Abstract
The habitat of the endemic, endangered, and medically important plant, Stachys cretica subsp. kutahyensis belongs to the family Lamiaceae, is threatened by human influence. This study used a shoot tip explant to standardize a simple and proper micropropagation system for relict endemic plant species. Murashige Skoog medium with different concentrations of gibberellic acid and juglone was used for in vitro germination. Seedlings from in vitro germinated plantlets were used as explant sources in tissue culture studies. The highest shoot average per explant (4.60 shoots) and shoot length (2.70 cm) was determined in MS medium with 2.0 mg/L 6-benzyl amino purine with 100% response in all treatments. This study used different rooting mediums: Murashige Skoog and modified Murashige Skoog media. The effects of Murashige Skoog and different concentrations of indole butyric acid, naphthaleneacetic acid, juglone, and modified MS, and different concentrations of juglone were investigated on rooting development. Elongated shoots were successfully rooted in the Mod MS medium with 10–7 M juglone. Rooted plantlets were gradually transferred to the soil by adapted external conditions. The transfer of S. cretica subsp. kutahyensis plants, adapted to the soil and field conditions (Tavşanlı/Nusretler), successfully carried out. This protocol can be used for conservation studies of endemic, endangered, and medicinally important plant species.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The distribution of endemic plants in the world is restricted to a geographical range, and their natural habitats are rapidly disturbed by environmental and human factors. As a result, endemic species encountered the threat of extinction (Sudhersan et al. 2003; Işık 2011). As it is known, endemic and medically important plants have an economic value used in cosmetics, medicine, food, paint, chemical industries, and many other fields (Efferth 2019; Akın 2020). Rare or endangered and threatened endemic species can be efficiently conserved with various cultural methods, such as ex-vitro and in vitro, with minimum plant material requirement, besides conventional propagation methods (Fay 1992; Cuenca et al. 1999; Rao 2004). Recently, plant biotechnology has emerged as an essential alternative to conventional propagation, in which plant-based in vitro systems are used, technology that facilitates the reproduction of plants and plant products and increases yield and quality. Tissue culture methods are the most effective plant propagation method compared to other vegetative propagation methods. For this reason, in vitro techniques used in plant biotechnology contribute to eliminating the danger of extinction of endangered species (Babaoğlu et al. 2002; Efferth 2019; Akın 2020).
A total of 125 taxa of Stachys are recorded from Türkiye, and 69 of them are endemic (Güner et al. 2023). The genus is well adapted to living in the Mediterranean in Türkiye (Bhattacharjee 1980; Güner 2022). Section Eriostomum contains 23 species (35 taxa) in Turkey (Akçiçek and Güner 2022). S. cretica subsp. kutahyensis Akçiçek belongs to the section. The plant is a locally endemic subspecies of West Anatolia (Tavşanlı Harmancık ‒ Dursunbey) with a narrow distribution in the provinces of Kütahya and Balıkesir in Turkey. The taxa grows at 235-870 m a.s.l. altitude. The number of mature individuals is less than 2500 and known in 3 locations. The taxa grows in stony places in Pinus nigra J.F. Arnold forest gaps openings and share its habitats with other species, including Campanula lyrata Lam. subsp. lyrata, Bromus sterilis L., Aegilops biuncialis Vis., Silene L., Picris L., Anthemis L., Sonchus asper subsp. glaucescens (Jord.) Ball, Alyssum L., and Papaver L. (Akçiçek 2010). Described as a new subspecies from Türkiye by Akçiçek (2010), S. cretica subsp. kutahyensis is a perennial herbaceous plant with basal-sterile rosettes and many stems from the base. Flowering time is from July to August (Fig. 1). On the other hand, Stachys L. species, valuable pharmacological properties, have broad uses in traditional medicine and have been showing antibacterial, anti-Helicobacter pylori, anti-inflammatory, anti-diarrheal, anticancer, and antioxidant properties (Salehi et al. 2007; Khanavi et al. 2009; Goren et al. 2011; Tundis et al. 2014; Tomou et al. 2020; Benabderrahim et al. 2021). The taxon's habitat is threatened by human influence in the areas (e.g., construction of roads, overgrazing, and deforestation) (Akçiçek 2010). Therefore, the IUCN (The International Union for Conservation of Nature) threat category is considered endangered (EN) (IUCN 2022).
