Abstract
Studies of Hirudinea species infesting elasmobranchs are scarce and scattered worldwide and they are inexistent off the Tunisian waters. In this work, we aimed to assess the occurrence of these parasites on two ray species in the Gulf of Tunis (Tunisia). Between 2017 and 2021, 254 fish specimens belonging to two Torpedinidae species (marbled electric ray, Torpedo marmorata Risso, 1810, and common torpedo, Torpedo torpedo (Linnaeus, 1758)) were examined for leech infestation. Morphological and molecular characterizations based on 18S rDNA and cytochrome c oxidase (COI) sequences allowed us to identify two leech species: Pontobdella muricata (Linnaeus, 1758) and a new Branchellion Savigny, 1822 species Branchellion tunisensis sp. n. This new species (B. tunisensis) exhibited distinctive traits unlike other Branchellion, including a transparent body marred with dark green along the abdomen and neck, long trachelosome (about 1/3 of the length of the body), distinctive two eye spots, the presence of a pair of lateral branchiae per somite except for the last 2 somites and cup-shaped posterior sucker. Molecular analysis of the 18S rDNA and COI gene fragments shows more than 10% divergence of B. tunisensis from other Branchellion spp. and high identity score of P. muricata with other sequenced isolates. This work allowed us to report the presence of P. muricata for the first time off the Tunisian coasts and to identify a new marine leech species parasitizing electric rays in the Gulf of Tunis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Leeches (Annelida: Clitellata: Hirudinea) (Sawyer 1986) are common worldwide (Yamauchi et al. 2008). Many leeches are blood-sucking on vertebrates or invertebrates, and the remaining are largely predators but rarely scavengers. Most of them inhabit freshwater, although there are marine and terrestrial species as well (Başusta et al. 2015). Hirudinea is a monophyletic group that includes 14 families (Sket and Trontelj 2008). Among these families, Piscicolidae and Glossiphoniidae are known to parasite predominantly freshwater or marine fishes (Govedich et al. 2004). Hirudinea is the only group of annelids known to include species that parasitize elasmobranchs (Caira and Healy 2004) and such parasitism is restricted to marine leeches of the family Piscicolidae (Benz and Bullard 2004; Utevsky and Trontelj 2004). In fact, elasmobranchs (sharks and rays) are host to a great variety of parasites in nature, particularly helminths (Merlo-Serna and García-Prieto 2016). More than 1500 helminth species including several leech species of the family Piscicolidae have been recorded in association with these hosts worldwide (Caira et al. 2012).
These parasites can potentially affect the health of fish in a variety of ways. Indeed, they feed on blood from sharks and rays and are known to be vectors of hematozoa (Caira and Healy 2004; Utevsky and Trontelj 2004). Moreover, marine leeches’ damage on areas of feeding or attachments on the fish reduces the economic value of the demanded fish. They act as vectors of potentially pathogenic organisms for the fish hosts (Bulguroğlu et al. 2014). Thus, marine leeches, especially those with parasitic habits, are important components of the ecosystem (Wunderlich et al. 2011). Investigations on these parasites from the marine environment remain relatively scarce and geographically scattered (Bakopoulos and Ksidia 2014). In Tunisia, the Gulf of Tunis provides an important habitat for Torpedinid ray species like Torpedo marmorata and T. torpedo (Azouz 1973; Hattour 2000; Zarrad et al. 2000). However, to our knowledge marine leeches have not previously been investigated in Tunisian waters. Thus, the aim of this work was to assess the occurrence of these parasites on the two most common ray species in the Gulf of Tunis.
Materials and methods
Fish sampling and identification
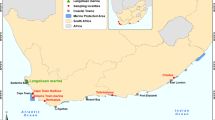
Between September 2017 and September 2021, 254 representatives of the family of Torpedinidae namely common torpedo Torpedo torpedo (Linnaeus, 1758) and marbled electric ray Torpedo marmorata Risso, 1810, were collected bi-monthly from fishermen working in the Gulf of Tunis (136°49’2.99” N, 10°18’10.80” E) as soon as they entered the bay of “La Goulette” city (Fig. 1; Table 1). Then, they were transported in ice to the laboratory where they were examined on the same day. The different host species were identified according to Fisher et al. (1987) and Séret (2006). Host nomenclature is according to Froese and Pauly (2023).
Study area
The Gulf of Tunis is characterized by rugged relief, bristling with shoals and reefs (Azouz 1973). The average salinity is around 37‰ throughout the year (Hattour 2000). The average surface temperature is between 13 °C in winter and 28 °C in summer (Zarrad et al. 2000). At a depth of 60 m, it drops and reaches 4 °C (Zarrad et al. 2000). In fact, this geographical area seems to be the favorite spot for Torpedinidae species with a fairly high abundance in comparison with the other gulfs in Tunisia.
