Abstract
Features of shape and size variability of blood ark (Anadara kagoshimensis) inhabiting biotopes in the northern part of the Black and Azov Seas were studied. Fourteen morphometric traits were measured for each blood ark shell. The principal component analysis extracted only one principal component (PC) whose eigenvalue is greater than 1.0. The PC 1 explained 84.5 % of the variation in morphometric traits and was interpreted to be an integral indicator of mollusk shell size. The variation of morphometric traits due to the size and asymmetry of the valves was subjected to correction using a Multiple General Linear Model with PC 1 as a continuous predictor and right or left valve as a discrete predictor. A cluster analysis was performed based on the residuals after size correction, as a result of which mollusk individuals were divided into four clusters. For mollusks from the Sea of Azov, a characteristic feature was an increased distance between pallial line and ventral shell margin. In turn, mollusks from the Black Sea exhibited an increase in the width of the posterior adductor scar. The observed morphological differences can be explained by different quantity and quality of food, since the soil composition in the biotopes studied is almost identical.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anadara kagoshimensis (Tokunaga, 1906) is a bivalve mollusk inhabiting the intertidal and subtidal zones (Bañón et al. 2015). The natural range of the species comprises the coasts of the central Indian Ocean and western Pacific, the coasts of India, Sri Lanka, Indonesia, Korea, China, Japan, and northern Australia (Poutiers 1998; Yurimoto et al. 2008). The current range includes the Indo-Pacific region, the Mediterranean basin, with the center of invasion in the Adriatic Sea, the Black Sea, and the Sea of Azov (Strafella et al. 2017). In the Mediterranean Sea, the invasive process of A. kagoshimensis began in 1969 (Ghisotti 1973), where it was introduced accidentally, most likely by shipping (Bañón et al. 2015). The current distribution in the invasion area includes the western and eastern Mediterranean, Catalan, Adriatic, Aegean, Marmara, Black and Azov Seas (Zaitsev and Ozturk 2001). This species in the Black Sea occurs in the depth range of 3–60 m (Marinov 1990). In the Sea of Azov, it was found at depths of up to 10–11 m (Chikhachev et al. 1994). Anadara kagoshimensis easily tolerates hypoxic and anoxic conditions (de Zwaan et al. 1995; Borodina and Soldatov 2019). The presence of hemoglobin in the mollusk tissues and in erythrocytes provides the ability to survive for a long time under hypoxic conditions when many species of other mollusks die (Weber et al. 1990; Shiganova 2008; Shiganova et al. 2012).
In its natural distribution, A. kagoshimensis prefers sandy and clay habitats in the intertidal and subtidal zones (Santhanam 2018). Anadara kagoshimensis is able to live on various substrates (sandy, rocky and muddy) (Rinaldi 1985; Streftaris and Zenetos 2006; Crocetta 2011), but prefers mainly the soft substrates (sandy or muddy) (Zaitzev and Mamaev 1997). For the situation of the Black Sea, the most favorable conditions for this species are formed in the organically enriched near-mouth areas of the western and eastern Black Sea shelf (Gomoiu 1984). The range of A. kagoshimensis distribution in the Black Sea depths includes the mussel belt (Revkov 2016) and the sandy substrate belt with a dominant Veneroida order of Bivalvia (Bondarev 2020). The two biocenosis belts in the Black Sea corresponding to facies complexes with dominant bivalves of Mytilloida and Veneroida are formed on the basis of their different ability to adapt to sediment conditions (Bondarev 2014). The ability to attach to all types of hard substrates with byssus is a competitive advantage of this mollusk (Ghisotti and Rinaldi 1976). Mussels, due to their anatomical features, are subject to inhibition in the zone of sand development (Zaika et al. 1990), while A. kagoshimensis has a psammic resistance, which allows expanding the boundaries of its distribution to sandy substrates, where Venerida dominates (Bondarev, 2014). Therefore, the distribution of A. kagoshimensis extends over two benthic belts (Bondarev 2020).
