Abstract
Macroinvertebrates occupy an important trophic level in riverine ecosystems based on their composition and diversity. In this study, we explored the biodiversity pattern of macroinvertebrates in relation to environmental parameters in a bid to assess the water quality of the Uwagbe River, Nigeria. Sampling was carried out in three well marked stations from March 2018 to February 2020 following standard procedures. The physico-chemical parameters recorded were within the acceptable limit by World Health Organization and Federal Environmental Protection Agency of Nigeria standards except the pH and DO of Stations 2 and 3 and BOD of Station 3. The Principal Component Analysis showed pH and DO to be positively associated with Station 1. 13 orders of macroinvertebrates, comprising of 32 families, 45 taxa and 4,796 individuals were recorded. The most dominant order was Diptera (26.7%) while the least was Arachnida (0.1%). The most predominant taxon was Lumbricus sp. (7.9%), while the least was Aeschna sp. (4.3%). Diversity indices showed that Station 1 had the highest indices for Margalef index, Shannon-Wiener diversity index (H), Equitability index (E) and Simpsons’ dominance index while Station 3 had indices with the lowest values. Canonical Correspondence Analysis ordination showed that chloride, alkalinity, BOD, nitrate, phosphate and water depth were strongly correlated with families such as Lumbricidae, Chironomidae, Potamonautidae, Tabanidae and Palaemonidae in Station 3. DO and pH were strongly associated with Amphipodae, Hydrophilidae, Gerridae and Libellulidae families in Station 1. The study revealed the significance of the utility of macroinvertebrates community structure and its relationship with environmental factors in assessing the level of perturbation in riverine ecosystems. The results provide insight on how river managers can put in place appropriate conservation processes to forestall the incessant level of perturbation occurring in riverine ecosystems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Macroinvertebrates occupy an important trophic level (primary consumers) in aquatic ecosystems (Amri et al. 2014). Information about macroinvertebrates are used for the determination of the health status of aquatic systems as well as their functionality (Edegbene and Arimoro 2012). Macroinvertebrates are strategic in profiling the ecological health status of freshwater environment as they have been widely explored using their biological indices/features such as their richness, abundance, diversity and distribution (Olatunji et al. 2023). Furthermore, these indices are influenced by the availability of food resources, sediment type, physico-chemical content, water quality and predation relationship in the environment that harbours the macroinvertebrates as well as their behavioural and adaptation patterns (Edegbene et al. 2021; Olatunji et al. 2023; Liang 2023). Macroinvertebrates have been used globally to monitor changes in aquatic ecosystems for decades (Arimoro and Ikomi 2009; Adu and Oyeniyi 2019; Edegbene et al. 2021; Edegbene 2022).
The adaptation of macroinvertebrates to varying degree of environmental factors, their sensitivity/tolerance level to pollution, abundance and diversity can be proxy to ascertain the condition of the environment (Adu and Oyeniyi 2019). According to Odume (2020), changes in the abundance of macroinvertebrates in a river can reflect the influence of anthropogenic activities on the river. Macroinvertebrates respond to environmental stress and pollutants differently and their diversity also forms important ecological profiling indices, hence their usefulness in biomonitoring aquatic ecosystems (Odume 2020). Furthermore, macroinvertebrates diversity indices form important conservative influence in biomonitoring and is a function of species richness, taxa composition, evenness, etc. (Tampo et al. 2020; Olatunji et al. 2023). For instance, the decrease in the taxonomic composition of macroinvertebrates is concomitant to degradation of the water quality of rivers (Amri et al. 2014). George et al. (2009) asserted that the diversity of insect orders correlates with physico-chemical parameters in freshwater bodies while decrease in the diversity of macroinvertebrates portends poor water quality. The relationships between physico-chemical parameters and macroinvertebrates have been widely discussed by freshwater scientists globally (Edokpayi et al. 2010; Amri et al. 2014; Forio et al. 2016; Odigie 2019; Edegbene et al. 2020; Aghajari et al. 2021; Liv et al. 2019). Hence, the wide exploration of macroinvertebrates as biomonitoring tools for assessing the ecological health status of riverine ecosystems (Olatunji et al. 2019).
Biomonitoring of rivers is a global activity towards understanding the ecological health status of aquatic ecosystems (Wang et al. 2023). The ability of macroinvertebrates to respond to different level of environmental stressors, little or no mobility, tolerance/sensitivity to pollution, easy/cheap mode of sampling, larger species richness and genetic diversity made them to be widely integrated for long- and short-term environmental biomonitoring (Omovoh et al. 2022; Edegbene 2022; Olatunji et al. 2023). Many biotic indices based on macroinvertebrates have been proposed in evaluating the health status of running waters in the Afrotropics (Akamagwuna et al. 2019; Edegbene 2020; Tampo et al. 2020; Katswangue et al. 2021; Edegbene 2022). Despite the several studies on the use of macroinvertebrates for developing biomonitoring tools in Africa, more studies are needed, especially in the current study area (the Uwagbe River) where study of this kind had not been done. This further justifies the use of macroinvertebrates for developing biomonitoring tools for effective river systems management and sustainability tracking in the Afrotropics.
