Abstract
Background
Current studies report mixed results in health status and health behaviors after a diagnosis of cancer. The aim of our study is to investigate potential differences in lifestyle factors among cancer survivors and cancer-free individuals in a prospective cohort study conducted in the United States.
Methods
Using data from the Prostate, Lung, Colorectal and Ovarian (PLCO) Trial, 10,133 cancer survivors were identified and compared to 81,992 participants without cancer to evaluate differences in body mass index (BMI), smoking, NSAID use, and physical activity.
Results
Cancer survivors, compared to the cancer-free, were significantly less likely to engage in physical activity (odds ratio (OR) = 0.82, 95% CI = 0.77–0.88). Compared to those who were obese at baseline, cancer survivors were more likely to be at normal BMI at follow-up compared to the cancer-free (OR = 1.90, 95% CI = 1.42–2.54). Cancer survivors were less likely to report regular aspirin use as compared to the cancer-free population (OR = 0.86, 95 % CI = 0.82–0.92). Of the current smokers, cancer survivors were more likely to be former smokers at follow-up compared to the cancer-free (OR = 1.50, 95% CI = 1.30–1.74).
Conclusion
Upon stratification by baseline health markers, cancer survivors practice healthier lifestyle habits such as smoking cessation and maintenance of a healthy weight. However, cancer survivors are less likely to be physically active as compared to cancer-free individuals, regardless of baseline practices.
Implications for cancer survivors
For cancer survivors who reported poor health status and behaviors at baseline, a cancer diagnosis may encourage the practice of healthier lifestyle behaviors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer survivors—with survival defined from the point of diagnosis through the end of that person’s life [1]—account for nearly 14.5 million people in the United States today [2]. By 2024, there will be a projected 19 million cancer survivors [3]. Between 2004 and 2010, 68% of those diagnosed with cancer survived at least 5 years past diagnosis [2], a major improvement from the 5-year survival from 1975 to 1977 of 49% [2]. As medical advances in cancer diagnosis and treatment occur, along with the aging of the population, the number of cancer survivors will continue to grow [4].
Cancer survivors typically have poorer health after diagnosis and treatment when compared to the general population [5]. Cancer survivors have risk of recurrence of the primary cancer and an increased risk of second primary cancer, as well as a greater risk for comorbid chronic conditions and premature mortality related to cancer and/or consequences of treatment [6–9]. Genetic predisposition, lifestyle factors and the effects of cancer treatment all contribute to the elevated risk of these adverse medical and health conditions [10]. As those diagnosed with cancer are living longer and are more prone to develop chronic disease, survivorship care and the identification of public health strategies to promote optimal health are becoming increasingly important [11]; thus, identifying ways to reduce secondary health problems of cancer survivors is becoming of great interest among researchers and clinicians.
Recommendations for healthy behaviors, such as meeting physical activity and nutritional guidelines, do not differ greatly between cancer survivors and those without cancer [4, 12]. However, with the adoption of these types of healthy behaviors—such as meeting physical activity and nutritional recommendations—cancer survivors may experience protection against cancer recurrence and other health problems [13, 14]. A national cross-sectional survey of cancer survivors showed that those who met a greater number of lifestyle behavior recommendations, including 5-a-day fruit and vegetable consumption and physical activity guidelines, had better health-related quality of life (HRQoL) [15]. A Cochrane review indicated that HRQoL measures for cancer survivors were improved with interventions promoting physical activity [16]. Conversely, obesity and failure to engage in behaviors that meet healthy lifestyle guidelines have been negatively associated with HRQoL measures in cancer survivors [17].
Tertiary prevention through lifestyle behavior changes—increasing physical activity, reducing alcohol intake, smoking cessation, improving diet and using preventive pharmaceuticals such as NSAIDs—can play a critical part in reducing the adverse sequelae from cancer and the associated treatment [18]. Current smoking post-diagnosis in colorectal cancer survivors has been associated with higher colorectal cancer-specific mortality and higher all-cause mortality [19]. In a meta-analysis of 22 prospective cohort studies, breast cancer survivors who were physically active post-diagnosis had a reduced risk of recurrence, new primary cancers and progression of the initial cancer [20]. Among breast and colorectal cancer survivors, the regular use of aspirin has been associated with decreased risk for recurrence and death due to cancer [21, 22].
Studies of cancer survivors demonstrate mixed results for lifestyle behaviors compared to those never diagnosed with cancer. A US population-based case control study of breast, prostate, and colorectal cancer based on the Behavioral Risk Factor Surveillance System (BRFSS) reported that breast cancer survivors exhibited lower rates of current smoking and were more likely to meet the daily fruit and vegetable recommendations when compared to non-cancer controls [23]. However, when compared to the cancer-free, cancer survivors from the Health Information National Trends Survey (HINTS) in the United States showed no statistically significant difference in the proportion of current smokers in a study evaluating tobacco use, fruit and vegetable intake, physical activity, and body mass index (BMI) [5]. Breast cancer survivors in the Danish Diet, Cancer and Health Cohort exhibited no reduction in BMI, and no reduced consumption of alcohol or tobacco compared to cancer-free women [24]. Cancer survivors in a UK population-based study were less likely to engage in physical activity and more likely to be sedentary, compared to cancer-free controls [25].