Anadara kagoshimensis belongs to warm-water forms of mollusks (Lutaenko 2006). The stable development of its settlements in the hard temperature conditions of the Black Sea and the Sea of Azov indicates the biological eurythermicity of the species and the ability to exist in a wide range of temperature changes (Revkov and Shcherban 2017). Anadara kagoshimensis is a euryhaline species (Broom 1982; Broom 1985). Although its maximum distribution corresponds to areas with salinity around 30 ‰, the mollusk tolerates desalination up to 10–11 ‰, and in the Adriatic Sea it is found even in brackish-water lagoons (Rinaldi 1985). Apoptosis, amino acid metabolism, and other biological processes mediate the immune regulation mechanisms of A. kagoshimensis that occurred after exposure to a sudden decrease in salinity. A sudden decrease in salinity (from 30 ‰ to 14 ‰) results in oxidative damage (Zhang et al. 2019). Anadara kagoshimensis is able to survive hypoxia (< 2 mg L− 1) for about two weeks in summer. As water salinity reduces, hypoxia survival time decreases (Suzuki et al. 2012). This species survives for 5–7 days under conditions of low oxygen concentrations in the medium (up to 0.5 ml L− 1) (Zaitzev and Mamaev 1997). This invasive bivalve can act as an ecological engineer that determines the structure, composition, and functioning of natural communities (Sousa et al. 2009). In the Adriatic and Black seas, the significant dispersion of A. kagoshimensis led to the decline of autochthonous bivalves such as Chamelea gallina (Linnaeus, 1758), Mya arenaria Linnaeus, 1758 and Cerastoderma glaucum (Bruguiere, 1789) in their habitats (Streftaris and Zenetos 2006; Kolyuchkina and Miljutin 2013). New communities dominated by the invasive species A. kagoshimensis were observed offshore in an area with increased accumulation of terrigenous sediments and a less favorable oxygen regime (Chikina 2009).
The macrozoobenthos of the Azov Sea lagoons showed a high resistance to invasion by A. kagoshimensis. The abundance and biomass of A. kagoshimensis were lower than that of the native dominant species. Anadara kagoshimensis does not form distinct communities. The effective colonization of A. kagoshimensis is inhibited by the lack of a free niche in the communities. Anadara kagoshimensis colonization in the lagoon is inhibited by the unsuitable habitat (combination of very soft muddy substrate and high-density seagrass meadows) (Kolyuchkina et al. 2019). The A. kagoshimensis invasion was suggested to promote the growth of natural bivalve communities by utilizing excess organic matter (Bondarev 2020).
The invasive species A. kagoshimensis in a new environment, which it finds in the Black Sea and Sea of Azov, is able to occupy a variety of habitats and has a significant competitive advantage in the comparison with representatives of the natural fauna. The shell of A. kagoshimensis is much thicker and harder than that in the Cerastoderma spp. mollusks of the same size. Therefore, Cerastoderma spp. are predominantly easily accessible preys for benthophagous species only during the first two years of life (Anistratenko and Haliman 2006; Finogenova et al. 2013).
The adaptive capabilities of the species also manifest themselves at the level of shell morphology of these animals. The study of the variability of shell shape and size is important for understanding the mechanisms of adaptation of invasive molluscks for living in different ecological conditions. Therefore, the aim of the study is to identify the features of variation in the shape and size of A. kagoshimensis inhabiting biotopes in the northern part of the Black Sea and the Sea of Azov.
Methods
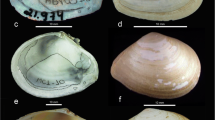
Mollusk shells were collected in 2020 in the Black Sea on the Dzharylgach Spit (46º04’ N, 32º32’ E) and in the Sea of Azov on the Fedotova Spit (46º13’ N, 35º14’ E). Fourteen morphometric traits were measured for each blood ark shell to the nearest 0.1 mm using a dial caliper (Fig. 1). Measurement error using these techniques does not contribute significantly to trait variation (Gardner 1995). The traits previously used by the authors in the morphological diagnosis of mollusk shells were used for the analysis (McDonald et al. 1991; Finogenova et al. 2013) (Fig. 1). The number of ribs on the valves of each blood ark was counted in addition to the mensural (measured) features. The left and right valves were measured from a sample of shells collected on the shore. Values for each of the 15 morphometric traits were log10-transformed before being used for statistical procedures. The differences in shape from the differences in body size were separated by a size correction procedure (McCoy et al. 2006). The method of sharing as a special technique of residual analysis based on a multivariate description of the body size. The multiple trait measurements were analyzed using principal component analysis (PCA) to yield the first principal component (PC 1), which was a quantitative measure of “body size” because, by definition, it summarizes isometric size alone (Jolicoeur 1963; Somers 1986; Diniz-Filho et al. 1994; Bookstein 1996). The morphometric traits were subjected to PCA. The PC 1 scores were applied as a measure of total shell sizes in the size correction procedure. A Multiple General Linear Model (GLM) was