The propagation of medicinally critical endemic plants with tissue culture techniques is used as an alternative method for conserving endemic plants. Recently, optimizing an in vitro propagation protocol for each plant species is gaining more and more importance (Akın 2020; Işıkalan et al. 2020).
In vitro multiplication protocols for rare, endangered, and endemic plant species make their reintroduction into their natural habitats possible and reduce extinction risk (Wochok 1981; Nadeem et al. 2000; Chandra et al. 2006). Until now, no study exists about the in vitro regeneration protocol of S. cretica subsp. kutahyensis. Therefore, the present study was designed to develop a successful in vitro regeneration system from shoot tips for the plant. We think that the data obtained from this study will contribute to overcome the difficulties in reproducing our endemic plant species, eliminating the threat of extinction, using it effectively in food and pharmacology, and thus protecting our endemic species as a gene source.
Materials and methods
Plant material, sterilization procedure, and In Vitro germination
Stachys cretica subsp. kutahyensis seeds were collected from populations at the entrance of Nusretler village, Tavşanlı district of Kütahya province (Türkiye), in June-July 2021 (39°39′168″N, 29°17′226″E) and brought to the laboratory. Dried seeds were disinfected with % three sodium hypochlorite (NaOCl) solution by adding a few drops of tween-20 for 20 min. Afterward, the sterilized seeds were rinsed thrice for 5 min with sterile distilled water. All media (pH 5.7) containing 3.0% sucrose and 0.7% agar were autoclave-sterilized at 121 °C for 20 min. Murashige Skoog (MS) nutrient medium without a plant growth regulator was used as a control for germination studies. In addition, to overcome the contamination problem, Plant Preservative Mixture (PPM 2 mL, 0.2% v/v) (Plant Cell Technology, Washington, D.C., USA), a broad-spectrum biocide used in plant tissue culture, which bites to sterilization, was added to the MS medium (Miyazaki et al. 2010; Uyanık 2017). In addition, three concentrations of Gibberellic acid (GA3 10, 25, 50 mg/L) and three concentrations of juglone (10–5, 10–6, 10–7 M) were added to the nutrient mediums germination medium. The seeds were germinated in a 16/8 photoperiod growth chamber at 25 °C ± 2 °C in MS medium supplemented with 3% sucrose and 0.7% agar. The same culture conditions were used for all experiments described in this study. Different concentrations of juglone and GA3 were added to the medium after autoclaving by passing through a 0.22-μm millipore filter. Each trial was repeated at least three times, ten seeds were used in each treatment, and the trials were repeated as far as seeds could be obtained. Seeds were subcultured every 30 days during the 60-day experimental period. Seeds germinated for 60 days were counted, and germination percentage (%) was calculated. The emergence of the radicle through the seed coat was considered the criterion for germination.
Culture medium and isolation of explants
Throughout the study, full-strength MS basal medium with varying concentrations of 6-benzyl amino purine (BAP) and kinetin (Ki) were used in vitro multiplication responses. After 60 days, the shoot tips of the seedlings germinated in vitro were placed in an MS medium containing different concentrations of BAP (0.5, 1.0, 2.0 mg/L) and Ki (0.5, 1.0, 2.0 mg/L), and the shoot formation stage was continued for eight weeks. The shoot tips (about 0.5–0.7 cm in length) were used as the explants to study this plant shoot multiplication. The shoots were subcultured at 3-week intervals.
For rooting studies, MS and modified MS (Mod MS) with different growth regulators were used as rooting mediums [Indole Butiric Acid (IBA), Naphthaleneacetic Acid (NAA), Juglone]. Since Akın et al. (2014) determined the positive effects of modification in MS medium on Erodium sibthorpianum Boiss., necessary changes were made in MS medium for the rooting of seedlings based on the relevant study. According to this study, while preparing the Mod MS nutrient medium, the amounts of ammonium + 4.38 mmol, nitrate + 4.38 mmol, calcium + 1.98 mmol, chloride + 3.96 mmol, and thiamine-HCl + 0.4 mg were increased in the MS basal nutrient medium. In comparison, the amount of nicotinic acid -0.475 mg was decreased. Sucrose (3.0%) was used as the carbon source in all experiments, and all media combinations were added 0.7% agar. The regenerated shoots (about 2 to 3 cm) were excised and transferred to MS medium without plant growth regulators (control), with various concentrations of juglone (10–6 and 10–7 M), IBA, and NAA (0.5, 1, and 2 mg/L) and Mod MS medium control, and with various concentrations of juglone (10–6 and 10–7 M) in 50 ml Magenta vessels to test the rooting potential. At the end of the experiment, shoot number per explant, shoot length (cm), shoot frequency (%), root length (cm), number of roots per shoot, and shoot rooting (%) were measured after eight weeks from the culture initiation.