Globally, the sustainability and management of freshwater ecosystems have become great challenges due to agricultural and industrial run off, waste disposal, dredging, oil exploration/exploitation, climate change and urbanization, leading to the pollution problems facing rivers and their inhabitant biota (Edegbene and Arimoro 2012; Olatunji et al. 2022; Qian 2023). Most of these disturbances are presently occurring in the catchments of the Uwagbe River, hence this current study is pertinent to forestall further degradation in the river.
The Uwagbe River is a very important freshwater system that serves various communities where it passes through in Esanland, Edo State, Nigeria. The Uwagbe River is used for several domestic and commercial undertakings such as drinking, washing, dredging, irrigation, fishing, domestic and religious activities. As a result of the increase in the population of the surrounding towns and urbanization challenges, many anthropogenic activities occur at different reaches of the river, increasing the level of pollution thereby reducing its health status. The aim of this research was to estimate the degree of the pollution in the Uwagbe River using macroinvertebrates and physico-chemical parameters.
Materials and methods
Research area and selection of station
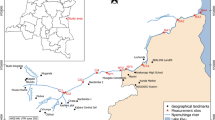
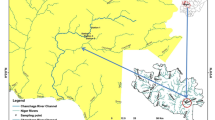
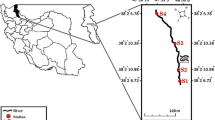
The Uwagbe River is positioned within the interception of latitude 6o30’53’’ N and longitude 6o4’54’’E of the equator (Fig. 1). The river flows from Urhio town through Ujiogba to Ugun towns in Esan West Local Government, Edo State, Nigeria. The Uwagbe River separates Ujiogba from Obazagbon town in Esan West and Uhunmwode Local Government of Edo State Nigeria, respectively. It serves various communities such as Uhi, Ughieghudu, Obazagbon, Ogwa, Ebelle and Okalo. The research area is in the tropical rainforest part of Nigeria distinguished by wet and dry seasons (Olatunji et al. 2022). The wet season spans from April to October and has a break in August while the dry season spans from November to March with dry harmattan sub-season in December and January (Alens 2017). The zone has afrotropical climate with average yearly temperature of 28 oC (22–34 oC), average yearly relative humidity of 69.5% (62–77%) and the rainfall extends from 2,000 to 2,500 mm/year (Alens 2017). The catchments of the study area are dominated by dense canopies of trees and other vegetation which include; Bambusa sp., Elaeis guineesis, Alocasia sp., Chromolaena odorata and Panicum maximum. Surface run-off and organic matter derived from the surrounding vegetation contribute to the allochthonous input resulting in increased deposition of organic particles in most of the reaches of the studied sites. The zone is characterized by the riverine structure composed of sand intermingled with lignite and spots of sand. Perturbation sources are animal and human faecal deposition, dredging, washing, bathing, agricultural run-off, decomposing religious sacrifices and domestic waste disposal.
Based on substrate type, human influence and accessibility during inundation period, three study locations representing upstream, midstream and downstream were carefully chosen for sampling. Station 1 is located at the upstream of the waterway, and the substratum are silt, sand and decaying macrophyte. This station is wadable, devoid of much human activities except tapping of palm wine and soaking/washing of cassava; the dominant riparian vegetation are Bambusa sp. and Elaeis guineesis while the aquatic vegetation in the station are Eichhornia crassippes, Salvinia sp., Nymphaea lotus. and Lemna sp. Station 1 was chosen as the control station because there was little or no anthropogenic presence/perturbation. Station 2 is 1,010 m away from Station 1 and is the mid-stream point on the river where the flow rate is minimal. The bed rock is made of silt, sand and decomposing macrophyte. This area is wadable with high level of human activities like fetching of water by villagers and tanker drivers, bathing, washing, grazing, and sand dredging. The dominant riparian vegetation are Zea mays, Raffia hookeri, Costus afar, Manihot sp., Discorea sp. and Elaeis guineesis while the aquatic vegetations there are Eichhornia crassippes and Lemna sp. Station 3 is 2100 m away from Station 2, the bed rock is made of silt, sand, stones and decaying macrophyte. The station is an open zone characterized by higher current velocity. This area has low level of human activities like farming and deposition of sacrifices by worshippers of the river deity. The dominant riparian vegetation includes Zea mays, Manihot sp., Bambusa sp., while the aquatic vegetation there are Eichhornia crassippes, Nymphaea lotus and Lemna sp. At this station, the river runs parallel to the Orhunworu River, but the two rivers do not mix.
Samples collection
Sampling of physico-chemical parameters and macroinvertebrates in all the stations were carried out monthly for 24 months between March 2018 and February 2020. Sampling was carried out between 8 am and 10 am while laboratory analysis was carried out in the laboratory immediately after arrival from the field with the exception of biological oxygen demand (carried out five days after arrival).