used for size correction procedure (García-Berthou 2001; Dahl and Peckarsky 2002; Longepierre et al. 2003; Wojczulanis-Jakubas et al. 2015). The residuals of the Multiple GLM were used in the cluster analysis. The importance of morphometric traits for distinguishing clusters was tested using discriminant analysis. The morphometric traits were subjected to PCA after size correction for each cluster separately. Procrustean analysis (Peres-Neto and Jackson 2001) was applied to find the degree of consistency of the configurations obtained after PCA. Procrustes analysis is a procedure that minimizes the sum of-squared differences between configurations in a multivariate Euclidean space. In a procrustean analysis to find the optimal superimposition, one configuration is kept as a reference while the other is rotated sequentially until the sum-of-the-squared residuals between the corresponding coordinates in both configurations (SS) is minimized. The greater the coherence between the datasets, the smaller the value of SS. The individual residuals between homologous traits can be interpreted separately, revealing the degree of their correspondence (Peres-Neto and Jackson 2001). The statistical calculations were performed with the vegan library (Oksanen et al. 2018) for a language and environment for statistical computing R (R Core Team 2020). Descriptive statistics of morphometric traits and the degree of correspondence to the laws of distribution were performed in the Statistica software (Statsoft).
Shell traits measured for each individual clam in Anadara kagoshimensis: H – shell height, mm; B/2 – valve width, mm; L – shell length, mm; a – ligament length, mm; b – length of the upper margin of the valve, mm; c – length from the anterior margin of the ligament to the apex, mm; d – length from dorsal margin of the ligament to the apex, mm; e – distance between the projection of anterior margin and ventral margin, mm; f – distance between pallial line and ventral shell margin, mm; h – width of posterior adductor scar, mm; i – width of anterior adductor scar, mm; j – umbo height, k – umbo length; l – thickness of the bivalve shell; m – ribs
Results
The coefficient of variation of morphometric characteristics of A. kagoshimensis shell was in the range 4.11–69.76 % (left shell) and 3.88–70.73 % (right shell) (Table 1). The individuals of this species usually have a larger left shell than the right one. But sampling collections among costal deposits yielded estimates of morphometric characteristics that were not statistically significantly different between the left and right sides of the shell in both size and level of variation. The number of ribs in the shell surface sculpture was the least variable feature. The most variable features were the length from the anterior margin of the ligament to the apex and the length of the ligament. The variability of other indices did not differ significantly from each other and was in the range 32.20–43.81 %.
The analysis of the histograms of the morphometric traits indicated their bimodal (for the shell height, trimodal) nature (Fig. 2). Accordingly, the model from the mixture of normal distributions was able to explain the observed histograms well. The distribution of the number of ribs in clams from the Fedotova spit deviated significantly from the binomial law as a random model for discrete traits (χ2 = 14.4, df = 4 p < 0.001). The deviation was due to a greater prevalence of individuals from 32 to 33 ribs than would be expected due to the random alternative. There were also significantly fewer individuals with marginal numbers of ribs per shell, 29, 30, and 35, than would be expected as a result of a random process. The modal value of the number of ribs is 32 for the population from Dzharylgach Spit, and in the population from Fedotova Spit the modal number of ribs on the shell is 33.
Histograms of morphometric traits of Anadara kagoshimensis shells. The red line indicates the histogram, which is formed by the mixture of three (for H) or two (for all other continuum traits) hypothetical normal distributions. The hypothetical binomial distribution is represented by the red line for the number of ribs: H – shell height, mm; B/2 – valve width, mm; L – shell length, mm; a – ligament length, mm; b – length of the upper margin of the valve, mm; c – length from the anterior margin of the ligament to the apex, mm; d – length from dorsal margin of the ligament to the apex, mm; e – distance between the projection of anterior margin and ventral margin, mm; f – distance between pallial line and ventral shell margin, mm; h – width of posterior adductor scar, mm; i – width of posterior adductor scar, mm; j – umbo height, k – umbo length; l – thickness of the bivalve shell; m – ribs (1 – Fedotov Spit, 2 – Dzharylgach Spit)
The observed bimodal distribution of morphometric traits can be explained by biotopic features. The morphometric traits of mollusks from the Fedotova Spit were significantly higher than those of mollusks from the Dzharylgach Spit. According to the level of variation, mollusks from Dzharylgach Spit exceeded mollusks from Fedotova Spit in such traits as ligament length and length from the anterior margin of the ligament to the apex. For other traits, the level of variation was not significantly different between biotopes.