As the previous research has produced mixed results on health behaviors by cancer survivors, the aim of our study is to investigate potential differences in key lifestyle factors (e.g., physical activity, smoking status, NSAID/aspirin use), among cancer survivors and cancer-free individuals in a large prospective cohort study conducted in the United States. We hypothesized that cancer survivors will report lower activity levels, higher BMI, higher rates of smoking cessation, and less NSAID use as compared to the cancer-free. We also investigated lifestyle factors between short-term (<5 years) and long-term (≥5 years) survivors, as few studies have comprehensively assessed whether differences exist in health behaviors by time since diagnosis.
Methods
Participants and data collection
Health behaviors of cancer survivors were compared to those of the cancer-free in the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial. The PLCO Cancer Screening Trial was implemented in the United States to evaluate the effect of screening for these cancers on cancer-related mortality [26]. Between November 1993 and July 2001, 154,897 men and women between the ages of 55 and 74 were randomized into either an intervention arm or a control arm of the PLCO trial. The trial was conducted at ten centers across the United States (located in Alabama, Colorado, Hawaii, Michigan, Minnesota, Missouri, Pennsylvania, Utah, Washington, D.C., and Wisconsin). Individuals in both arms of the trial continued their routine health care. Participants in the intervention arm received additional screening exams for prostate, lung, colorectal, and ovarian cancer. A self-administered baseline questionnaire was completed at entry by all participants. The follow-up questionnaire was provided to participants between 2006 and 2008, with an average of 10 years between randomization and follow-up completion. Informed consent was obtained in written form from all research participants. Each study center obtained ethical approval for human subject research.
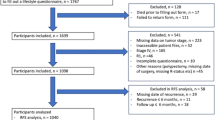
The data used in this study include information from randomization through December 31, 2009. Of the 154,897 participants enrolled in the study, 97,406 completed both baseline and follow-up questionnaires and had no cancer diagnosis prior to study enrollment. Those identified as having no confirmed cancer diagnosis at follow-up were designated the cancer-free group. Cancer survivors were identified as having a confirmed cancer diagnosis between enrollment and follow-up and must have had a confirmed cancer behavior of “malignant, primary site.” Those diagnosed with cancer during the study, but after the completion of the follow-up questionnaire, were excluded from the study because the aim of this study is to assess behaviors after cancer diagnosis (4389). Of the 11,528 PLCO participants who had completed a baseline questionnaire and had a cancer diagnosis, 11,025 participants completed the supplemental questionnaire. Lastly, those diagnosed with “in situ” cancers or “uncertain or borderline” cancers, 892 individuals, were omitted for this analysis. We analyzed data for 81,992 cancer-free individuals and 10,133 cancer survivors (Fig. 1). The cancer diagnoses by site were as follows: 4275 prostate, 1720 breast, 858 colon, 731 hematopoietic, 412 lung, 396 bladder, 384 melanoma, 363 endometrial, 232 renal, 163 head and neck, 119 ovarian, 90 thyroid, 89 upper gastrointestinal, 42 pancreatic, 12 biliary, 9 glioma, 8 liver cancers, and 258 from other sites (Fig. 1). If cancer survivors were diagnosed with more than one cancer, they were grouped according to first cancer diagnosis.
Although the intervention arm was screened for prostate, lung, colorectal, and ovarian cancers, in both arms, the incidence rate of cancer was similar. Of the 10,133 cancer survivors, 5343 were from the intervention arm and 4790 were from the control arm. For specific cancer site, the number of controls vs interventions were as follows: 2346 vs 1929 for prostate cancer, 231 vs 181 for lung cancer, 379 vs 479 for colorectal cancer, 73 vs 46 for ovarian cancer, 24 vs 18 for pancreatic cancer, 220 vs 164 for melanoma, 176 vs 193 for bladder cancer, 867 vs 853 for breast cancer, 381 vs 350 for hematopoietic cancers, 4 vs 4 for liver cancer, 48 vs 41 for upper gastrointestinal cancers, 5 vs 7 for biliary cancers, and 142 vs 116 for other cancer sites.
Annual updates about cancer diagnosis, type of cancer, date of diagnosis, and hospital or clinic of diagnosis were collected from study participants. All self-reported cancers were confirmed from medical records, and the data abstraction of these records included diagnosis, cancer behavior, morphology, and grade as assigned by the study abstractor based on the ICD-O-2 code [27]. The baseline questionnaire collected self-reported information on personal sociodemographic characteristics, tobacco, family history of cancer, height, weight, aspirin and ibuprofen use, and medical history. The follow-up questionnaire included self-reported information on the following: income level, religion at birth, work status, height, weight, family history of cancer, health history, nonsteroidal anti-inflammatory drug (NSAID) and aspirin use, physical activity, and tobacco use. To assess smoking status, participants were asked the following: at baseline, “Have you ever smoked cigarettes regularly for 6 months or longer?” and at follow-up, “Have you ever smoked at least 100 cigarettes in your lifetime?” Questions with more detail about physical activity and NSAID use and some smoking behaviors were only collected at follow-up.