Regarding the physico-chemical parameters, at each of the sampling stations, in situ parameters such as pH, temperature, flow velocity and electrical conductivity (EC) were recorded and 500 ml plastic bottles washed with sulphuric acid were used to collect water samples 30 cm beneath the water surface in the direction against the water current for laboratory analysis on a monthly basis as earlier employed by Edegbene et al. (2019) and Olatunji et al. (2023). Physico-chemical parameters were measured following methods described in APHA (2015). Air and water temperatures were measured with mercury-in-glass thermometer. Water depth was measured with standardized stick calibrated in centimetres and flow velocity was determined by employing surface float method by timing a stopwatch. Electrical conductivity and pH were measured with HANNA Probe Analyzer. Dissolved Oxygen (DO) and Biochemical Oxygen Demand (BOD) were determined using Winklers’ Titrimetric method (APHA, 2015). Phosphate, nitrate and sulphate were measured using spectrophotometric method according to APHA (2015). Alkalinity was determined by titration using Phenolphthalein indicator method as earlier described by Edegbene et al. (2015).
Alongside physico-chemical parameters, macroinvertebrates were also collected on monthly basis. Macroinvertebrates samples were collected from five biotopes – pool, run, riffle, sediment and bank root per station. A kick net (30 × 30 cm range and 500 μm mesh dimension) was used for kick sampling of macroinvertebrates at each station in all the biotopes except depositional (Arimoro et al. 2015; Edegbene 2022). Sampling was conducted by disturbing the substratum and emergent vegetation that house all the biotopes and toiling the kick net against the water current in a zig-zag form to dislodge the macroinvertebrates that were anchored to the substratum and vegetation. The net was positioned to collect the macroinvertebrates washed by the current. The biotopes received many kicks that lasted for six minutes to get a monthly sample. Van Veen grab was used to obtain macroinvertebrates from the depositional biotope as described by Olatunji et al. (2023). In order to reduce variation, macroinvertebrates were obtained from the same environment of riffle zones of the river in parts with expanse canopy coverage and riparian vegetation. All macroinvertebrates collected in the five biotopes at each station were summed together to get a composite sample. After each sampling exercise, macroinvertebrates were kept in 10% formalin for further analysis in the laboratory. All macroinvertebrates were named and enumerated in the laboratory under a dissecting microscope and available identification guides by Gerber and Gabriel (2002), and Arimoro and James (2008).
Data analysis
SPSS statistical software (version 16.0) was used for the statistical analysis of the physico-chemical parameters (SPSS Inc 2007). Descriptive statistics: the standard deviation, mean, minimum and maximum of the physico-chemical parameters at each station were computed. The means obtained from the physico-chemical parameters were compared within the stations with one-way analysis of variance (ANOVA). In order to determine the significant ANOVA (p < 0.05), Tukey’s post hoc Honestly Significant Difference (HSD) test was calculated. Principal Component Analysis (PCA) was carried out to relate physico-chemical parameters with the sampled stations using PAST v4.03 software package (Zuur et al. 2007). The abundance of macroinvertebrates was presented in tabular form and the spatial and temporal variation in abundance and composition of macroinvertebrates were shown on a bar chart. ANOVA was performed to reveal the significance of the monthly composition of macroinvertebrates in the research area among the stations and months sampled. Faunal diversity and dominance analysis involving taxa richness (Margalef index), diversity (Shannon-Weiner index, H), evenness (E) and Simpson dominance (D) indices were analyzed using PAST v4.03 software package (Zuur et al. 2007). Canonical Correspondence Analysis (CCA) ordination was constructed to clarify the connection of physico-chemical parameters and macroinvertebrate taxa among the sampled stations using PAST v4.03 software package. Before CCA ordination was constructed, the 14 physico-chemical parameters analyzed were log transformed log (x + 1) to normalize the parameters prior to examination thereby preventing unjustified effects of farthest values on the triplot. In addition, Monte Carlo P- test@999 permutation was calculated to know the level of significance of the first two canonical axes of the CCA.
Results
Physico-chemical parameters
The mean and standard deviation of the physico-chemical parameters within the sampled stations and their significant differences are displayed in Table 1. It shows that Station 3 had significantly higher values of flow velocity, transparency, water depth, nitrate, phosphate, sulphate, alkalinity and BOD than Station 1 and Station 2. As shown by Tukey’s HSD test for multiple comparisons carried out between stations, Station 3 means were significantly different from the means of Station 1 and Station 2. pH and DO significantly increased from Station 1 to Station 3. Conductivity was significantly highest in Station 2 compared to Station 1 and Station 3.
PCA: UWAGBE
The relationship of physico-chemical parameters in the sampled stations within the sampling period showed that the first axis had a percentage variance of 81.787% of the entire PCA value with an Eigenvalue of 11.4502 (Fig. 2). The second axis of the PCA had a percentage variance of 18.213% of the entire PCA value with an Eigenvalue of 2.54981 (Fig. 2). Physico-chemical parameters such as phosphate, alkaline, water depth, sulphate, flow velocity, nitrate, DO, BOD, pH and chloride were positioned on the first axis of the PCA (Fig. 2). Transparency, water temperature, sulphate, flow velocity and phosphate were positively correlated with Station 3 (Fig. 2). Physico-chemical parameters such as water and air temperature, alkaline, conductivity, BOD and chloride are positively correlated with Station 2 (Fig. 2). Physico-chemical parameters such as pH and DO were associated with Station 1 (Fig. 2).