The PCA of the morphometric traits extracted only one component whose eigenvalue exceeds unity (Table 2). This component was able to explain 84.5 % of the variation in morphometric traits. The PC 1 was related to all morphometric traits, as confirmed by the highly significant correlation coefficients. This revealed that the PC 1 was an integral indicator of mollusk shell size. The shell sizes expressed by the integral index PC 1 were significantly different between biotopes (Fig. 3). Shells from Fedotova spit were generally larger in size than those from Dzharylgach Spit (F = 121.1, p < 0.001).
The variation of morphometric traits due to the size and asymmetry of the valves was subjected to correction using a Multiple GLM with PC 1 as a continuous predictor and right or left valve as a discrete predictor (Table 3). These predictors were found to be able to explain 8 to 98 % of the variation in morphometric traits. Size is a statistically significant predictor for all traits. The shell asymmetry was manifested in the fact that the height and width of the left valve were greater than those of the right one. In turn, the ligament length, the length of the upper margin of the valve, the length from the anterior margin of the valve to the apex, and the width of the posterior locking muscle mark on the right side were greater. Even after size correction, some indices differed in size dependence. Thus, as the size of the shell on the left side increased, such indices as the length of the valve, ligament length, and length from the anterior margin to the apex increased.
The residuals of the Multiple GLM represent the morphometric characteristics, from which the size component of variation was extracted. Based on the residuals, a cluster analysis was performed, as a result of which mollusk individuals were divided into four clusters (Fig. 4). A discriminant analysis was performed to interpret these clusters with the residuals of regression models as predictors (Table 4). The analysis showed that the clusters can be differentiated quite well using canonical variables (Fig. 5). Canonical variable 1 is most sensitive to the opposite variation of the length from distance between the projection of anterior margin and ventral margin in relation to the length of the upper margin of the valve, the width of posterior adductor scar, and the number of ribs. Thus, individuals from the cluster 2 have a greater length of the upper margin of the valve, the width of posterior adductor scar, and the number of ribs, while individuals from the cluster 1 have a greater length from the anterior margin of the ligament to the apex. The canonical variable 2 is most sensitive to the opposite dynamics on one side of the length from the anterior margin of the valve to the apex, distance between the projection of anterior margin and ventral margin, and the distance from the mantle margin to the distance between pallial line and ventral shell margin of the shell, the ligament length, and the width of posterior adductor scar on the other side. Accordingly, individuals from cluster 4 show larger values of the length from the anterior margin of the shell to the apex, the length from the anterior margin to the apex, and the distance from the mantle margin to the margin of the shell. In turn, mollusks from different biotopes are represented by all clusters. Nevertheless, the clusters exhibit a certain level of predominance in a biotope (Fig. 6). The specimens of mollusks from the Fedotova Spit are predominantly represented by the clusters 2 and 4, and the specimens of mollusks from the Dzharylgach Spit are predominantly represented by the clusters 1 and 3. Thus, the selected clusters were clearly distinguished by a set of morphometric traits that reflect the functional state of the mollusks, which allows to consider the clusters as ecomorphotypes.
Procrustes analysis allowed to compare factor solutions between individual ecomorphotype (Fig. 7). The ecomorphotype factor structures statistically significantly correlated in the range of 0.54–0.77, indicating a considerable level of the similarity between morphometric traits in the members of different ecomorphotype. The highest individual residuals are found for ligament length, length of the upper margin of the valve, length from the anterior margin of the ligament to the apex, length from dorsal margin of the ligament to the apex, and distance between the projection of anterior margin and ventral margin (Fig. 8). These features contribute most to the difference between the ecomorphotype. In turn, traits such as distance between pallial line and ventral shell margin and width of posterior adductor scars are relatively invariant and play an important role in matching the shape of mollusks from different ecomorphotype.