Measures
The baseline questionnaire was used to examine the sociodemographic characteristics of participants. BMI data were obtained from the follow-up questionnaire. Participants were asked about physical activity over the year prior to completion of the follow-up questionnaire. Participants were queried about the type of physical activity they engaged in, duration of time spent doing the activity, and the average frequency of participating in the activity each week. To estimate meeting physical activity recommendations, a rough mean was created from the reported number of days per week of moderate activity and the reported duration of each session of moderate activity. The original responses for frequency of moderate activity were collected as follows: “0 = none or <1 day/week,” “1 = 2–3 days/week,” “2 = 4–5 days/week,” and “3 = 6–7 days/week.” Duration of moderate activity was collected as follows: “0 = none or <15 min,” “1 = 16–19 min,” “2 = 20–29 min,” “3 = 30–39 min,” and “4 = 40+ min.” A rough mean was calculated for each response option as follows: moderate duration (0 = 0, 1 = 2.5, 2 = 4.5, and 3 = 6.5) and moderate duration frequency (0 = 0, 1 = 17.5, 2 = 24.5, 3 = 34.5, and 4 = 40). The same procedure was conducted for the strenuous activity and strenuous activity duration. Two variables were then created for those who reported over 150 min of moderate and 75 min of strenuous activity. Questions about aspirin and NSAID use included current intake, strength of medication, and how many years medication had been taken. Low intake of aspirin and NSAIDs was defined as less than once per week and high intake was defined as at least once per week. Most recent smoking status was obtained from the follow-up questionnaire.
Statistical analysis
Differences between cancer survivors and those without cancer were compared. Participant characteristics and health behaviors at baseline and follow-up were examined using descriptive statistics. t tests were performed for all continuous data categories. Multivariable logistic regression was used to analyze differences between cancer survivors and cancer-free with stratification by BMI at baseline, smoking status at baseline, education level at baseline, sex, income at follow-up, and age at follow-up. The mean household income in the United States for 2009 was $54,925/year [28]. This approximate value was used to create the household income level stratification (<$50,000 vs ≥$50,000). Further analysis was performed with stratification into short-term (< 5 years) and long-term (≥ 5 years) survivors. Years of survival were calculated by taking the number of days to follow-up questionnaire completion from randomization minus the number of days to diagnosis of cancer from randomization. A significance level of α = 0.05 was used for analyses. To correct for the false discovery rate in multiple comparisons, the Benjamini-Hochberg method was used with a 0.05 point-wise threshold [29]. Following this correction, all p values equal to or greater than 0.021 were considered to be non-significant. All significant findings are bolded in the tables (Tables 1, 2, 3, and 4; data not shown) to indicate remaining significance following this correction method. Stata (version 14.0, College Station TX) was used for data analysis.
Results
Demographics
At randomization, cancer survivors were older compared to the cancer-free individuals (p < 0.001; Table 1). The majority of cancer survivors were male as compared to the cancer-free population (p < 0.001; Table 1). White, non-Hispanic individuals constituted roughly 90% of the population for both cancer-free and cancer survivors. Education level varied somewhat, trending toward slightly higher education levels in cancer survivors at enrollment (p < 0.001; Table 1). No statistically significant difference was seen in income level at follow-up between the groups.
Body mass index
At the end of the last follow-up, roughly 33% of both cancer-free individuals and cancer survivors had a normal BMI of between 18.5 and 25 kg/m2 (Table 1). The majority of both cancer survivors and those without cancer were overweight (BMI between 25 and 30 kg/m2) or obese (BMI greater than or equal to 30 kg/m2). Cancer survivors were significantly more likely to be underweight as compared to the cancer-free (OR = 1.33, 95% CI = 1.07–1.65; Table 2). Between groups, there was no statistically significant difference in likelihood of being overweight or obese at follow-up. However, cancer survivors who were obese at baseline were significantly more likely to be at a normal BMI at follow-up than the cancer-free who were obese at baseline (OR = 1.90, 95% CI = 1.42–2.54; Table 3). Of those who were overweight at baseline, cancer survivors were significantly more likely to be at a normal BMI at follow-up when compared to the cancer-free (OR = 1.15, 95% CI = 1.05–1.26, Table 3) but were also significantly more likely to be obese at follow-up compared to the cancer-free (OR = 1.11, 95% CI = 1.01–1.18; Table 3). Upon stratification by income level at follow-up, cancer survivors who made less than $50,000 a year were significantly more likely to be underweight as compared to the cancer-free (OR = 1.38, 95% CI = 1.03–1.85; data not shown).