Parasite extraction and morphology
The leech species were transported alive along with their freshly captured hosts to the research laboratory. They were picked up from the dorsal and ventral surfaces of their hosts and observed under a stereo microscope. Then, they were preserved in 70% ethanol without relaxation. Subsequently, the fixed parasites were examined again under a stereo microscope. Length and width, diameters of oral and caudal suckers of parasites were measured. Annulation on the body surface of parasites was separated as a1, a2 and a3 annulus. After, somites on these annuli were examined in terms of morphological characteristics and then these somites were counted according to dorsal, lateral and ventral regions. Parasites morphometry and identification follow Richardson (1949), and Llewellyn (1966) keys.
DNA extraction, amplification, and sequencing
Genomic DNA from leech specimens was extracted from the caudal sucker using a DNeasy Blood & Tissue Kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s instructions. Briefly, fragments from three specimens of each leech species were macerated and transferred in a sterile Eppendorf tube containing 200 µl of lysis buffer and 20 ul of proteinase K (20 mg/ml). The mixture was incubated at 56 °C for 2 to 4 h until the tissue was compltetely lysed. The DNA was purified and washed as recommended by the manufacture. Quantification of the DNA was done with a Nano Drop (ND-1000, USA). DNA concentrated at 50–100 ng was preserved at -20 °C until use.
For PCR amplification, different universal primers were used for screening Helminth organisms. These primers were used to target partial fragments of the 18S rDNA, ITS and COI region. For the 18S rDNA, two couple of primers were used to amplify this region:
-
1 F:5′-TACCTGGTTGATCCTGCCAGTAG‐3′, 5R:5′-CTTGGCAAATGCTTTCGC‐3′ (Giribet et al. 1996);
-
3 F:5′-GTTCGATTCCGGAGAGGGA‐3, ′9R: 5′‐GATCCTTCCGCAGGTTCACCTAC‐3′ (Giribet et al. 1996).
For ITS sequence amplification, we have used forward ITS-1-F: 5’-TTTCCGTAGGTGAACCT-3’ (Cunningham 1997) and reverse ITS-2-R: 5’-GGTAATCACGCTTGAATC-3’ (Matejusová et al. 2001).
The COI sequence was amplified using the universal primers LCO1490: 5′-GGTCAACAAATCATAAAGATATTGG-3′ and HCO2198: 5′-TAAACTTCAGGGTGACCAAAAAATCA-3′ (Folmer et al. 1994).
PCR conditions were as follows: initial denaturation at 95 °C for 5 min, followed by 35 cycles of 94 °C for 20 s, with an annealing temperature of 50–54 °C for 20–60 s, and an extension step of 72 °C for 2 min and a final extension of 72 °C for 10 min. The PCR products were subjected to sequencing by the commercial sequencing company (Macrogen Inc., Seoul, South Korea) for both directions using the same PCR primers. The obtained sequences were deposited in GenBank and used to query similar sequences using the Basic Local Alignment Search Tool (BLAST) tool. Sequences were selected for alignment using the Clustal x2.1.0.12 program applying the default parameters (Larkin et al. 2007).
Phylogenetic analyses were performed with Maximum Likelihood (ML) and Neighbor Joining using the Mega V7 (Kumar et al. 2016). The ML method was conducted based on the General Time Reversible model with Gamma distributed rate, and Invariant sites (GTR + G + I) determined using ModelTest (Darriba et al. 2012). Nodal support was obtained for a bootstrap analysis with 1,000 replicates. All distance values among the dataset sequences were calculated using the MEGA V7. Genetic divergences between sequences were conducted using the Kimura 2-parameter model (Kimura 1980). The rate variation among sites was modeled with a gamma distribution (shape parameter = 5). The analysis involved 20 nucleotide sequences of 18S and 16 sequences of COI. All positions with less than 95% site coverage were eliminated. That is, fewer than 5% alignment gaps, missing data, and ambiguous bases were allowed at any position.
Statistical analysis
Rates of infestation were evaluated using statistical indices frequently used in parasite ecology. The terms prevalence (P%), mean intensity (MI) and abundance (A) were used as defined by Margolis et al. (1982) and modified by Bush et al. (1997).
Statistical analyses were carried out using excel 2020 software. Prevalence of parasite infection was compared among all four seasons using a Chi-square test. Statistical significance was accepted when p ≤ 0.05.
Results
The examination of two Torpedinidae species (marbled electric ray, Torpedo marmorata and common torpedo, T. torpedo ) from the Tunisian coast allowed us to collect two Hirudinea species: Branchellion tunisensis sp. n. and Pontobdella muricata (Linnaeus, 1758) (Table 1).
General morphological description
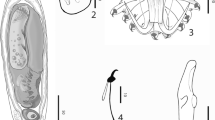
Branchellion tunisensis sp. n. (Fig. 2)
Diagnosis. Transparent body with dark green stains along the abdomen and neck. Long trachelosome, about 1/3 of the length of the body. One pair of lateral branchiae per somite except the last two somites. The presence of two distinguishable eye spots on the anterior sucker and the circular concave almost cup-shaped posterior sucker.