Pairwise procrustean rotation of solutions derived from principal component analyses (PCA) in the space of principal components 1 and 2: the blue arrows indicate procrustes errors (large errors correspond to large arrows) calculated by rotating traits in 9,999 permutations and comparing traits positions of two PCA for the corresponding ecomorphotypes until finding positions with least differences; procrustes sum of squares (SS) and correlation in symmetric procrustean rotation is presented under each pairwise diagram, H – shell height, mm; B/2 – valve width, mm; L – shell length, mm; a – ligament length, mm; b – length of the upper margin of the valve, mm; c – length from the anterior margin of the ligament to the apex, mm; d – length from dorsal margin of the ligament to the apex, mm; e – distance between the projection of anterior margin and ventral margin, mm; f – distance between pallial line and ventral shell margin, mm; h – width of posterior adductor scar, mm; i – width of posterior adductor scar, mm; j – umbo height, k – umbo length; l – thickness of the bivalve shell; m – ribs
Procrustes residuals of the traits: the x-axis represents H – shell height, mm; B/2 – valve width, mm; L – shell length, mm; a – ligament length, mm; b – length of the upper margin of the valve, mm; c – length from the anterior margin of the ligament to the apex, mm; d – length from dorsal margin of the ligament to the apex, mm; e – distance between the projection of anterior margin and ventral margin, mm; f – distance between pallial line and ventral shell margin, mm; h – width of posterior adductor scar, mm; i – width of posterior adductor scar, mm; j – umbo height, k – umbo length; l – thickness of the bivalve shell; m – ribs; the y-axis represents the procrustean residuals of the traits
Discussion
The shells of A. kagoshimensis show size variability, shell asymmetry and asymmetry variability which is related to size. The number of ribs is least affected by these predictors. The right and left valves do not differ in this parameter. The shell sculpture in mollusks from the Black Sea is represented by 30–36 (usually 34–35) (Anistratenko et al. 2014) or 31–32 (Lutaenko 2006) radial ribs. In mollusks from the Sea of Azov there are 31–35 (usually 32–33) (Anistratenko et al. 2014), in forms from the Adriatic Sea – 30–31 (Lutaenko 2006) or 32–34 (Strafella et al. 2017). According to our data, the number of ribs in mollusks from the Fedotova Spit varies from 30 to 36 (usually 32–34), and 30–35 ribs (usually 32–34) were found in mollusks from the Dzharylgach Spit. The number of mollusk valve ribs is subject to geographic variability . However, the clinal variability of this trait was difficult to reveal (Clarke 1965). The presence of ribs and their relief increase the strength of the shell at equal thickness and also serve as a defense against drilling predators by the exaptation mechanism (Klompmaker and Kelley 2015). The level of shell sculpture development and thickness are directly related and determined by environmental energy (Mandic and Piller 2001). A decrease in the level of shell sculpture development indicates a decrease in environmental energy as depth increases (Kauffman 1965). Some deep-sea bivalves usually have a thin-walled shell with a low relief or smooth surface. The thin-walled shell is typical of most mollusk species that live in the zone of no significant fluctuations in the water column temperature (Bondarev 2013).
Mollusks from different biotopes differ significantly in size. The individuals may differ in morphology due to genetic features, environmental influences on development, or as a consequence of sexual dimorphism (McCoy et al. 2006). The phenotypic traits within or between populations can influence the rate and direction of evolution, determine population dynamics, and influence the results of ecological interactions (Peacor and Werner 2001; Werner and Peacor 2003; Utsumi et al. 2010). The evidence from the long-term and large-scale studies suggests that interactions mediated by morphological traits can influence community dynamics (Bolker et al. 2003). The average size of individuals in a population depends on the growth rate of individuals and the age structure. Obviously, the average size of individuals in the population will be the larger the greater the growth rate of individuals and more representatives of the older age categories. Anadara kagoshimensis has a long ontogeny and a low lethality of adults. The sexual maturity is reached at a shell length of about 1–2 cm (Chikina et al. 2003; Sahin et al. 2006). The size of an organism is related to the lifespan, the size of the local range and other aspects of the life span and ecology of the species (Peters 1983; Brown et al. 2004). The relationship between body size and population size is an important link between individual, population levels, and the structure and dynamics of ecological communities (Abele 1976; Woodward et al. 2005). Body size is one of the primary determinants of metabolism and resource use (Peters 1983; Brown et al. 2004). In the Black Sea compared to other areas of the World Ocean, the growth rate of A. kagoshimensis is somewhat higher, which is explained by more favorable feeding conditions (Sahin et al. 2006). According to the type of