Principal Component Analysis of selected physico-chemical parameters of Uwagbe River, Nigeria from March 2018 to February 2020. Note: DIO: Dissolved oxygen, WAT; Water temperature; ART: Air temperature; TRN: Transparency; SUP: Sulphate; FLV: Flow velocity; PHS: Phosphate; NIT: Nitrate; ALK: Alkaline; BOD: Biochemical oxygen demand; CND: Conductivity; PH: pH; WAD: Water depth; CHL: Chloride
Thirteen (13) orders of macroinvertebrates comprising of 32 families consisting of 45 taxa and 4,796 individuals were documented in this research (Table 2). Station 1 had the highest abundance of taxa with 2,006, followed by Sstation 3 with 1,558 and Station 2 with 1,232 individuals.
The order Odonata had five families and the family Aeschnidae had the highestnumber of individuals (320) represented by Aeschna sp. while Anax sp. Ischnura sp. of the family Coenagrionidae had the least abundance (2) among the order Odonata. Order Coleoptera was represented by two families, namely Hydrophilidae and Dystiscidae. Cybister sp. was the most abundant (77) while Helochares sp. was the least abundant (7). In all stations, the number of macroinvertebrates recorded in Station 1 was more than those of Stations 2 and 3. The order Hemiptera was represented by four families, namely Nepidae, Gerridae, Veliidae and Pleodae. Nepa sp. was the most abundant (98) while Microvelia sp. was the least abundant (14). Pleo sp. (Pleodae) was not seen in Stations 2 and 3. The order Diptera was composed of four families, namely Chironomidae, Tipulidae, Simulidae and Tabanidae. Chironomus nilodorum was the most abundant (321) taxon in the order Diptera while Simulium sp. was the least (50) abundant. Ephemeroptera was represented by four families, namely Baetidae, Oligoneuridae, Heptageniidae and Trichorytidae. Afronurus sp. was the most abundant (227) taxon while Elassoneuria sp. was the least abundant (27). Baetis tricaudatus (Baetidae) and Elassoneuria sp. (Oligoneuridae) were not collected from Stations 2 and 3. Plecoptera, Trichoptera and Lepidoptera were represented by Neopeta sp., Aethaloptera sp. and Nymphula stratiotata, respectively, and they were all present only in Station 1 (Table 2).
The order Decapoda was represented by three families, namely Atyidae, Potamonautidae and Palaemonidae. Caridina africana was the most abundant taxa (268) while Potamonautes sp. was the least abundant (76). Caridina africana and Euryrhynchina sp. were more abundant in Station 2 (179 and 97 individuals) than Station 1 (36 and 16 individuals) and Station 3 (53 and 14 individuals), respectively.
The order Mollusca was represented only by Potadoma moerchi of family Thiaridae, it was present only in Station 3. The order Oligochaeta was represented by three families, namely Lumbricidae, Naididae and Tubificidae. Lumbricus sp. was the most abundant taxon (379) while Stylaris sp. was the least abundant (11). Nais sp., Stylaris sp. and Tubifex tubifex were absent in Station 1. The order Arachnida was only represented by Arrenurus sp. of family Arrenuridae and was present only in Station 3.
The dominant order numerically was Diptera (26.7%) followed by Odonata (18.2%), Oligochaeta (15.6%), Ephemeroptera (11.9%), Decapoda (11.8%), Coleoptera (4.6%), Hemiptera (4.3%), Plecoptera (2.5%), Trichoptera (1.8%), Amphipoda (1.2%), Mollusca (1.0%), Lepidoptera (0.2%) and Arachnida (0.1%) accordingly. The prominent taxa collected were Lumbricus sp. (7.9%), Chironomus nilodorum (6.7%), Caridina africana (5.6%), Tabanus sp. (4.8%), Afronurus sp. (4.7%), Baetis tricaudatus (4.5%) and Aeschna sp. (4.3%).
Monthly and seasonal variations of macroinvertebrates
Overall, more macroinvertebrates (81.7%) were collected in the wet season (April-October) than in the dry season (18.3%) (November-March). The spatio-temporal analysis showed that the highest number of macroinvertebrates were documented for Station 1 in July 2018 (194) and the lowest were documented for Stations 1 and 2 (13) in February 2020 and December 2018, respectively (Fig. 3).
In July, 2018, the highest number of macrobenthos were collected (9.2%) while in December, 2018 the least number was obtained (1.15%). Odonata, Trichoptera, Coleoptera and Decapoda were most abundant in July, 2018. Hemiptera, Ephemiroptera and Diptera were most abundant in August, 2018. Lepidoptera was most abundant in October, 2018 and July, 2019. Amphipoda was most abundant in September, 2018 and Plecoptera in July 2019. Oligochaeta and Mollusca were most abundant in August, 2019 while Arachnida occurred sporadically in 2018 and 2019 together (Table 3).
