudov L, Snyder CF, Merrill JK, Wollins DS, Shulman LN. American Society of Clinical Oncology Clinical Expert Statement on Cancer Survivorship Care Planning. J Oncol Pract. 2014;10(6):345–51.
National Comprehensive Cancer Network. Survivorship (Version 2.2017)2017. 2017. https://www.nccn.org/professionals/physician_gls/pdf/survivorship.pdf
Runowicz CD, Leach CR, Henry NL, Henry KS, Mackey HT, Cowens-Alvarado RL, et al. American Cancer Society/American Society of Clinical Oncology Breast Cancer Survivorship Care Guideline. CA Cancer J Clin. 2016;66(1):43–73.
American College of Surgeons. Cancer Program standards: Ensuring Patient-Centered Care. 2016. https://www.facs.org/quality-programs/cancer/coc/standards
McCabe MS, Partridge AH, Grunfeld E, Hudson MM. Risk-based health care, the cancer survivor, the oncologist, and the primary care physician. Semin Oncol. 2013;40(6):804–12.
Handley NR, Schuchter LM, Bekelman JE. Best practices for reducing unplanned acute care for patients with cancer. J Oncol Pract. 2018;14(5):306–13.
Colligan EM, Ewald E, Ruiz S, Spafford M, Cross-Barnet C, Parashuram S. Innovative oncology care models improve end-of-life quality, reduce utilization and spending. Health Aff (Millwood). 2017;36(3):433–40.
Kline RM, Arora NK, Bradley CJ, Brauer ER, Graves DL, Lunsford NB, et al. Long-term survivorship care after cancer treatment - Summary of a 2017 National Cancer Policy Forum Workshop. J Natl Cancer Inst. 2018;110(12):1300–10.
Commission on Cancer. American College of Surgeons. Optimal Resources for Cancer Care. 2020. https://doi.org/www.facs.org/qualityprograms/cancer/coc/standards/2020
Ganz PA, Levit LA. Charting a new course for the delivery of high-quality cancer care. J Clin Oncol. 2013;31(36):4485–7.
Kline R, Adelson K, Kirshner JJ, Strawbridge LM, Devita M, Sinanis N, et al. The Oncology Care Model: Perspectives From the Centers for Medicare & Medicaid Services and Participating Oncology Practices in Academia and the Community. Am Soc Clin Oncol Educ Book. 2017;37:460–6.
Hill RE, Wakefield CE, Cohn RJ, Fardell JE, Brierley ME, Kothe E, et al. Survivorship care plans in cancer: a meta-analysis and systematic review of care plan outcomes. Oncologist. 2020;25(2):e351–72.
Hua A, Sesto ME, Zhang X, Wassenaar TR, Tevaarwerk AJ. Impact of survivorship care plans and planning on breast, colon, and prostate cancer survivors in a community oncology practice. J Cancer Educ. 2020;35(2):249–55.
Warren JL, Mariotto A, Melbert D, Schrag D, Doria-Rose P, Penson D, et al. Sensitivity of Medicare Claims to Identify Cancer Recurrence in Elderly Colorectal and Breast Cancer Patients. Med Care. 2016;54(8):e47-54.
Funding
KK was supported by a Mentored Research Scholar Grants in Applied and Clinical Research, MRSG-18–020-01-CPPB, from the American Cancer Society. The funding source had no role in the design or conduct of the study, analysis or interpretation of the data, and preparation or review of the manuscript.
Author information
Authors and Affiliations
Contributions
KK designed the research, analyzed the data, and wrote manuscript. SB, GB, AC, and GR designed the research, contributed to writing the manuscript, and revising. All authors approved the final version.
Corresponding author
Ethics declarations
Ethics approval
The study was deemed exempt by the University of Alabama at Birmingham Institutional Review Boards.
Consent for publication
Not applicable.
Conflict of interest and disclosures
There are no competing interests to declare and no disclosures.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kenzik, K.M., Rocque, G., Williams, G.R. et al. Primary care and preventable hospitalizations among Medicare beneficiaries with non-metastatic breast cancer. J Cancer Surviv 16, 853–864 (2022). https://doi.org/10.1007/s11764-021-01079-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11764-021-01079-7