Description
External morphology (based on 5 specimens)
Total length 16–23 mm; maximum width (excluding branchiae) 1.75–2.8.
Regarding the color, when pulled, the body is transparent with some stains of dark green colors along the abdomen and neck (Fig. 2a). At rest, these parts become darker. The clittelar region is transparent. As for the two suckers, they are transparent except for the capules (secondary suckers) which have a darker color. The skin of the body is smooth without any tubercules. The body is distinctly divided into trachelosome and urosome and 2 suckers (Fig. 2a).
The anterior sucker is flat, disc-like shaped and is directed ventrally, its width is equal to the maximum width of the abdomen. On the outer side, two elongated eye spots are clearly separated from each other and covered with brownish pigments and are visible at 2/3rd of the length of the sucker (Fig. 2b). Circular and flattened secondary suckers cover its ventral side.
The urosome terminates in a large and ventrally directed sucker. The posterior sucker is centrally attached, circular concave almost cup-shaped and exactly terminal, with membrane-like papillae at its external extremities and circular secondary suckers, of the same shape as on the anterior sucker, at its ventral side (Fig. 2c).
The trachelosome is fusiform, relatively of the same width as the urosome and is about 1/3 of the length of the body. It is distinctly divided into a head, including the eccentrically attached anterior sucker and multiple somites.
The clittelar region counts two segments smaller in width than the neck. It presents a construction separating the last somite of the trachelosome and the first somite of the urosome.
The urosome is distinctly annulated, more conspicuously on the dorsal side. It is slightly conical where the segments widen gradually from the first somite. The different abdominal somites are approximately the same length except for the first two, thinner and smaller. The urosome consists throughout its length of gill-bearing somites, each final annuli bearing a pulsatile vesicle at the base of the branchia on either side except the last two somites that do not bear any gill. Each branchia has a broad proximal region while their anterior region is more rounded.
Type-host: Torpedo torpedo (Linnaeus, 1758). English common name: Common torpedo.
Type-locality: La Goulette (Gulf of Tunis) (136°49’2.99” N, 10°18’10.80” E), Tunisia.
Type-material. A holotype (Fig. 2a) was deposited in the parasitological collection of the Zoology Department Museum, College of Science, King Saud University, Riyadh, Saudi Arabia, with the number (LeeTun221018). Partial sequences of the 18S rDNA- ITS and COI were deposited in GenBank under the accession numbers OR519898 and OR506480 respectively.
Prevalence: 8.03% (9/112)
Number of collected specimens: 11
Site on host: Dorsal and ventral surface.
Etymology. The name of this leech is related to Tunisia and to the Gulf of Tunis.
Remarks. The external diagnostic characters proved that B. tunisensis sp. n. is different from the other known Branchellion species (see discussion).
Pontobdella muricata (Linnaeus, 1758) (Fig. 3)
The morphological features of the collected specimens of P. muricata (Fig. 3) during this study are in accordance with the description of Sawyer (1986). The size of the collected specimens varies between 4 and 4.7 cm long and the maximum width, the urosome, varies between 0.8 and 1 cm. The trachelosomal region compromises about ¼ of the total length, while the urosomal region compromises the remaining ¾ of the total length.
The upper part (trachelosome and clitellum) of the body along with the two suckers of this species have a yellow color while the abdomen has a brown or olive green color (Fig. 3).
The body is fusiform, narrowing gradually towards its ends (Fig. 3). It is clearly divided into five regions: the oral sucker, the trachelosome, the clitellum (with the genital openings), the urosome and the posterior sucker. The head region counts segments I-VI, the neck region segments VII-IX and the clitellum segments X-XII. Segmentations of the abdomen region are not well visible on the harvested specimens.
Pontobdella muricata have no eyes. The anterior sucker is cup-shaped (Fig. 3) and attached eccentrically so that the dorsal surface is longer than the ventral. It presents on its edges a rounded marginal fringe. This sucker is significantly wider than the anterior part of the leech, measuring 3.2 mm. At the level of the mouth, there are no jaws but an exsertile trunk. There are no visible gills. The posterior sucker is very deeply cupped and is a little smaller than the anterior sucker measuring 3 mm.
The skin is rough and strongly warty (Fig. 3), and the rings have tubercles or globular papillae, ending in weaker points on the 3rd ring of each segment. The summit of the tubercles bears stiff projections like thorns and arranged in a circle.