Acclimatization
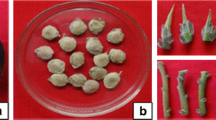
For the ex-vitro acclimatization, rooted plantlets of S. cretica subsp. kutahyensis were rinsed with distilled water to remove residual rooting medium, were transplanted to pots containing peat + vermiculite (3:1, v/v), and covered by a transparent polythene bag, and then placed in a growth chamber at room temperature (25 °C ± 2 °C) for fifteen days and, plants were gradually adapted to culture room conditions. (Fig. 2a, b). After that, acclimatized plantlets were transferred to the field conditions (Tavşanlı/Nusretler,) where they were grown naturally (Fig. 3). Then, plant survival (%) was measured.
Statistical analysis
The experiments were conducted using a completely randomized design with at least three replicated (Magenta vessels) with ten explants per vessel. Collected data were subjected to t-tests and ANOVA, and Tukey HSD multiple range test at the p = 0.05 level by using the JMP 6 SAS program (2005) (Kocaçalışkan and Bingöl 2017). Statistical analysis was performed separately for MS and Mod MS medium.
Results and discussion
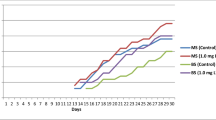
In order to obtain sterile seedlings during the propagation stage in vitro, the conditions in which the plant seeds show optimum germination should be determined (Fig. 3). In our study, the effects of MS nutrient media containing GA3 (10, 25, 50 mg/L), and juglone (10–5, 10–6, 10–7 M) on seed germination percentage was investigated to overcome the germination barrier in seeds since seeds did not germinate on filter paper moistened with distilled water in Petri dishes. However, 10–5 M juglone completely inhibited seed germination. As seen in Fig. 4, 10–7 M juglone (75%) treatments increased the germination percentages of S. cretica subsp. kutahyensis. On the contrary, it was determined that the germination percentage decreased to 40.00%, 37.50%, and 25.00%, respectively, at MS 10, 25, and 50 mg/L GA3 concentrations, and in conclusion, increasing GA3 concentrations inhibited germination. According to this result, 10–7 M juglone treatments were selected as the optimum for the germination of the plant (Fig. 4 A). In contrast, 10–5 juglone inhibited seed germination significantly. Several studies have demonstrated that when juglone is used at the proper concentration, it positively affects seed germination depending on the plant species (Kocaçalışkan and Terzi 2001; Akin and Kocacaliskan 2016). Besides, juglone is similarly not solely an allelopathically important chemical but also an important allelochemical in its antiviral, antibacterial, and antifungal properties (Clark et al. 1990; Arasoglu et al. 2016, 2017).
MS basal salts are one of the most widely used nutrient mediums in tissue culture studies (Perica 2003; Wala and Jasrai 2003; Erdağ and Emek 2005; Akbar and Roy 2006; Khawar et al. 2008). Knowing the seed germination requirements is helpful for threatened, rare, and endemic plant species. In our study, it was determined that the effects of different growth regulators that we added to the MS medium for germination were statistically significant for root development (F = 9.3667; p < 0.005). It has been determined that the MS medium containing 10–7 M juglone treatment increased the root and shoot elongation of S. cretica subsp. kutahyensis (Fig. 4 B).
Previous studies have shown that the allelopathic effects of juglones are often toxic, but it is also beneficial to a third plant species at lower concentrations and can be a stimulatory effect on plant growth (Kocaçalışkan and Terzi 2001; Akin and Kocacaliskan 2016; Milewska-Hendel et al. 2017). A similar result was reported by Akin and Kocacaliskan (2016) that juglone significantly increased the seed germination percentages of Aubrieta olympica Boiss. and Arabis drabiformis Boiss. at 10–5 and 10–6 M concentrations. However, only 10–5 M concentration increased the seedling growth of plants.