Analysis of variance revealed that differences in the monthly composition of macrobenthic invertebrates in the research area were highly significant (p < 0.05) among the stations sampled, but no significant difference was revealed among the monthly samples collected during the study period by the Tukey HSD test.
Dominance and diversity indices
The dominance and diversity indices for Stations 1, 2 and 3 are presented in Table 4. Margalefs index showed maximum species richness for the macrozoobenthos collected in Station 1 (4.73), followed by Station 2 (4.49) and Station 3 (4.49). Shannon-Wiener diversity index (H) showed that Station 1 had the highest index (3.22), followed by Station 2 (3.06) while Station 3 had the lowest value (2.89). Statistically, the indices were significantly different between all the stations (p < 0.05). The evenness index (E) showed that Station 1 had the maximum evenness of distribution (0.68) and Station 3 had the minimum evenness of distribution (0.53). The maximum Simpsons’ dominance was recorded in Station 1 (0.95) while Station 3 had the minimum (0.93).
Multivariate analysis related to selected physico-chemical parameters and macroinvertebrates composition
The Canonical Correspondence Analysis (CCA) ordination showed positive relationships among species abundances and calculated ecological variables (Fig. 3). 83.51% of the data variation was accounted for by the first canonical axis while the second axis accounted for 16.49%. The Eigenvalue of axis 1 was 0.5946 while that of axis 2 was 0.1174. Monte Carlo permutation test at 999 permutation argument showed that the first and second axes were not significant (p > 0.05) in the CCA correlation among macrobenthic invertebrate families and physico-chemical parameters concentration. Chlorine, alkalinity, BOD, water depth, nitrate and phosphate were positively correlated with these macrobenthos families – Lumbricidae, Chironomidae, Potamonautidae, Tabanidae and Palaemonidae in Stations 2 and 3. pH and DO were correlated with Amphipodae, Hydrophilidae, Gerridae and Libellulidae families in Station 1 (Fig. 4).
Discussion
Water quality
The composition of aquatic macroinvertebrates, their distribution and abundance are influenced by the geomorphic, physico-chemical and biotic factors, namely water quality, food supply, predation, competition and nature of substrate (Akamagwuna et al. 2019; Olatunji and Anani 2020; Edegbene et al. 2021; Wen et al. 2024). The outcomes of the present study area showed that all the physico-chemical parameters recorded were within the recommended limits of World Health Organisation’s water quality standard for potable water (WHO 2004) and Nigerian water quality standard for inland surface water (FMENV 1991) except the pH and DO of Stations 2 and 3 in some of the months sampled. Additionally, the BOD of Station 3 from July to September 2018 were higher than the recommended limits of FMENV (1991) and WHO (2004).
Considering the mean values of of all physico-chemical parameters analysed, it was only the pH of Station 3 that was below the recommended limits of WHO (2004) and FMENV (1991), all other parameterss were within the permissible limits of WHO (2004) and FMENV (1991). Overall, the DO and pH values of Station 3 were significantly lower than those of Stations 1 and 2, indicating the deteriorating state of the water in Station 3 due to anthropogenic activities. The anthropogenic activities in Station 3 were peculiar compared to the other stations considering the deposition of sacrifices in the river by worshippers of the river deity and the river running parallel to the Orhiomwhan River in the station which might explain the lower pH, higher nutrients and BOD recorded there compared to others. Other studies in the Afrotropics and Asia have reported similar results (Arimoro et al. 2014; Keke et al. 2021; Edegbene et al. 2021; Xue et al. 2023).
The PCA showed that parameters such as sulphate, phosphate and electrical conductivity were positively correlated to Stations 2 and 3, while pH and DO were positively correlated to Station 1. The 14 physico-chemical parameters explained 53.5% of all the variance. According to Liu et al. (2021), such variance value is moderate enough to make reliable and useful deduction about the characteristics of the total dataset.
Macroinvertebrates assemblage and diversity
The composition of macroinvertebrates consisting of 32 families, 45 taxa and 4,756 individuals collected from the Uwagbe River is within the range observed by Edegbene and Arimoro (2012), Arimoro et al. (2015) and Odigie (2019). The diverse nature of the macroinvertebrates collected was also likely due to the different niche compartments within the study sites and the behavioural and adaptation patterns of the taxa collected.
Karrouch et al. (2017) reported that water bodies with high quality physico-chemical parameters influenced the growth and abundance of aquatic macroinvertebrates. The order Diptera dominated in terms of taxa and individuals collected, this was in line with the works of Olomukoro and Dirisu (2014) and Edegbene (2022). The physiological and morphological adaptations of the dipteran larvae to different aquatic microhabitats (niches) could contribute to their high abundance in the Uwagbe River. Odigie (2015) observed that chironomid in the order Diptera colonized diverse aquatic niches including perturbed water due to their ability to use inherent haemoglobin to attract oxygen from water.
Potadoma moerchi was only found in Station 3, which may be due to macrophyte vegetation providing them with shelter and sustenance as they are periphytic in nature (Tampo et al. 2021). Furthermore, the presence of stones in the substrate of Station 3 may also be the reason P. moerchi was predominant in the station as stones contain calcareous shells building materials (Edegbene and Arimoro 2012).