Molecular characterization
Partial sequences of the 18S rDNA gene and cytochrome c oxidase subunit I (COI) were obtained for each species. For Pontobdella muricata, the obtained sequences of 18S rDNA (1462 bp) and COI (629 bp) were submitted in GenBank under accession numbers OR500914 and OR501021, respectively. These two sequences show 99.7% and 99.52% of identity with P. muricata (accession number KY659070) reported by Bottari et al. (2017) from the Tyrrhenian Sea on the brown ray, Raja miraletus (Linnaeus, 1758), and the spotted ray, R. montagui (Fowler, 1910) (Table 2). For Branchellion tunisensis sp. n. the partial sequences of 18S rDNA-ITS2 (1564 bp) and COI (678 bp) were also submitted to GenBank under the accession numbers OR519898 and OR506480, respectively. Both sequences showed a low degree of identity with already published sequences in GeneBank. The highest identity was obtained with Branchellion lobata Moore, 1952 with 87.9% for the 18S rDNA (accession number OX387249) and 89.38% for COI (GenBank Entry, OX387263). A slightly lower identity was observed also with Branchellion parkeri Richardson, 1949 (89.17%) for the COI gene.
Phylogenetic analysis inferred by maximum likelihood based on 18S rDNA (Fig. 4a) and COI (Fig. 4b) gives trees with similar topology. Both trees show clustering of the collected P. muricata with the sequenced P. muricata isolates (GenBank entries, KY659070 and AY336029) and two Stibarobdella species with strong bootstrap support. The second species, B. tunisensis sp. n. cluster with other seven Brachellion spp., including B. lobata, B. torpedinis with high nodal support.
Phylogenetic trees of the 18S rDNA (a) and the COI (b) genes based on Maximum Likelihood method showing the relative positions of the two newly sequenced species Branchellion tunisensis sp. n. and Pontobdella muricata (in bold) with related Piscicolidae species. Node labels show the bootstrap support of the consensus trees
Parasitological indexes of the collected species
The analysis of the parasitological indexes of the collected leeches shows that the prevalence of B. tunisensis sp. n. (P = 8.03%) is higher than the prevalence of P. muricata (P = 3.52%) (Table 1).
The prevalence of B. tunisensis increased during winter (P = 16%) and spring (P = 19.35%) while the prevalence of P. muricata increased during autumn (P = 3.33%) and winter (P = 13.33%) (Table 3).
No significant differences were detected between the four seasons in the prevalence of P. muricata (Table 3). However, we noted a significant difference between autumn/winter, Autumn/spring, winter/spring and spring/summer in the prevalence of B. tunisensis (Table 3). Furthermore, B. tunisensis is the only species to possess a mean intensity higher than 1 (MI = 1.2) recorded during the spring season.
Macroscopic observation of lesions caused by the Hirudinea species on their hosts
Both B. tunisensis sp. n. and P. muricata were fixed on the dorsal and the ventral surfaces of their respective hosts. During the extraction, we were able to notice the occurrence of many lesions near the edges of both body surfaces of their hosts (Fig. 5).
The trauma left by B. tunisensis was much less conspicuous macroscopically with no hemorrhage or lesion. While the trauma left by P. muricata on T. marmorata was more evident. In fact, skin petechias, hemorrhages and swelling were observed in the attachment site of this leech species.
After the detachment of the leech a roundish shallow trauma is observed and two typical lesions on the epidermal tissues of T. marmorata were found. The major lesion was caused by oral sucker and the minor lesion was induced by caudal sucker of leeches (Fig. 5). On the anterior surface of the skin, we noted the presence of hemorrhage where the parasites were fixed with no evidence of the lesion reaching the muscle tissue.
Discussion
Leech species are common on chondrichthyans, and many species have been reported from the skins of sharks and rays including thornback ray (Raja clavata Linnaeus, 1758), sand-tiger shark (Carcharias taurus Rafinesque, 1810) and Argentina angel shark (Squatina argentina Marini, 1930) from various locations such as the Indian Ocean, the Atlantic Ocean, Japan, and southern Brazil (Oka 1910; Soto 2000; Wunderlich et al. 2011).
During our work, we were able to characterize a new leech species B. tunisensis sp. n. found on Torpedo torpedo and to record the occurrence of Pontobdella muricata on T. marmorata. Indeed, T. marmorata is well known to host Branchellion torpedinis Savigny, 1822 (Narváez and Osaer 2017) and P. muricata Linnaeus, 1758 (Saglam et al. 2003; Bolognini et al. 2017).
The Pontobdellinae (Piscicolidae) encompasses the majority of marine leeches, which are characterized by a large size, the presence of tubercles on their body, and parasitism on Chondrichthyans (Utevsky et al. 2007). Pontobdella muricata is an ectoparasite of benthic elasmobranch species rarely found on bony fish (Minelli 1979). It was reported from several host species, namely Dasyatis pastinaca (Linnaeus, 1758) (Bakopoulos and Ksidia 2014; Başusta et al. 2015), Dipturus batis (Linnaeus, 1758) (Olsson 1876), Myliobatis aquila (Linnaeus, 1758) (Bolognini et al. 2017), Raja clavata Linnaeus, 1758 (Saglam et al. 2003; Oktener and Utevsky 2010; Gaevskaya 2012; Bakopoulos and Ksidia 2014; Bolognini et al. 2017), R. miraletus Linnaeus, 1758, R. montagui Fowler, 1910 (Bottari et al. 2017), and T. marmorata (Saglam et al. 2003; Bolognini et al. 2017). This species presents a large geographical distribution in the East banks of the Mediterranean (Greece and Turkey) (Saglam et al. 2003; Oktener and Utevsky 2010; Gaevskaya 2012; Bakopoulos and Ksidia 2014), in the Adriatic Sea (Minelli 2008; Bolognini et al. 2017) and in the Tyrrhenian Sea (Bottari et al. 2017). However, this is its first record in the Tunisian coasts.