The manipulation of plant growth regulator ratios in plant tissue cultures affects growth, differentiation, and developmental processes (Ramabulana et al. 2021; Sudheer et al. 2022). In this research, the plant growth regulators (PGRs) considerably affected the morphogenetic responses of S. cretica subsp. kutahyensis under in vitro conditions. The influence of different concentrations of BAP and Ki plant growth regulators on the shoot differentiations is given in Table 1. Eight weeks after culture initiation, shoot development was seen at all BAP and Ki concentrations. The highest shoot average per explant (4.60 shoots) and shoot length (2.70 cm) was obtained from MS medium supplemented with 2.0 mg/L BAP with 100% response in all treatments (Table 1; Fig. 3). In contrast, the lowest shoot average was observed in MS medium containing 0.5 mg/L BAP and 0.5 mg/L Ki (1.69 and 1.93 cm, respectively). When BAP and Ki growth regulators were compared with each other, Ki was found to be less effective in causing shoot initiation (Table 1). Among the cytokinins, the superior effects of BAP for shoot multiplication than Ki have been reported by Anish et al. (2008), Gümüşçü et al. (2008), Akın and Kocaçalışkan (2011), Senapati et al. (2013), and Moola and Kumari (2019). BAP is a cytokine class hormone that stimulates the differentiation of cells in the meristematic tissues and promotes the growth of lateral shoots, apical dominance, and expansion of leaves (Decker et al. 2006; Yuniastuti et al. 2018). The increased shoot induction by BAP may be due to its effectiveness in stimulating plant tissues to metabolize its natural endogenous hormone to induce shoot organogenesis (Ahmed and Anis 2014; Moola and Kumari 2019). Similar to the results of our research, it has been demonstrated by many researchers that BAP is the most effective cytokinin on shoot formation for many plant species and can be used successfully in many species in shoot formation (Ram and Shekhawat 2011; Mayerni et al. 2020). In conclusion, our study shows that a culture medium containing specific growth regulators at certain concentrations affects organogenesis. BAP also plays an engagement and distinctive role in stimulating organogenesis and shoot proliferation.
The induction of adventitious roots is a crucial and complex process for vegetative propagation and a critical step for successful clonal propagation (Díaz-Sala 2021). Selecting a nutrient medium is one of the most important factors affecting success in tissue culture studies. This research investigated the rooting efficiency of micro-shoots in two different mediums (MS, Mod MS), and various auxins, namely NAA, IBA, and juglone. Shoots taken to in vitro rooting medium exhibited different responses according to the treatments (Table 2). The number of roots per shoot, root length, and rooting rate changed significantly with different concentrations of juglone. The maximum number of roots per shoot (4.20 roots/shoot), root length (3.30 cm), and rooting percentage (100%) were changed significantly with MS containing 10–6 M juglone (Table 2). Adding 10–6 M juglone to the MS medium increased root parameters. However, micro-shoots treated with juglone produced more roots than cuttings treated with no auxin, IBA, or NAA. However, the physiological mechanism of juglone action in plants is unknown (Bamel and Gupta 2022). Previous works have shown that juglone allelopathic effects are often toxic but are also beneficial at low concentrations (Kocaçalışkan and Terzi 2001; Akin and Kocacaliskan 2016; Milewska-Hendel et al. 2017). Bamel and Gupta (2022) have shown that low levels (10–9 and 10–8 M) of juglone promoted rhizogenesis in tomatoes. Solanum lycopersicum L. plants, treated with juglone (10–4 M), increased the development of adventitious roots, resulting in visibly longer trichomes. The results show that if the concentration is not lethal and possibly the duration of action is not too long, juglone can have a positive effect on plant growth (Milewska-Hendel et al. 2017).