Tobacco use
A higher number of cancer survivors were former smokers at follow-up as compared to the cancer-free, while a lower number of cancer survivors were never smokers as compared to the cancer-free (p < 0.001; Table 2). Cancer survivors who smoked at baseline were significantly more likely to be former smokers at follow-up compared to the cancer-free who smoked at baseline (OR = 1.50, 95% CI = 1.30–1.74; Table 4). Cancer survivors who made less than $50,000 a year were significantly more likely to be current smokers as compared to the cancer-free (OR = 1.18, 95% CI = 1.04–1.33; data not shown). Cancer survivors with a high school education or less were also significantly more likely to be current smokers as compared to the cancer-free (OR = 1.22, 95% CI = 1.03–1.45; data not shown).
Physical activity
A higher proportion of cancer survivors (12.5%) reported having not participated in physical activity at least once a month for the last 12 months compared to the cancer-free individuals (11.0%) (p < 0.001; Table 1). Almost 60% of cancer survivors reported being less active at follow-up than they were 10 years ago (p < 0.001) and were more likely to report being less active at follow-up than 10 years ago compared to those without cancer (OR = 1.28, 95% CI = 1.22–1.34; Table 2). Cancer survivors were less likely to participate in moderate and strenuous exercise each week than the cancer-free (p < 0.001; Table 1). Cancer survivors were also less likely to meet the physical activity recommendations of at least 150 min of moderate activity a week or 75 min of vigorous activity per week (OR = 0.89, 95% CI = 0.83-0.94; OR = 0.87, 95% CI = 0.83-0.92, respectively Table 2). Of those who were overweight and obese at baseline, cancer survivors, when compared to the cancer-free, were significantly more likely to report being less active than they were 10 years ago (OR = 1.26. 95% CI = 1.18–1.09; OR = 1.31, 95% CI = 1.18–1.46, respectively; Table 3). Similarly, of those who were current and former smokers at baseline, cancer survivors were significantly more likely than the cancer-free to be less active as compared to 10 years ago (OR = 1.27, 95% CI = 1.08–1.50; OR =1.25, 95% C.I. = 1.16–1.34, respectively; Table 4). Upon stratification by income, cancer survivors in both income groups (<$50,000/year and ≥$50,000/year) were significantly less likely to have been physically active at least once a month in the last 12 months than those without cancer (OR = 0.80, 95% CI = 0.73–0.87; OR = 0.89, 95% CI = 0.81–0.98, respectively; data not shown).
NSAID and aspirin use
No difference was seen for intake or frequency of intake of NSAIDS between cancer survivors and cancer-free use (Table 2). A difference was seen in likelihood of aspirin use, where cancer survivors were significantly less likely to use either adult (325 mg) or baby strength (81 mg) aspirin as compared to those without cancer (OR = 0.83, 95% CI = 0.78–0.89; OR = 0.90, 95% CI = 0.85–0.96, respectively; Table 2). Similarly, cancer survivors were less likely to report any frequency of aspirin intake than were the cancer-free. No differences were seen in NSAID use between cancer survivors and the cancer-free after stratification by income level, education level, or age. Both income groups for cancer survivors were significantly less likely to report high aspirin use as compared to the cancer-free (less than $50,000/year: OR = 0.87, 95% CI = 0.81–0.94; $50,000 and more/year: OR = 0.83, 95% CI = 0.78–0.90).
Short-term vs long-term cancer survivors
No statistically significant differences were observed in BMI, smoking status, or NSAID use between short-term and long-term survivors (data not shown). After controlling for age at follow-up, sex, race, education, and income level at follow-up, short-term cancer survivors were more likely to engage in moderate physical activity 6 to 7 days a week as compared to long-term survivors (OR = 1.36, 95% CI = 1.13–1.63; data not shown). Short-term survivors were also more likely to engage in strenuous physical activity 2 to 3 days a week as compared to long-term survivors (OR = 1.19, 95% CI = 1.09–1.31; data not shown).
Discussion
With the differing data on the adoption of healthier lifestyle habits by cancer survivors, our goal was to better understand the behavior of cancer survivors compared to those without cancer. The health behaviors evaluated in this study (smoking, physical activity, body mass index, and NSAID/aspirin use) have been associated with many of the prevalent chronic diseases in the United States [30–33]. As cancer survivors have been found to have a higher prevalence of chronic conditions compared to cancer-free individuals [34, 35], the adoption of healthful lifestyle behaviors is an important aspect in survivorship care, and public health efforts promoting the optimal health of cancer survivors should address these issues.
In almost all aspects of physical activity, the cancer-free population reported higher levels of activity and longer durations of time spent in physical activity. Although it might be expected that a diagnosis of cancer would motivate behavior change, recent studies found little support for this. In the NHANES study, cancer survivors were more likely to be sedentary (measured as time spent sitting or reclining) [36], and cancer survivors in the English longitudinal study of aging were not only more likely to be sedentary (hardly ever or never participated in mildly energetic activity), but also less likely to be physically active (moderate to vigorous activity at least once a week) [25]. A prospective study of breast cancer survivors in the Kaiser Permanente Northern California Medical Care Program found a decrease in moderate physical activity and recreational activities 6 months after diagnosis as compared to baseline activity levels [37]. Similarly, colorectal cancer survivors in the Eindhoven Cancer Registry who experienced comorbidities associated with cancer were less likely to engage in physical activity [38]. Lower levels of physical activity may be a result of frailty or from chronic fatigue reported by cancer survivors [39–41]. A postal questionnaire-survey looking at exercise barriers in cancer survivors found that fatigue and illness were prominent barriers to engaging in physical exercise; however, the majority of these participants also reported an interest in participating in an exercise program [42].