Branchellion Savigny, 1822 species are parasites of rays and sharks (Pauls and Provenzano 1999). These species are distributed in distinct marine realms (Sawyer 1986): B. angeli Sigalas, 1921 in the South-Western Indian Ocean and North Atlantic; B. borealis Leigh-Sharpe, 1933 known only in the English Channel; B. lobata Moore, 1952 in the Eastern Pacific; B. parkeri Richardson, 1949 in the Indo-Pacific; B. plicobranchus Sanjeeva Raj, 1954 in the Indian Ocean; B. ravenelii (Girard, 1850) in the Western Atlantic, B. spindolaorum in the Eastern Pacific (Ruiz-Escobar and Oceguera-Figueroa 2019), the type-species B. torpedinis Savigny, 1822 in the Atlantic (Ruiz-Escobar and Oceguera-Figueroa 2019) and B. brevicaudata in the West Pacific (Jimi et al. 2023). Among the nine known species of this genus (Jimi et al. 2023), only one species (B. torpedinis) was found in the Mediterranean (Başusta et al. 2015).
The molecular characterization of B. tunisensis sp. n. based on the 18S rDNA and COI did not show identity with any of the deposited leech sequences. The most related sequences belong to other Branchellion spp. and exhibited a high genetic divergence (more than 10%) with other published sequences of Branchellion spp. including B. lobata which shows less than 89% of identity for the 18S rDNA and 89.38% for the COI with almost 100% coverage, and B. pakeri with lower than 89% of identity for the COI gene and less than 70% coverage for the 18S rDNA. This result supports the membership of this new species to the genus Branchellion and its divergence from all the species sequenced till now. Moreover, we noted some morphological characteristics of this new species that differentiate it from the most related Branchellion spp., mainly B. lobata and B. pakeri. According to Richardson (1949), B. pakeri has 31 pairs of fully developed gills (although a minute reduced lobe represents a gill on xiii a2), and is unique in the possession of only 10 pairs of pulsatile vesicles, while the abdomen of B. tunisensis sp. n. consists throughout its length of gill-bearing somites, with a pulsatile vesicle at the base of the gill on either side except for the last two segments that are not bearing any gill or pulsatile vesicles. On the other hand, B. lobata is characterized by a short trachelosome and a much longer urosome, the branchiae leaf-like morphology and the caudal sucker heart-like morphology (Moore 1952) whereas B. tunisensis is characterized by a long trachelosome (1/3 of the body length) and the oral sucker counts secondary suckers, the posterior sucker is circular concave and almost cup-shaped and the branchiae are characterized by a broad proximal region and a rounded anterior region. Regarding the eye spots, the anterior sucker of B. parkeri bears two transverse, band-like, black pigment patches, one on either side of the mid-dorsal line, and several irregular radiating areas marked by a black pigment (Richardson 1949), B. lobata has no distinct eye spots but the presence of a pair of irregular groups of ocellar elements (Moore 1952), while on the posterior sucker of B. tunisensis two rectangular lateral eye spots are observed. Moreover, B. parkeri is mostly known in New Zealand (Richardson 1949; Hewitt and Hine 1972) and in Tasmania (Ingram 1957) attacking Callorhinchidae, Rajidae, and Triakidae species and B. lobata has a wide geographic distribution in the Eastern Pacific (Ruiz-Escobar and Oceguera-Figueroa 2019).
Pontobdella muricata was mainly attached to the lower part of the disk of T. marmorata both on the ventral and dorsal areas. Our results are in concordance with Bolognini et al. (2017) who proved that the attachment site of P. muricata varies with the host and that the tail/disc area is the preferred site of attachment of this leech on T. marmorata. On the other hand, B. tunisensis sp. n. was fixed on the upper and lower part of the disk of T. torpedo on both ventral and dorsal areas and it did not show any preferences for a special site of fixation. Marine leech species are well known to inflict damage on areas of feeding or attachments on the fish and to play an important role as vectors of potentially pathogenic organisms for their hosts (Bulguroğlu et al. 2014). The examination of feeding sites of P. muricata has disclosed skin petechiae, hemorrhage, and swelling consistent with the results of Sawyer (1986), Saglam et al. (2003), Bakopoulos and Ksidia (2014), and Bolognini et al. (2017). According to Bakopoulos and Ksidia (2014), the lesions induced by this parasite consist of rounded wounds with red exposed muscles in the center. On the other hand, the lesion left by B. tunisensis on T. torpedo was less visible with no notable hemorrhage.