The micro-shoots of S. cretica subsp. kutahyensis also successfully established roots in a modified MS medium. The combination of modified MS medium with juglone, 10–7 M juglone showed a higher response in root length (6.28 cm) and root number (7.00; Table 2, Fig. 3). The rooting rate has been determined as 100% in all Mod MS treatments. According to the results in Table 2, Mod MS medium impacted the rooting parameters of the plant. According to the data analysis results, a statistically significant difference was found between Mod MS and MS medium in terms of root length (F = 93.1329; p < 0.05) and root number (F = 23.1840; p < 0.05) (Table 3). The success of plant tissue culture technology in propagation has been greatly influenced by the chemical composition of the in vitro culture medium (Nas and Read 2004; Akın et al. 2014). Therefore, we compared the effect of MS and Mod MS media on the rooting of seedlings, and the results showed that the Mod MS medium was superior to the MS medium. The modified rooting medium contains higher nitrogen and calcium than the MS medium. In a study conducted by Baba (1995) on Stachys tmolea Boiss., which is endemic to Turkey and economically important, 0.04 NAA/2 BA hormones were added to the modified MS (No. 2) nutrient medium obtained by revising inorganic and organic substances, and it has been shown that it is a suitable nutrient medium for rooting. Nitrogen significantly affects plant growth, cell number, and cell size among the mobile elements. It is essential for the molecular structure of nucleic acids, proteins, and some hormones. Nitrogen is also essential in improving tissue growth in cultures (Wu et al. 2005; Akın et al. 2014). Also, calcium is a nutrient element that mediates signal transmission of many hormones, especially auxin, responses to various stress factors, the development of plant and growth, and especially root morphogenesis (Wu et al. 2005; Sathyanarayana and Varghese Dalia 2007; Zhang et al. 2020). The root system plays an essential role in absorbing nutrients and water from the soil, which are vital to the survival of plants (Zhang et al. 2020). Additional vitamin requirements of plants vary according to the type of plant and type of culture. Experimentation shows that some vitamins can be removed from recommended mediums or their concentrations can be changed (George et al. 2008). Our study showed that the optimum growth rate of S. cretica subsp. kutahyensis root formation on MS salts requires the addition of thiamine with a fivefold increased amount. In addition, the level of nicotinic acid was reduced to half that used by Murashige and Skoog (1962). Consequently, an optimal concentration of root growth increasing the effect of juglone, vitamins, and macronutrients can cause roots to form in S. cretica subsp. kutahyensis.
Rooted plants were transplanted to the soil by adapted external conditions. The transfer of S. cretica subsp. kutahyensis plants, adapted to the soil and field conditions (Tavşanlı/Nusretler), successfully carried out, and the survival rate of regenerated plantlets transferred to the soil was 92%.
Conclusion
The in vitro plant tissue culture techniques reported here are an important tool for rapidly multiplying rare, endemic, endangered, and medicinal plant species under threat. This study investigated the possibilities of plant regeneration through tissue culture technology. In conclusion, 10–6 M and even 10–7 M juglone may be used as a component of MS medium in tissue culture for efficient propagation of the plant species studied, which are under extinction risk. Our results concluded that Mod MS medium is more effective on root parameters than standard MS medium, and juglone was found to increase root growth at both 10–6 and 10–7 M concentrations. The amount of juglone to be applied in the right proportions in Mod MS medium increases plant root parameters. Conclusively, this study describes a proper and straightforward regeneration protocol for the first time for this species.
Data Availability
The data that support the findings of this study are available on request from the corresponding author.
References