The spatio-temporal dynamics of the population of macroinvertebrates showed that more organisms were collected in the wet season (81.7%) than in the dry season (18.3%). This is in congruence with the results of Abebe et al. (2009) for Borkena river, Ethiopia and Edegbene (2022) for the Owan River, Southern Nigeria. Edegbene and Arimoro (2012) had earlier reported that wide distribution of macroinvertebrate in the wet season may be occasioned by flood and erosion which disperse some of the macroinvertebrates to the riparian zone of the river.
Overall, the number of macroinvertebrates recorded in the Uwagbe River is likely connected to the predominance of certain taxa such as Lumbricus sp and Chironomus sp. which can survive in the river with various environmental variables. For instance, the presence of Baetis tricaudatus and Aeschna sp. in Station 1 (pollution sensitive taxa) and Chironomus sp. and Lumbricus sp. in Station 3 (tolerant taxa) showed the diversity of macroinvertebrates across the different stations studied in this research (Edegbene and Arimoro 2012; Arimoro et al. 2015; Akamagwuna et al. 2019; Edegbene et al. 2023). For diversity indices, Margalef’s index was highest in Station 1 (4.738) and lowest in Station 3 (4.365) showing that Station 1 was richest in species while Station 3 had the least diversity of macroinvertebrates. This conformed with the works of Odigie (2019) in a river system in Benin, Edo State, Nigeria. Based on the values of the diversity indices, it is conclusive that the sampled stations were subjected to varied levels of disturbances.
Simpson’s dominance index (D) showed that Station 3 with the lowest dominance value of 0.9252 was more diverse in taxa composition compared to the other stations. Station 1 had the most dominant species with Simpson’s index of 0.9476 due to the predominance of Baetis tricaudatus. This was connected to the less perturbed state of Station 1 as a result of high number of pollution sensitive species in this station and agrees with the result of Edegbene et al. (2021) in riverine ecosystems in Southern Nigeria. Shannon-Weiner diversity index decreased from Station 1 (3.202) to Station 3 (2.839) as earlier reported by Arimoro (2009) and Arimoro et al. (2015) in the Ogba River, Southern Nigeria, where diversity of macroinvertebrate taxa decreased from the least impacted to the most impacted sites.
Macroinvertebrates and environmental factors interactions
The CCA analysis conducted for this study showed significant interactions between macroinvertebrates’ abundance and environmental parameters (Edegbene et al. 2019). The CCA triplot showed that some pollution tolerant families such as Simulidae, Lumbricidae, Chironomidae, Potamonautidae and Palaemonidae were positively associated with increased nutrients, salinity and alkalinity in Stations 2 and 3. Similarly, other studies (e.g. Olomukoro and Disu 2014; Arimoro et al. 2015) had attributed the deteriorating state of river ecosystems to the presence of pollution tolerant taxa. Most macroinvertebrates families sensitive to pollution such as Hydrophilidae, Gerridae, Libellulidae and Amphipodae were collected in Station 1, the least perturbed site. These macroinvertebrates were positively correlated with increased DO in Station 1. Edegbene and Arimoro (2012) showed that these species were associated with clean water conditions and had earlier been reported to positively correlate with increasing DO concentration.
Conclusion
The research work is a comprehensive report on the effects of anthropogenic influences on the Uwagbe River in Edo State, Nigeria through studying crucial physico-chemical parameters and macroinvertebrate community structure. The results of the study showed that the downstream of the river is prone to pollution from anthropogenic activities emanating from agricultural activities, dredging, bunkering and sacrificial items from traditionalists. This had resulted in the deterioration of the health status for the river. Station 1 was the least perturbed, while Station 2 was the moderately perturbed and Station 3 was the heavily disturbed with serious public health concern. In order to conserve the biodiversity of the river, biomonitoring using macroinvertebrate fauna is non-negotiable. We recommend further research should be carried out along the entire stretch of the river to confirm the current study results.