While there was no difference in likelihood of being overweight or obese between cancer survivors and the cancer-free after adjusting for race, sex, education at baseline, age at follow-up, and income at follow-up, the stratification by baseline health status provides evidence that cancer survivors who were overweight or obese prior to cancer diagnosis may be more likely to practice healthier lifestyle behaviors after diagnosis such as weight management. A similar finding was seen in a prospective population-based cohort study from the United States and United Kingdom which found that cancer survivors who were obese prior to cancer diagnosis were more likely to lose weight than obese adults without cancer [43]. However, as cachexia is a common adverse effect of cancer diagnosis and treatment, the weight loss seen in overweight and obese cancer survivors may be due to this condition rather than practice of healthier behaviors [44]. Additionally, while cancer survivors seemed to achieve healthier BMIs at follow-up than the cancer-free, the cancer survivors in this study follow the current trend of obesity in the United States: as of 2010, two in three adults are considered overweight or obese [45].
The diagnosis of cancer appears to improve the likelihood of smoking cessation in participants that were current smokers at baseline. A study from the Cancer Prevention Study-II Nutrition Cohort found that the quit rates of current smokers over the course of 2 years was higher in those diagnosed with cancer as compared to smokers without cancer [46]. Cancer site may also play a role in smoking cessation; a matched comparison study from the California Cancer Registry found that current smokers who were diagnosed with bladder cancer were significantly more likely to quit smoking compared to smokers in the general population [47]. As our study showed that cancer survivors had a higher intention to quit smoking as compared to the cancer-free, the diagnosis of cancer may present an ideal opportunity to encourage this behavior change.
The use of NSAIDs after a cancer diagnosis has been shown to reduce mortality in several types of cancer including those of the upper aerodigestive tract, colorectal, and breast [48–50]. However, no significant difference was shown in NSAID use between cancer survivors and the cancer-free. These results may be due to controlling for confounders, specifically age. In 2010, over 45% of individuals over the age of 65 were experiencing at least 2 chronic conditions [51]. As NSAIDs are a widely accepted form of pain and inflammation management, and since the mean age of both groups at follow-up was over 65 years, the presence of chronic conditions may be influencing NSAID use.
Cancer survivors were significantly less likely to use aspirin as compared to those without cancer after adjusting for age at follow-up, race, sex, income at follow-up, and education level. This is concerning as significant prevention benefits have been associated with aspirin use in cancer survivors. Specifically, findings from a prospective cohort study found that the risk of recurrence and mortality greatly decreased in colorectal cancer survivors who regularly take aspirin [52, 53]. A systematic review and meta-analysis of aspirin treatment of cancer found that aspirin reduced the risk of metastatic spread [54]. Additionally, regular aspirin use is a key intervention strategy for many chronic conditions such as heart attack and stroke [31].
Strengths of this study include the utilization of data from a large, national prospective cohort which may limit selection bias and minimize temporal ambiguity. The longitudinal nature of this study is necessary to support the causal link between cancer diagnosis and limited adoption of improved health behaviors by cancer survivors. Participants in the PLCO trial were closely followed over the course of the study through administration of questionnaires and annual updates. Participants diagnosed with cancer during the study were actively tracked to obtain and collect accurate medical records related to diagnostic follow-up and treatment [55]. Furthermore, the ability to account for the smoking status and BMI prior to cancer diagnosis strengthened the results found in this study.
As cancer diagnosis continued throughout the follow-up period, the reporting of health behaviors at follow-up may have been affected by the difference in time since cancer diagnosis. Responder bias is an inherent limitation of self-reported data. There is a well-known downward bias of weight status in self-reports, which has remained a stable trend over the last decade [56] and a systematic review of direct versus self-reported measures of physical activity found self-reports measured both higher and lower than direct measures [57]. This study did not consider comorbid conditions among participants, which may have an influence on all health behaviors and status at follow-up. This is an area, however, for future investigation.
In conclusion, the findings of this study provide evidence that while cancer survivors may make some improvements to practice healthier lifestyle behaviors as compared to those without cancer, there is still concern that cancer survivors appear to be engaging in less physical activity than those without cancer and are not meeting recommendations for aspirin and NSAID intake. As cancer survival rates are increasing, the consideration of health and quality of life post-diagnosis are exceedingly important. The American Society of Clinical Oncology includes guidance on health promotion activities, diet, and exercise as a part of high-quality survivorship care [58] and health behavior counseling and other support should be prioritized as a key component to comprehensive survivorship care. The low adoption of healthy behaviors may be amenable to interventions. Specifically, successful behavior change by cancer survivors has been seen in interventions based on social cognitive theory [59]. Rehabilitation and intervention programs should specifically target health behavior changes, and health care providers should encourage behavior change in cancer survivors, which may improve long-term survival and quality of life.