Ahmed R, Anis M (2014) Rapid in vitro propagation system through shoot tip cultures of Vitex trifolia L.-an important multipurpose plant of the Pacific traditional Medicine. Physiol Mol Biol Plants 20:385–392. https://doi.org/10.1007/s12298-014-0233-7
Akbar MA, Roy SK (2006) Effects of liquid medium on rooting and acclimation of regenerated microshoots of Banana (Musa sapientum L.) cv. Sagar. Plant Tissue Cult Biotech 16(1):11–18. https://doi.org/10.3329/ptcb.v16i1.1100
Akçiçek E (2010) A new subspecies of Stachys cretica (section Eriostomum, Lamiaceae) from Turkey. Turk J Bot 34(2):131–136. https://doi.org/10.3906/bot-0911-225
Akçiçek E, Güner Ö (2022) A new subspecies of Stachys cretica (Lamiaceae) from western Turkey. Phytotaxa 539(3):257–264. https://doi.org/10.11646/phytotaxa.539.3.4
Akın B (2020) Tissue culture techniques of medicinal ve aromatic plants: History, cultivation and micropropagation. J Sci Rep - A 45:253–266
Akın B, Kocaçalışkan İ (2011) In vitro propagation of Arabis drabiformis Boiss. (Brassiaceae) an endemic rare species of Uludağ mountain (Bursa-Turkey). Afr J Biotechnol 10:18356–18361. https://doi.org/10.5897/AJB11.2831
Akın B, Kocaçalışkan I, Güleryüz G (2014) Micropropagation of Erodium sibthorpianum subsp. sibthorpianum, an endemic threatened species of Uludağ Mountain (Bursa-Turkey). Turkish J Botany 38(1):148–155. https://doi.org/10.3906/bot-1304-24
Akin B, Kocacaliskan I (2016) Effect of juglone on seed germination and seedling growth of endemic species Aubrietaolympica and Arabisdrabiformis in tissue culture conditions. Phyton Annales Rei Botanicae 56(1):121–128. https://doi.org/10.12905/0380
Anish NP, Dan M, Bejoy M (2008) Conservation using in vitro progenies of the threatened ginger- Boesenbergia pulcherrima (Wall.) Kuntze. Int J Bot 4(1):93–98. https://doi.org/10.3923/ijb.2008.93.98
Arasoglu T, Mansuroglu B, Derman S, Gumus B, Kocyigit B, Acar T, Kocacaliskan I (2016) Enhancement of antifungal activity of juglone (5-Hydroxy-1, 4naphthoquinone) using a poly (d, l-lactic-co-glycolic acid)(PLGA) nanoparticle system. J Agric Food Chem 64(38):7087–7094. https://doi.org/10.1021/acs.jafc.6b03309
Arasoglu T, Derman S, Mansuroglu B, Yelkenci G, Kocyigit B, Gumus B, Acar T, Kocacaliskan I (2017) Synthesis, characterization and antibacterial activity of juglone encapsulated PLGA nanoparticles. J Appl Microbiol 123(6):1407–1419. https://doi.org/10.1111/jam.13601
Baba B (1995) Ekonomik öneme sahip endemiklerin doku kültür teknikleri ile çoğaltılması. Doktora tezi, Ege Üniversitesi, Fen Bilimleri Enstitüsü, İzmir, 58 s (in Turkish)
Babaoğlu M, Gürel E, Özcan S (2002) Bitki biyoteknolojisi: I. Doku kültürü ve uygulamaları. Selçuk Üniversitesi Vakfı Yayınları, Konya (in Turkish)
Bamel K, Gupta R (2022) Juglone promotes shooting and inhibits rooting in leaf explants of in vitro raised tomato (Solanum lycopersicum L. var. Pusa Ruby) seedlings. In Vitro Cell Dev Biol - Plant 58:942–949. https://doi.org/10.1007/s11627-022-10277-6
Benabderrahim MA, Sarikurkcu C, Elfalleh W, Ozer MS, Ceylan O (2021) Phenolic composition and biological activities of Turkish endemic plant: Stachys cretica subsp. kutahyensis. S Afr J Bot 138:124–128. https://doi.org/10.1016/j.sajb.2020.12.012
Bhattacharjee R (1980) Taxonomic studies in Stachys II: A new infrageneric classification of Stachys L. Notes from Royal Botanic Garden 38(1):65–96
Chandra B, Palni LMS, Nandi SK (2006) Propagation and conservation of Picrorhiza kurrooa Royle ex Benth.: an endangered Himalayan medicinal herb of high commercial value. Biodivers Conserv 15(7):2325–2338. https://doi.org/10.1007/s10531-005-0770-z
Clark AM, Jurgens TM, Hufford CD (1990) Antimicrobial activity of juglone. Phytother Res 4(1):11–14. https://doi.org/10.1002/ptr.2650040104
Cuenca S, Amo-Marco JB, Parra R (1999) Micropropagation from inflorescence stems of Spanish endemic plant Centaurea paui Loscos ex Willk. (Compositae). Plant Cell Rep 18:674–679. https://doi.org/10.1007/s002990050641
Decker EL, Frank W, Sarnighausen E, Reski R (2006) Moss systems biology en route: phytohormones in physcomitrella development. Plant Biol 8:397–406. https://doi.org/10.1055/s-2006-923952