The study of parasitological indices shows that the two leech species exhibited relatively high infestation rates. However, B. tunisensis sp. n. presented a higher prevalence and mean intensity (P = 8.03%; MI = 1.2) than P. muricata (P = 3.52%; MI = 1). This may be due to the difference in size of the two species and the impact induced by these two species on their hosts. Indeed, B. tunisensis is smaller in size than P. muricata which can induce a less intra-specific competition for food and space. Moreover, we noted that the indices of increased B. tunisensis during winter and spring while those of P. muricata increased during autumn and winter. According to Bolognini et al. (2017), this peak corresponds with the reproduction period of the hosts. Indeed, according to Quignard and Capapé (1974), the mating period of T. torpedo occurs from January to May in Tunisian waters, while it occurs at the end of autumn and in the winter for T. marmorata (Capapé 1979). However, B. tunisensis was also found during the autumn season and it exhibited a slightly higher mean intensity (MI = 1.2) recorded during the spring season. These results may suggest that this species has a longer period of fixation on its host.
The present study allowed us to report for the first time the presence of P. muricata along the Tunisian coasts and to identify a new species of Branchellion, increasing the number of known species belonging to this genus to ten. The preliminarily morphological identification and the molecular characterization of the collected specimens confirm that B. tunisensis sp. n. is a new species. In addition, it seems very interesting to study the presence of these parasite species on other host species and from different locations along the Tunisian coast. Indeed, our findings prove that our knowledge about the diversity and geographical distribution of leech species is far from being complete and studies of these parasites are still needed.
References
Azouz A (1973) Les fonds chalutables de la région nord de la Tunisie. Les potentialités De La pêche, écologie et répartition bathymétique des poissons. Bull lnst Natn Scien Tech Mer 3:1–4
Bakopoulos V, Ksidia V (2014) Pontobdella muricata infection of Raja clavata and Dasyatis pastinaca off the coast of Lesvos, Greece. J Mar Biol Assoc UK 94:405–409. https://doi.org/10.1017/S0025315413000830
Başusta N, Ilaria De Meo I, Miglietta C, Mutlu E, Olguner MT, Şahin A, Balaban A, Deval MC, Yurtseven UU, Patania A (2015) Some marine leeches and first record of Branchellion torpedinis Savigny, 1822 (Annelida, Hirudinea, Piscicolidae) from elasmobranchs in Turkish waters, with new host records. Mar Biodivers 46(3):713–716. https://doi.org/10.1007/s12526-015-0411-z
Benz GW, Bullard SA (2004) Metazoan parasites and associates of chondrichthyans with emphasis on taxa harmful to captive hosts. In: Smith M, Warmolts D, Thoney D, Hueter R (eds) The elasmobranch husbandry manual: captive care of sharks, rays and their relatives. Special Publication of the Ohio Biological Survey, Ohio, pp 325–416
Bolognini L, Leoni S, Polidori P, Grati F, Scarcella G, Pellini G, Domenichetti F, Ferrà C, Fabi G (2017) Occurrence of the leech, Pontobdella muricata Linnaeus, on elasmobranch species in the Northern and Central Adriatic Sea. J Parasitol 102(6):643–645. https://doi.org/10.1645/15-826
Bottari T, Profeta A, Rinelli P, Gaglio G, La Spada G, Smedile F, Giordano D (2017) On the presence of Pontobdella muricata (Hirudinea: Piscicolidae) on some elasmobranchs of the Tyrrhenian Sea (Central Mediterranean). Acta Adriat 58(2):225–234
Bulguroğlu SY, Korun J, Gökoğlu M, Özvarol Y (2014) The marine leech Stibarobdella moorei (Oka, 1910) (Hirudinea, Piscicolidae) parasitic on the thornback ray Raja clavata Linnaeus, 1758 and angel shark Squatina squatina (Linnaeus, 1758) in Antalya Bay, Mediterranean Sea of Turkey. Helminthologia 51(3):250–252. https://doi.org/10.2478/s11687-014-0237-4
Bush AO, Lafferty KD, Lotz JM, Shostak AW (1997) Parasitology meets ecology on its own terms: Margolis et al., revisited. J Parasitol 83:575–583
Caira JN, Healy CJ (2004) Elasmobranchs as hosts of metazoan parasites. In: Carrier JC, Musick JA, Heithaus MR (eds) Biology of sharks and their relatives. CRC, New York, pp 523–551
Caira JN, Healy CJ, Jensen K (2012) An updated look at elasmobranchs as hosts of metazoan parasites. In: Carrier JC, Musick JA, Heithaus MR (eds) Biology of sharks and their relatives, 2nd edn. CRC, Boca Raton, pp 547–578