References
Underwood JM, Townsend JS, Stewart SL, Buchannan N, Ekwueme DU, Hawkins NA, et al. Surveillance of demographic characteristics and health behaviors among adult cancer survivors--behavioral risk factor surveillance system, United States, 2009. MMWR Surveill Summ. 2012;61(1):1–23.
American Cancer Society. Cancer statistics, 2015. CA Cancer J Clin [Internet] Atlanta. 2015;65(1):5–29.
DeSantis CE, Lin CC, Mariotto AB, Siegel RL, Stein KD, Kramer JL, et al. Cancer treatment and survivorship statistics, 2014. CA cancer J Clin [internet]. Atlanta. 2014;64(4):252–71.
Rock CL, Doyle C, Demark-Wahnefried W, Meyerhardt J, Courneya KS, Schwartz AL, et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin [Internet]. 2012;62(4):242–74. Available from: http://doi.wiley.com/10.3322/caac.21142
Mayer DK, Terrin NC, Menon U, Kreps GL, McCance K, Parsons SK, et al. Health behaviors in cancer survivors. Oncol Nurs Forum. 2007;34(3):643–51.
Buchanan ND, King JB, Rodriguez JL, White A, Trivers KF, Forsythe LP, et al. Changes among US cancer survivors: comparing demographic, diagnostic, and health care findings from the 1992 and 2010 National Health Interview Surveys. ISRN Oncol. 2013;2013(4):1–9.
Low CA, Beckjord E, Bovbjerg DH, Dew MA, Posluszny DM, Schmidt JE, et al. Correlates of positive health behaviors in cancer survivors: results from the 2010 LIVESTRONG survey. J Psychosoc Oncol [Internet]. 2014;32(6):678–95. Available from: http://www.tandfonline.com/doi/abs/10.1080/07347332.2014.955243
Beckjord EB, Reynolds KA, van Londen GJ, Burns R, Singh R, Arvey SR, et al. Population-level trends in post-treatment cancer survivors’ concerns and associated receipt of care: results from the 2006 and 2010 LIVESTRONG surveys. J Psychosoc Oncol [Internet]. 2013;32(2):125–51.
Eakin EG, Youlden DR, Baade PD, Lawler SP, Reeves MM, Heyworth JS, et al. Health behaviors of cancer survivors: data from an Australian population-based survey. Cancer Causes Control. 2007;18(8):881–94.
Mosher CE, Lipkus I, Sloane R, Snyder DC, Lobach DF, Demark-Wahnefried W. Long-term outcomes of the FRESH START trial: exploring the role of self-efficacy in cancer survivors’ maintenance of dietary practices and physical activity. Psychooncology [Internet]. 2013 Apr;22(4):876–85. doi:10.1002/pon.3089.
Karlsen RV, Bidstrup PE, Christensen J, Larsen SB, Tjønneland A, Dalton SO, et al. Men with cancer change their health behaviour: a prospective study from the Danish diet, cancer and health study. Br J Cancer [Internet]. 2012;107(1):201–6. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3389426&tool=pmcentrez&rendertype=abstract
Kushi LH, Doyle C, McCullough M, Rock CL, Demark-Wahnefried W, Bandera EV, et al. American Cancer Society guidelines on nutrition and physical activity for cancer prevention. CA Cancer J Clin. 2012;62(1):30–67.
Carmack CL, Basen-Engquist K, Gritz ER. Survivors at higher risk for adverse late outcomes due to psychosocial and behavioral risk factors. Cancer Epidemiol Biomark Prev. 2011;20(10):2068–77.
Demark-Wahnefried W, Jones LW. Promoting a healthy lifestyle among cancer survivors. Hematol Oncol Clin North Am. 2008;22(2):319–42.
Blanchard CM, Courneya KS, Stein K. Cancer survivors’ adherence to lifestyle behavior recommendations and associations with health-related quality of life: results from the American Cancer Society's SCS-II. J Clin Oncol. 2008;26(13):2198–204.
Mishra SI, Scherer RW, Geigle PM, Berlanstein DR, Topaloglu O, Gotay CC, et al. Exercise interventions on health-related quality of life for cancer survivors. Cochrane Database Syst Rev. 2012;8(8):CD007566.
Koutoukidis DA, Knobf MT, Lanceley A. Obesity, diet, physical activity, and health-related quality of life in endometrial cancer survivors. Nutr Rev [Internet]. 2015;73(6):399–408. Available from: http://nutritionreviews.oxfordjournals.org/cgi/doi/10.1093/nutrit/nuu063
Bellizzi KM, Rowland JH, Jeffery DD, McNeel T. Health behaviors of cancer survivors: examining opportunities for cancer control intervention. J Clin Oncol. 2005;23(34):8884–93.