Díaz-Sala C (2021) Adventitious root formation in tree species. Plants 10(3):486. https://doi.org/10.3390/plants10030486
Efferth T (2019) Biotechnology applications of plant callus cultures. Engineering 5(1):50–59. https://doi.org/10.1016/j.eng.2018.11.006
Erdağ B, Emek Y (2005) In vitro micropropagation of Anthemis xylopoda O. Schwarz, a critically endangered species from Turkey. Pak J Biol Sci 8(5):691–695. https://doi.org/10.3923/pjbs.2005.691.695
Fay MF (1992) Conservation of rare and endangered plants using in vitro methods. In Vitro Cell Dev-Biol 28:1–4. https://doi.org/10.1007/BF02632183
George EF, Hall MA, de Klerk GJM (2008) The components of plant tissue culture media II. Organic additions, osmotic and pH effects, and support systems. In: George EF, Hall MA, de Klerk GJM (eds) Plant propagation by tissue culture, vol 1. The Background, Springer Dordrecht, pp 115–173. https://doi.org/10.1007/978-1-4020-5005-3_4
Goren AC, Piozzi F, Akcicek E, Kılıç T, Çarıkçı S, Mozioğlu E, Setzer WN (2011) Essential oil composition of twenty-two Stachys species (mountain tea) and their biological activities. Phytochem Lett 4(4):448–453. https://doi.org/10.1016/j.phytol.2011.04.013
Gümüşçü A, Çöçü S, Uranbey S, İpek A, Çalışkan M, Arslan N (2008) In vitro micro-propagation of endangered ornamental plant Neotchihatchewia isatidea (Boiss.) Rauschert. Afr J Biotechnol 7(3):234–238. https://doi.org/10.5897/AJB07.721
Güner Ö (2022) Stachys istanbulensis (Lamiaceae) a new species from Turkey: evidence from morphological, micromorphological and molecular analysis. Turk J Bot 46:624–635. https://doi.org/10.55730/1300-008X.2737
Güner Ö, Özdöl T, Yıldırım H (2023) A New Rupicolous Species from West of Türkiye: Stachys cuhacioglui (Lamiaceae). Türler ve Habitatlar 4(2):98–109. https://doi.org/10.53803/turvehab.1339346
Işık K (2011) Rare and endemic species: why are they prone to extinction? Turk J Bot 35(4):411–417. https://doi.org/10.3906/bot-1012-90
Işıkalan Ç, Orcan P, Akbaş F, Namlı S, Kuru İS, Buluş Ş (2020) Effect of cytokinins on in vitro propagation of Ajuga xylorrhiza Kit Tan (Critically Endangered), Endemic to Turkey. In Vitro Cell Dev Biol-Plant 56:911–914. https://doi.org/10.1007/s11627-020-10082-z
IUCN (2022) The IUCN Red List Guidelines version 2022–15.1. https://www.iucnredlist.org. Cited July 2022
JMP (2005) JMP SAS Statistical Analysis System. Cary, North Carolina, USA
Khanavi M, Hajimahmoodi M, Cheraghi-Niroomand M, Kargar Z, Ajani Y, Hadjiakhoondi A, Oveisi MR (2009) Comparison of the antioxidant activity and total phenolic contents in some Stachys species. Afr J Biotechnol 8(6):1143–1147
Khawar KM, Ozel CA, Ulug A, Sur I, Kızılates E, Uzuntas F, Arslan O (2008) In vitro adventitious shoot proliferation of İsatic aucheri Boiss. (Turkish Woad) from petiole explants. Res J Agric Biol Sci 4(4):327–330
Kocaçalışkan İ, Bingöl NA (2017) Biyoistatistik. Nobel Akademik Yayıncılık, Ankara (in Turkish)
Kocaçalışkan I, Terzi I (2001) Allelopathic effects of walnut leaf extracts and juglone on seed germination and seedling growth. J Hortic Sci Biotechnol 76(4):436–440. https://doi.org/10.1080/14620316.2001.11511390
Mayerni R, Warnita A, Chan SROS (2020) Direct organogenesis in local clones of Patchouli Plant (Pogostemon cablin Benth) in vitro. Indones J Crop Sci (JERAMI) 3(1):16–19. https://doi.org/10.25077/jijcs.3.1.16-19.2020
Milewska-Hendel A, Polak M, Sala K, Zieleźnik-Rusinowska P, Gawecki R, Kurczyńska E (2017) Morpho-histological analysis of tomato (Solanum lycopersicum L.) plants after treatment with juglone. Acta Agrobot 70(2):1701. https://doi.org/10.5586/aa.1701
Miyazaki J, Tan BH, Errington SG (2010) Eradication of endophytic bacteria via treatment for axillary buds of Petunia hybrida using Plant Preservative Mixture (PPM™). Plant Cell Tiss Organ Cult 102:365–372. https://doi.org/10.1007/s11240-010-9741-5
Moola AK, Kumari BDR (2019) Direct regeneration of plantlets from shoot tip explants of a vulnerable medicinal plant – Celastrus paniculatus Willd. J Applied Horticult 21(3):189–194. https://doi.org/10.37855/jah.2019.v21i03.32
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15(3):473–497. https://doi.org/10.1111/j.1399-3054.1962.tb08052.x