Capapé C (1979) La Torpille marbrée, Torpedo marmorata Risso, 1810 (Pisces, Rajiformes) des côtes tunisiennes: nouvelles données sur l’écologie et la biologie de la reproduction de l’espèce, avec une comparaison entre les populations méditerranéennes et atlantiques. Ann Sci Nat 13(1):79–97
Cunningham CW (1997) Is congruence between data partitions a reliable predictor of phylogenetic accuracy? Empirically testing an iterative procedure for choosing among phylogenetic methods. Syst Biol 46(3):464–478. https://doi.org/10.1093/sysbio/46.3.46444
Darriba D, Taboada GL, Doallo R, Posada D (2012) jModel Test 2: more models, new heuristics and parallel computing. Nat Methods 9:772. https://doi.org/10.1038/nmeth.210999
Fischer W, Bauchot ML, Schneider M (1987) Fiches FAO d’identification des espèces pour les besoins de la pêche. (Révision 1). Méditerranée et Mer Noire. Rome: FAO. Zone de Pêche 37:761–1529
Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R (1994) DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol Mar Biol Biotechnol 3(5):294–299
Froese R, Pauly D (2023) FishBase.http://www.fishbase.org. Accessed 19 Feb 2023
Gaevskaya AV (2012) Parasites and diseases of fishes in the Black Sea and the Sea of Azov. EKOSI– Gidrofizika, Sevastopol
Giribet G, Carranza S, Baguñà J, Riutort M, Ribera C (1996) First molecular evidence for the existence of a Tardigrada + Arthropoda clade. Mol Biol Evol 13(1):76–84. https://doi.org/10.1093/oxfordjournals.molbev.a025573
Govedich FR, Moser WE, Davies RW (2004) Annelida: Clitellata, Hirudinea, Euhirudinea. In: Yule CM, Sen YH (eds) Freshwater invertebrates of the Malaysian region. Academy of Sciences Malaysia, Kuala Lumpur, pp 175–190
Hattour A (2000) Contribution à l’étude des poissons pélagiques des eaux tunisiennes. Ph.D. dissertation, Faculty of sciences of Tunis, Tunisia
Hewitt GC, Hine PM (1972) Checklist of parasites of New Zealand fishes and of their hosts. N Z J Mar Freshw Res 6(1–2):69–114. https://doi.org/10.1080/00288330.1977.9515410
Ingram DM (1957) Some Tasmanian Hirudinea. Pap Proc R Soc Tasmania 91:191–232
Jimi N, Shinji J, Hookabe N, Okanishi M, Woo SP, Nakano T (2023) A new species of Branchellion (Hirudinea: Piscicolidae) parasitizing the gills of short-tail stingrays (Batoidea: Dasyatidae) from the West Pacific. Zool Sci 40(4):308–313. https://doi.org/10.2108/zs220057
Kimura M (1980) A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 16:111–120. https://doi.org/10.1007/BF01731581
Kumar S, Stecher G, Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33:1870–1874. https://doi.org/10.1093/molbev/msw0544
Larkin MA, Blackshields G, Brown NP, Chenna R, Mc Gettigan PA, Mc William H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG (2007) Clustal W and Clustal X version 2.0. Bioinformatics 23:2947–2948. https://doi.org/10.1093/bioinformatics/btm404
Llewellyn LC (1966) Pontobdellinae (Piscicolidae: Hirudinea) in the British Museum (Natural History), with a review of the subfamily. Bull Brit Mus (Nat Hist) Zool 14(7):389–439
Margolis L, Esche GW, Holmes JC, Kuris AM, Schrad GA (1982) The use of ecological terms in parasitology (report of an ad hoc committee of the American Society of Parasitologists). J Parasitol 68:131–133
Matejusová I, Koubková B, D’Amelio S, Cunningham C (2001) Genetic characterization of six species of diplozoids (Monogenea; Diplozoidae). Parasitology 123(5):465–474. https://doi.org/10.1017/S0031182001008617
Merlo-Serna AI, García-Prieto L (2016) A checklist of helminth parasites of Elasmobranchii in Mexico. ZooKeys 563:73–128. https://doi.org/10.3897/zookeys.563.6067
Minelli A (1979) Fauna d’Italia: Hirudinea. Calderini, Bologna
Minelli A (2008) Hirudinea. In: Relini G (ed) Biologia Arina Mediterranea – Checklist della flora e della fauna deimariitaliani (parte I). Genova, Italy, pp 82–85
Moore JP (1952) New Piscicolidae (leeches) from the pacific and their anatomy. Occas Pap Bernice P Bishop Mus 21:17–44
Narváez K, Osaer F (2017) The marine leech Branchellion torpedinis parasitic on the angel shark Squatina squatina and the marbled electric ray Torpedo marmorata. Mar Biodivers 47(3):987–990. https://doi.org/10.1007/s12526-016-0444-y
Oka A (1910) Synopsis der japanischen Hirudineen, mit Diagnosen der neuen Species. Annot Zool Jpn 7:165–183