Yang B, Jacobs EJ, Gapstur SM, Stevens V, Campbell PT. Active smoking and mortality among colorectal cancer survivors: the cancer prevention study II nutrition cohort. J Clin Oncol. 2015;33(8):885–93.
Lahart IM, Metsios GS, Nevill AM, Carmichael AR. Physical activity, risk of death and recurrence in breast cancer survivors: a systematic review and meta-analysis of epidemiological studies. Acta Oncol. 2015;54(5):635–54.
Holmes MD, Chen WY, Li L, Hertzmark E, Spiegelman D, Hankinson SE. Aspirin intake and survival after breast cancer. J Clin Oncol. 2010;28(9):1467–72.
Chan AT, Ogino S, Fuchs CS. Aspirin use and survival after diagnosis of colorectal cancer. JAMA. 2009;302(6):649–58.
LeMasters TJ, Madhavan SS, Sambamoorthi U, Kurian S. Health behaviors among breast, prostate, and colorectal cancer survivors: a US population-based case-control study, with comparisons by cancer type and gender. J Cancer Surviv. 2014;8(3):336–48.
Bidstrup PE, Dalton SO, Christensen J, Tjonneland A, Larsen SB, Karlsen R, et al. Changes in body mass index and alcohol and tobacco consumption among breast cancer survivors and cancer-free women: a prospective study in the Danish diet, cancer and health cohort. Acta Oncol. 2013;52(2):327–35.
Williams K, Steptoe A, Wardle J. Is a cancer diagnosis a trigger for health behaviour change? Findings from a prospective, population-based study. Br J Cancer. 2013;108(11):2407–12.
Gohagan JK, Prorok PC, Hayes RB, Kramer BS. The prostate, lung, colorectal and ovarian (PLCO) cancer screening trial of the National Cancer Institute: history, organization, and status. Control Clin Trials. 2000;21(6 Suppl):251S–72S.
Bazzi L, Lamerato EL, Varner J, Shambaugh V, Cordes JE, Ragard LR' et al. Lessons in medical record abstraction from the prostate, lung, colorectal, and ovarian (PLCO) national screening trial. Rev Recent Clin Trials. 2015;10(3):200–5.
US. Bureau of the Census. Real Median Household income in the United States [MEHOINUSA672N] [Internet]. FRED, Federal Reserve Bank of St. Louis. 2016 [cited 2016 Feb 23].
Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing [Internet]. J R Stat Soc. Series B (Methodological). 1995. p. 289–300.
Lee I-M, Shiroma JE, Lobelo F, Puska P, Blair SN, Katzmarzyk PT. Impact of physical inactivity on the World’s major non-communicable diseases. Lancet. 2012;380(9838):219–29.
Bauer UE, Briss PA, Goodman R A, Bowman BA. Prevention of chronic disease in the twenty-first century: elimination of the leading preventable causes of premature death and disability in the USA. Lancet. 2014;384(9937):45–52.
De Leon SF, Shih SC. Tracking the delivery of prevention-oriented care among primary care providers who have adopted electronic health records. J Am Med Informatics Assoc. 2011;18(Supplement 1):i91–5.
Hennekens CH, Sechenova O, Hollar D, Serebruany VL. Dose of aspirin in the treatment and prevention of cardiovascular disease: current and future directions. J Cardiovasc Pharmacol Ther. 2006;11(3):170–6.
Berry NM, Miller MD, Woodman RJ, Coveney J, Dollman J, Mackenzie CR, et al. Differences in chronic conditions and lifestyle behaviour between people with a history of cancer and matched controls. Med J Aust. 2014;201(2):96–100.
Oeffinger KC, Mertens AC, Sklar CA, Kawashima T, Hudson MM, Meadows AT, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355(15):1572–82.
Kim RB, Phillips A, Herrick K, Helou M, Rafie C, Anscher MS, et al. Physical activity and sedentary behavior of cancer survivors and non-cancer individuals: results from a National Survey. PLoS One. 2013;8(3):9–11.
Kwan ML, Sternfeld B, Ergas IJ, Timperi AW, Roh JM, Hong C-C, et al. Change in physical activity during active treatment in a prospective study of breast cancer survivors. Breast Cancer Res Treat. 2012;131(2):679–90.
Buffart LM, Thong MSY, Schep G, Chinapaw MJM, Brug J, de Poll-Franse LV v. Self-reported physical activity: its correlates and relationship with health-related quality of life in a large cohort of colorectal cancer survivors. Lucia a, editor. PLoS One [Internet]. 2012;7(5):e36164. Available from: http://dx.plos.org/10.1371/journal.pone.0036164
Prue G, Rankin J, Allen J, Gracey J, Cramp F, appraisal C-r f: A c. Eur J Cancer. 2006;42(7):846–63.
Denlinger CS, Ligibel JA, Are M, Baker KS, Demark-Wahnefried W, Friedman DL, et al. Survivorship: fatigue, version 1.2014. J Natl Compr Canc Netw. 2014;12(6):876–87.