Nadeem M, Palni LMS, Purohit AN, Pandey H, Nandi SK (2000) Propagation and conservation of Podophyllum hexandrum Royle: an important medicinal herb. Biol Conserv 92:121–129. https://doi.org/10.1016/S0006-3207(99)00059-2
Nas MN, Read PE (2004) A hypothesis for the development of a defined tissue culture medium of higher plants and micropropagation of hazelnuts. Sci Hortic 101:189–200. https://doi.org/10.1016/j.scienta.2003.10.004
Perica MC (2003) In vitro propagation of Centaurea rupestris L. Acta Biol Cracov Bot 45(2):127–130
Ram K, Shekhawat NS (2011) Micropropagation of commercially cultivated Henna (Lawsonia inermis) using nodal explants. Physiol Mol Biol Plants 17:281–289. https://doi.org/10.1007/s12298-011-0069-3
Ramabulana AT, Steenkamp PA, Madala NE, Dubery IA (2021) Application of plant growth regulators modulates the profile of chlorogenic acids in cultured Bidens pilosa Cells. Plants (basel) 10(3):437. https://doi.org/10.3390/plants10030437
Rao NK (2004) Plant genetic resources: Advancing conservation and use through biotechnology. Afr J Biotechnol 3(2):136–145. https://doi.org/10.5897/AJB2004.000-2025
Salehi P, Sonboli A, Asghari B (2007) Chemical composition of the essential oil of Stachys acerosa and its antibacterial and antioxidant activities. Chem Nat Compd 43(3):339–341. https://doi.org/10.1007/s10600-007-0126-x
Sathyanarayana BN, Varghese Dalia B (2007) Plant Tissue Culture: Practices and New Experimental Protocols. IK International Publishing House, New Delhi, India
Senapati SK, Aparajita S, Rout GR (2013) Micropropagation and assessment of genetic stability in Celastrus paniculatus: An endangered medicinal plant. Biologia 68:627–632. https://doi.org/10.2478/s11756-013-0187-1
Sudheer WN, Praveen N, Al-Khayri JM, Jain SM (2022) Role of plant tissue culture medium components. In: Kumar A, Modi A, Singh M (eds) Rai A C. Advances in plant tissue culture, Academic Press, pp 51–83
Sudhersan C, AboEl-Nil M, Hussain J (2003) Tissue culture technology for the conservation and propagation of certain native plants. J Arid Environ 54:133–147. https://doi.org/10.1006/jare.2001.0884
Tomou EM, Barda C, Skaltsan H (2020) Genus Stachys: A review of traditional uses, phytochemistry and bioactivity. Medicines 7(10):63. https://doi.org/10.3390/medicines7100063
Tundis R, Peruzzi L, Menichini F (2014) Phytochemical and biological studies of Stachys species in relation to chemotaxonomy: a review. Phytochemistry 102:7–39. https://doi.org/10.1016/j.phytochem.2014.01.023
Uyanık M (2017) Türkiye’de tehlike altindaki bazi endemik Salvia türlerinin in vitro çoğaltimi ve tarla şartlarina adaptasyonu. Doktora tezi, Ankara Üniversitesi, Fen Bilimleri Enstitüsü, Ankara, 77 (in Turkish)
Wala BB, Jasrai YT (2003) Micropropagation of an endangered medicinal plant: Curculigo orchioides Gaertn. Plant Tissue Culture 13(1):13–19
Wochok ZS (1981) The role of tissue culture in preserving threatened and endangered plant species. Biol Conserv 20(2):83–89. https://doi.org/10.1016/0006-3207(81)90019-7
Wu Y, Yi G, Yang H, Zhou B, Zeng J (2005) Basal medium with modified nitrogen source and other factors influence the rooting of banana. HortScience 40:428–430. https://doi.org/10.21273/HORTSCI.40.2.428
Yuniastuti E, Widodo CE, Delfianti MNI (2018) Effect of benzyl amino purine and indole-3-acetic acid on propagation of Sterculia foetida in vitro IOP Conf Ser. Earth Environ Sci 142:012011. https://doi.org/10.1088/1755-1315/142/1/012011
Zhang XP, Ma CX, Sun LR, Hao FS (2020) Roles and mechanisms of Ca2+ in regulating primary root growth of plants. Plant Signal Behav 15(5):1748283. https://doi.org/10.1080/15592324.2020.1748283
Funding
No funds, grants, or other support were received.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interests
The authors have no relevant financial or non-financial interests to disclore.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Akın, B., Bingöl, N.A. & Bulman, G.C. A first approach for the micropropagation of threatened endemic subspecies of Stachys cretica subsp. kutahyensis. Biologia 79, 1653–1661 (2024). https://doi.org/10.1007/s11756-024-01640-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11756-024-01640-6