Oktener A, Utevsky SY (2010) New information on the hosts and distribution of the marine fish leeches Trachelobdella lubrica and Pontobdella muricata (Clitellata, Hirudinida). Vestn Zool 44:373–376. https://doi.org/10.2478/v10058-010-0023-9
Olsson P (1876) Bidrag till Skandinaviens Helminth fauna. Kongl Vetensk Acad Handl 14(1):1–35
Pauls SM, Provenzano FR (1999) Branchellion torpedinis Savingy, 1822 (Hirudinea, Piscicolidae) first report of marine leech from Venezuela. Acta Biol Venez 19(1):73–76
Quignard JP, Capapé C (1974) Recherches sur la biologie d’un sélacien du golfe de Tunis, Torpedo torpedo Linné, 1758. Bull lnst Natn Scien Tech Mer Salarnmbô 3(1–4):99–129
Richardson LR (1949) Studies on New Zealand Hirudinea: Part II—Branchellion parkeri, a new Ichthyobdellid leech. Zoology publications from Victoria University of Wellington, New Zealand, pp 1–11
Ruiz-Escobar F, Oceguera-Figueroa A (2019) A new species of Branchellion Savigny, 1822 (Hirudinida: Piscicolidae), a marine leech parasitic on the giant electric ray Narcine entemedor Jordan & Starks (Batoidea: Narcinidae) off Oaxaca, Mexico. Syst Parasitol 96(7):575–584. https://doi.org/10.1007/s11230-019-09872-w
Saglam N, Oguz MC, Celik ES, Doyuk SA, Usta A (2003) Pontobdella muricata and Trachelobdella lubrica (Hirudinea: Piscicolidae) on some marine fish in the Dardanelles, Turkey. J Mar Biol Assoc UK 83(6):1315–1316. https://doi.org/10.1017/S00253154030087499
Sawyer RT (1986) Leech biology and behavior. feeding biology, ecology, and systematics. Oxford Scientific Publications, Oxford
Séret B (2006) Guide d’identification des principales espèces de requins et de raies de l’Atlantique oriental tropical, à l’usage des enquêteurs et biologistes des pêches. IRD and MNHN, Paris
Sket B, Trontelj P (2008) Global diversity of leeches (Hirudinea) in freshwater. In: Balian EV, Lévêque C, Segers H, Martens K (eds) Freshwater animal diversity assessment. Developments in hydrobiology. Springer, Dordrecht. https://doi.org/10.1007/978-1-4020-8259-7155
Soto JMR (2000) Marine leech, Stibarobdella macrothela (Schmarda, 1861) (Hirudinea, Piscicolidae), parasitic on the whaler shark, Carcharhinus brachyurus (Günther, 1870) (Chondrichthyes, Carcharhinidae), in southern Brazilian waters. Rev Bras Biol 60(4):713–714. https://doi.org/10.1590/S0034-71082000000400024
Utevsky SY, Trontelj P (2004) Phylogenetic relationships of fish leeches (Hirudinea, Piscicolidae) based on mitochondrial DNA sequences and morphological data. Zool Scr 33:375–385. https://doi.org/10.1111/j.0300-3256.2004.00156.x
Utevsky SY, Utevsky AY, Schiaparelli S, Trontelj P (2007) Molecular phylogeny of pontobdelline leeches and their place in the descent of fish leeches (Hirudinea, Piscicolidae). Zool Scr 36:271–280
Wunderlich AC, Gadig OBF, Vaske-Júnior T, Pinheiro MAA (2011) Annelida, Hirudinida, Stibarobdella moorei (Oka, 1910): new distribution and host records. Check List 7(3):360–362
Yamauchi T, Ota Y, Nagasawa K (2008) Stibarobdella macrothela (Annelida, Hirudinida, Piscicolidae) from elasmobranchs in Japanese waters, with new host records. Biogeography 10:53–57
Zarrad R, El Abed A, Missaoui H, Gharbi H, Ben Abdallah L (2000) Analyse descriptive de la pecherie du golfe de Tunis. Bull lnst Natn Sci Tech Mer 27:27–34
Acknowledgements
The authors thank the fishermen for their help to collect the fish species.
The authors extended their appreciation to the Researchers Supporting Project number (RSP2024R75), King Saud University, Riyadh, Saudi Arabia. Part of this work was supported by RAS (project 121030100028-0“ Regularities of formation and anthropogenic transformation of biodiversity and biological resources of the Azov-Black Sea basin and other areas of the World Ocean” (Russian Federation).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
This research was conducted humanely, in line with the “Guidelines for Ethical Treatment of Animals in Applied Animal Behaviour and Welfare Research” that have been developed by the International Society for Applied Ethology.
Competing Interests
No financial interests are directly or indirectly related to the work submitted for publication.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Youssef, F., Benmansour, B., Yurakhno, V.M. et al. New marine leech species of Branchellion Savigny, 1822 (Hirudinida: Piscicolidae) and new host record of Pontobdella muricata in the Gulf of Tunis. Biologia 79, 1695–1704 (2024). https://doi.org/10.1007/s11756-024-01639-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11756-024-01639-z