Brown JC, Harhay MO, Harhay MN. The prognostic importance of frailty in cancer survivors. J Am Geriatr Soc [Internet]. 2015;63(12):2538–43.
Blaney JM, Lowe-Strong A, Rankin-Watt J, Campbell A, Gracey JH. Cancer survivors’ exercise barriers, facilitators and preferences in the context of fatigue, quality of life and physical activity participation: a questionnaire-survey. Psychooncology [Internet]. 2013;22:186–94. Available from: http://eprints.ulster.ac.uk/11010/1/Janine_survey_paper.pdf
Jackson SE, Williams K, Steptoe A, Wardle J. The impact of a cancer diagnosis on weight change: findings from prospective, population-based cohorts in the UK and the US. BMC Cancer. 2014;14(1):926.
Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol Elsevier Ltd; 2011;12(5):489–495.
Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011-2012. Jama. 2014;311(8):806–14.
Westmaas JL, Newton CC, Stevens VL, Flanders WD, Gapstur SM, Jacobs EJ. Does a recent cancer diagnosis predict smoking cessation? An analysis from a large prospective US cohort. J Clin Oncol. 2015;33(15):1647–52.
Bassett JC, Gore JL, Chi AC, Kwan L, McCarthy W, Chamie K, et al. Impact of a bladder cancer diagnosis on smoking behavior. J Clin Oncol. 2012;30(15):1871–8.
Macfarlane TV., Murchie P, Watson MC. Aspirin and other non-steroidal anti-inflammatory drug prescriptions and survival after the diagnosis of head and neck and oesophageal cancer. Cancer Epidemiol. 2015;39(6):1015–1022.
Hua X, Adams S, Phipps A, Cohen S, Burnett-Hartman A, Hardikar S, et al. Pre- and post-diagnostic non-steroidal anti-inflammatory drug use and colorectal cancer survival in Seattle Colon cancer family registry. Cancer Epidemiol Biomarkers Prev 2016;25(3):559–559.
Huang XZ, Gao P, Sun JX, Song YX, Tsai CC, Liu J, et al. Aspirin and nonsteroidal anti-inflammatory drugs after but not before diagnosis are associated with improved breast cancer survival: a meta-analysis. Cancer Causes Control. 2015;26(4):589–600.
Freid VM, Bernstein AB, Bush MA. Multiple chronic conditions among adults aged 45 and over: trends over the past 10 years. NCHS Data Brief. 2012;(100):1–8.
McCowan C, Munro AJ, Donnan PT, Steele RJC. Use of aspirin post-diagnosis in a cohort of patients with colorectal cancer and its association with all-cause and colorectal cancer specific mortality. Eur J Cancer. 2013;49(5):1049–57.
Young A, Weltzien E, Kwan M, Castillo A, Caan B, Kroenke CH. Pre- to post-diagnosis weight change and associations with physical functional limitations in breast cancer survivors. J Cancer Surviv. 2014;8(4):539–47.
Elwood PC, Morgan G, Pickering JE, Galante J, Weightman AL, Morris D, et al. Aspirin in the treatment of cancer: reductions in metastatic spread and in mortality: a systematic review and meta-analyses of published studies. PLoS One. 2016;11(4):e0152402.
Prorok PC, Andriole GL, Bresalier RS, Buys SS, Chia D, Crawford ED, et al. Design of the Prostate, lung, colorectal and ovarian (PLCO) cancer screening trial. Control Clin Trials. 2000;21(6 Suppl):273S–309S.
Hattori A, Sturm R. The obesity epidemic and changes in self-report biases in BMI. Obesity. 2013;21(4):856–60.
Prince S, Adamo K, Hamel M, Hardt J, Gorber S, Tremblay M. A comparison of direct versus self-report measures for assessing physical activity in adults: a systematic review. Int J Behav Nutr Phys Act. 2008;5:56.
American Society of Clinical Oncology. Providing High Quality Survivorship Care in Practice: An ASCO Guide. Am Soc Clin Oncol. 2014
Stacey FG, James EL, Chapman K, Courneya KS, Lubans DR. A systematic review and meta-analysis of social cognitive theory-based physical activity and/or nutrition behavior change interventions for cancer survivors. J Cancer Surviv. 2015;9(2):305–38.
Acknowledgments
A special thanks to the National Cancer Institute for granting access to the data collected by the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
NCI Extramural Program (DCP) and NCI Intramural Program (DCEG). DCP provided funding for the trial and biospecimen collection, and DCEG provided funding for biospecimen storage. This work was supported by contracts from the Division of Cancer Prevention, National Cancer Institute, NIH, DHHS under Award Number N01-CN-25524 and by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR001067.
Conflict of interest
The authors declare no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Rights and permissions
About this article
Cite this article
Hawkins, M.L., Buys, S.S., Gren, L.H. et al. Do cancer survivors develop healthier lifestyle behaviors than the cancer-free population in the PLCO study?. J Cancer Surviv 11, 233–245 (2017). https://doi.org/10.1007/s11764-016-0581-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11764-016-0581-0