References
Abebe B, Legesse W, Triest L, Kloos H (2009) Urban impact on ecological integrity of nearby rivers in developing countries: the Borkena River in Highland Ethiopia. Env Monit Assess 153:44–61. https://doi.org/10.1007/s10661-008-0379-x
Adu BW, Oyeniyi EA (2019) Water quality parameters and aquatic insect diversity in aahoo stream, Southwestern Nigeria. J Basic Appl Zool 80:15. https://doi.org/10.1186/s41936-019-0085-3
Aghajari K, Safaie M, Valinassab T, Noorinezhad M, Mortazavi M (2021) Assessing the diversity of macroinvertebrates communities and their relationship with environmental factors in the Persian Gulf and the Gulf Oman. Iran J Fish Sci 20(6):1704–1726
Akamagwuna FC, Mensah PK, Nnadozie CF, Odume ON (2019) Traits-based responses of Ephemeroptera, Plecoptera and Trichoptera to sediment stress in the Tsitsa River and its tributaries, Eastern Cape, South Africa. Riv Res Appl 35(7):999–1012. https://doi.org/10.1002/rra.3458
Alens OP (2017) Assessment of the use of surface water and its environmental health effects in Ekpoma, Nigeria. Int J Dev Sustain 6(12):2147–2161
American Public Health Association (APHA) (2015) American Water Works Association, Water Pollution Control Federation. Standard methods for the examination of water and waste water 13th Edn. Washinton DC
Amri N, Jamil S, Abdolbarghian S (2014) Diversity of macrobenthos communities and their relationships with environmental factors in Jagroud river, Iran. Res Environ 4(2):95–103
Arimoro FO (2009) Impact of rubber effluent discharges on the water quality and macroinvertebrtae community assemblages in a forest stream in Niger Delta. Chemosphere 77:440–449. https://doi.org/10.1016/j.chemosphere.2009.06.031
Arimoro FO, Ikomi RB (2009) Ecological integrity of Upper Warri River, Niger Delta using aquatic insects as bioindicators. Ecol Indic 9:455–461. https://doi.org/10.1016/j.ecolind.2008.06.006
Arimoro FO, James HM (2008) Preliminary pictorial guide to the macroinvertebrates of Delta State Rivers, Southern Nigeria. Albany Museum, Grahamstown
Arimoro FO, Ikomi RB, Nwadukwe FO, Eruotor OD, Edegbene AO (2014) Fluctuating salinity levels and an increasing pollution gradient on fish community structure and trophic levels in a small creek in the Niger Delta, Nigeria. Int Aquat Res 6(4):187–202. https://doi.org/10.1007/s40071-014-0078-2
Arimoro FO, Odume ON, Uhunoma SI, Edegbene AO (2015) Anthropogenic impact on water chemistry and benthic macroinvertebrate associated changes in a southern Nigeria stream. Env Monit Assess 187(2):14. https://doi.org/10.1007/s10661-014-4251-2
Edegbene O (2022) Assessing the health of forested riverine systems in the Niger Delta area of Nigeria- a macroinvertebrate based multimetric index approach. Env Sci Poll Res 29(2):15068–15080. https://doi.org/10.1007/s11356-021-16748-4
Edegbene AO, Arimoro FO (2012) Ecological status of Owan River, Southern Nigeria Using Aquatic Insects as Bioindicators. J Aquat Sci 27(2):99–111
Edegbene AO, Arimoro FO, Odoh O, Ogidiaka E (2015) Effect of anthropogenicity on the composition and diversity of aquatic insects of a municipal river in North Central Nigeria. Biosci Res Today World 1(1):69–80
Edegbene AO, Arimoro FO, Odume ON (2020) Exploring the distribution patterns of macroinvertebrate signature traits and ecological preferences and their responses to urban and agricultural pollution in selected rivers in the Niger Delta ecoregion. Nigeria Aquat Ecol 54(2):553–573. https://doi.org/10.1007/s10452-020-09759-9
Edegbene O, Omovoh G, Osimen E, Ogidiaka E, Jegbefuma L, Olatunji E, Nwaka K (2021) Assessing the ecological health of a river in North-West Nigeria using macroinvertebrates structural assemblage and environmental factors. Asian J Geol Res 4(4):1–16
Edokpayi CA, Olowoporoku AO, Uwadiae RE (2010) The hydrochemistry and microbentic characteristics of urban draining Creek. Int J Biodiv Conserv 2(8):196–203. https://doi.org/10.5897/IJBC.9000136
Forio MA, Echelpoel WV, Dominguez-Granda L, Mereta ST, Ambelu A, Houng TH, Boets P, Goethous P (2016) Analysing the effects of water quality on the occurrence of freshwater macroinvertebrates taxa among tropical river basins from different continents. AI Comm 29(2016):665–685
George AD, Abowei JF, Alfred-Ockiya JF (2009) The distribution, abundance and seasonality of benthic macroinvertebrate in Okpoka Creek sediment, Niger Delta, Nigeria. Res J Appl Sci Eng Tech 2(1):11–18
Gerber A, Gabriel MJM (2002) Aquatic invertebrates of South African Rivers – field guide. Resource quality services, Department of Water Affairs, Pretoria
Karrouch EC, Jewitt G, Karr JR (2017) Studies on the hydrochemistry of Kangimi Reservoir, Kaduna State Nigeria. Afr J Ecol 43:7–13
Katswangene P, Oleko W, Kazadi M (2021) Spatiotemporal portrait of the quality of water supplied by REGIDESO, Democratic Republic of Congo. Adv Micr 11(5):225–242
Keke UN, Omoigberale MO, Ezenwa I, Yusuf A, Biose E, Nweke N, Edegbene AO, Arimoro FO (2021) Macroinvertebrate communities and physicochemical characteristics along an anthropogenic stress gradient in a Southern Nigeria Stream: implications for ecological restoration. Environ Sustain Indic 21:82–97. https://doi.org/10.1016/j.indic.2021.100157
Liang J (2023) An analysis of the linkage between marine industry and regional economy-taking the three coastal economic zones as an example. J Sea Res 193:102371. https://doi.org/10.1016/j.seares.2023.102371
Liv X, Zhag J, Shi W, Wang M, Chen K, Wang L (2019) Priority pollutants in water and sediments of river for control basing on benthic invertebrate community structure. Water 11:12–67. https://doi.org/10.3390/w11061267
Odigie J (2019) Application of water quality and pollution tolerance indexes as effective tools for river management. Punjab Univ J Zool 34(2):105–113
Odume ON (2020) Searching for urban pollution signature and sensitive macroinvertebrate traits and ecological preferences in a river in the Eastern Cape of South Africa. Ecol Indic 108:105759. https://doi.org/10.1016/j.ecolind.2019.105759
Olatunji EO, Anani OA (2020) Bacteriological and physicochemical evaluation of River Ela, Edo State Nigeria: water quality and perceived community health concerns. J Biol Innov 9(5):736–749
Olatunji EO, Isola OB, Fakeye OD (2019) Characterization and identification of bacterial isolates from different drinking water sources in Uromi, Edo State,Nigeria. SAU Sci Tech J 4(1):28–43
Olatunji EO, Anani OA, Osimen EC, Omojoyegbe RT (2022) Public health concerns and water quality integrity of selected water sources in a peri-urban community of South-South Nigeria: a comparative investigation. Sust Wat Res Managt 8:1–10
Olatunji EO, Elakhame LA, Osimen EC, Edegbene AO (2023) Assessing the effect of environmental stressors on the community structure of macroinvertebrate and water quality of River Ugbalo, Nigeria. Pollution 9(4):1317–1332. https://doi.org/10.22059/poll.2023.354819.1771
Omovoh BO, Arimoro FO, Anyanwale AV, Egwin EC, Omovoh GO, Akamagwuna FC, Zakari H, Edegbene AO (2022) Macroinvertebrates of Wupa River, Abuja, Nigeria: do environmental variables pattern their assemblages? Bio Insights 1:1–9. https://doi.org/10.55085/bi.2022.612
Qian X (2023) Research on the coordinated development model of marine ecological environment protection and economic sustainable development. J Sea Res 193:102377. https://doi.org/10.1016/j.seares.2023.102377
Tampo L, Lazar I, Kabore I, Oueda A, Akpatuku V (2020) A multimetric index for assessment of aquatic ecosystem health based on macroinvertebrates for the Zio River basin in Togo. Limnologica 83:125783. https://doi.org/10.1016/j.limno.2020.125783
Tampo L, Alhassan IK, Oueda A, Bawa LM, d- Boundjou G (2021) Benthic macroinvertebrate as ecological indicators; their sensitivity to the water quality and human disturbances in a tropical river. Front Wat 42:662765. https://doi.org/10.3389/frwa.2021.662765
Wang J, Wang Z, Wang Q, Zhou Z (2023) Ecological index analysis of growth and development of Procambarus Clarkii based on biological characteristics. J Sea Res 192:102363. https://doi.org/10.1016/j.seares.2023.102363
Wen Z, Han J, Shang Y, Tao H, Fang C, Lyu L, Song K (2024) Spatial variations of DOM in a diverse range of lakes across various frozen ground zones in China: insights into molecular composition. Wat Res 252:121204. https://doi.org/10.1016/j.watres.2024.121204
World Health Organisation (2004) Guidelines for drinking water quality, 2nd edn. World Health Organization, Geneva
Xue Y, Ma Y, Long G, He H, Li Z, Yan Z, Zhu B (2023) Evaluation of water quality pollution and analysis of vertical distribution characteristics of typical rivers in the Pearl River Delta, South China. J Sea Res 193:102380. https://doi.org/10.1016/j.seares.2023.102380
Zuur AF, Ieno EN, Smith GM (2007) Analysing ecological data. Springer Science. Business Media, LCC, New York, NY 10013, USA
Acknowledgements
We acknowledge the staff of the Hydrobiology Laboratory, Ambrose Alli University, Ekpoma for their support in the laboratory. We thank Dr Joshua Izegaegbe for the support received from him during the identification stage and we also appreciate Mr. Eugene Festus an aquatic research technologist whose assistance at the research locations was inestimable.
Funding
The authors received no funding for this research.
Author information
Authors and Affiliations
Contributions
Olatunji EO, Elakhame LA, Osimen EC and Edegbene AO contributed to conceptualization and design of the study. Olatunji EO, Osimen EC and Edegbene AO organized the database. Olatunji EO collected the samples. Olatunji EO and Edegbene AO performed the data analysis. Olatunji EO and Osimen EC wrote the original draft of the manuscript. Elakhame LA, Tampo L and Edegbene AO wrote segments of the manuscript. Elakhame LA, Osimen EC and Edegbene AO supervised the entire work. All the authors participated in the revision of the manuscript, read and approved the submitted version.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interest.
Declaration of competing interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Ethics approval
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Olatunji, O.E., Elakhame, L.A., Osimen, E.C. et al. Responses of macrobenthic invertebrates’ diversity to environmental factors in a tropical freshwater river in Edo State, Nigeria. Biologia (2024). https://doi.org/10.1007/s11756-024-01781-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11756-024-01781-8