feeding, Anadara spp. belong to the sestonophagous filtrators. The maximum age of A. kagoshimensis (7 years) was recorded in the population of the eastern part of the Anatolian coast (Sşahin et al. 2009), and the greatest length of the Black Sea specimens (up to 85 mm) was also recorded there (Sşahin et al. 2009). The length of the A. kagoshimensis was in the range of 4.5–71.8 mm in the Middle Black Sea (Aydin et al. 2014). On the Caucasian coast and off the coast of Bulgaria, the length of the A. kagoshimensis shell did not exceed 60 mm (Marinov 1990; Zolotarev and Terentyev 2012), in the Kerch Strait – 65 mm (Anistratenko and Haliman 2006). The length of A. kagoshimensis shell was in the range of 5–48.2 mm at Kozacha Bay (Crimea) (Bondarev 2020), the maximum length was 54 mm in Sevastopol Bay (Revkov 2016; Revkov and Shcherban 2017). In the Sea of Azov, the maximum age of mollusks is estimated at 5–6 years with an average length of ~ 50 mm (Chikhachev et al. 1994). I. P. Bondarev (Bondarev 2020) suggested that A. kagoshimensis reaches the maximum size and greatest age under optimal conditions. Thus, the aquatic life conditions at Fedotova spit are closer to the optimal than those at Dzharylgach Spit. As the ground silted up, the average values of the height-to-length ratio of the shell of A. kagoshimensis decreases, and the average thickness-to-length ratio increases. Anadara kagoshimensis forms a flatter shell on sandy soil (Finogenova 2016). The studied populations are not significantly different in the H/L ratio (F = 0.47, p < 0.001), which may indicate a similar soil composition in these biotopes. The comparison of our data with the results given by Finogenova for Kinburn and Tendrovsky Spits (Finogenova 2016) indicates a greater elongation of mollusks from the populations we studied. The soils in the area of the Kinburn and Tendrovsky Spits were reported as muddy, while in our study area the soils were sandy (Zenkovich 1958), which may explain the observed differences in the degree of elongation of the shells.
The left valve in A. kagoshimensis is generally larger than the right valve, as indicated by traits such as shell height and width. A non-trivial result is that some morphometric traits of the left valve are smaller than of the right valve. This result can be explained by the greater asymmetry of the left valve as a result of greater elongation of the valve posterior part. The above features of the shell morphology were subjected to a size correction procedure using the Multiple GLM approach. The cluster analysis of individual clam shells was performed after the size correction. This approach was not previously used in the morphometric studies of A. kagoshimensis in the Black Sea and the Sea of Azov. As a rule, collections from a single point of space were considered as a separate cluster, the shells from which were further subjected to the methods of multivariate statistical analysis. Our approach makes it possible to obtain two fundamentally new results. First of all, the samples from a single location are not homogeneous, but are compositions of several ecomorphotypes. The differences between the locations consist in the specific ratio of representatives of the different ecomorphotypes. Ecomorphotypes can be assumed to occupy certain ecological conditions in the marine habitat. The biotopic diversity near different locations leads to the formation of specific spectra of the ecomorphotypes that were detected. Nevertheless, for the Azov and Black Seas, specific trends in the transformation of the shell shape can be revealed.
For mollusks from the Sea of Azov, a characteristic feature is an increased distance between pallial line and ventral shell margin. In turn, mollusks from the Black Sea exhibit an increase in width of posterior adductor scar. It should be noted that Bivalves regulate the intake of particles suspended in the water depending on their quality and quantity. Changing the clearance rate controls the capture of particles. Pallial organs sort out a part of captured particles as pseudofaeces (Ward and Shumway 2004). Functional capacity is determined by pallial organs size, so pallial organs size varies with water turbidity (Theisen 1982; Payne et al. 1995; Barillé et al. 2000; Honkoop et al. 2003). The large adductor muscle is used to efficiently eject pseudofaeces (Yoshino et al. 2013). The observed differences between mollusks from different biotopes may be suggested to be related to the level of water turbidity in them. In addition to the level of water turbidity, the degree of development of the pallial organs and the adductor muscle can be influenced by the ratio of organic and inorganic materials in the suspended matter (Yoshino et al. 2013). Some bivalves, such as Cerastoderma edule (Linnaeus, 1758), exhibit a strategy of high intensity filtration and high particle selection, which is associated with the production of pseudofaeces is adaptive in terms of energy efficiency under conditions of low food value of the substances suspended in the water (Iglesias et al. 1992; Urrutia et al. 1997). Thus, the higher growth rate of mollusks in the Sea of Azov and the characteristic features of morphology may be suggested to be due to a higher quality of food in this body of water than in the Black Sea.
The clusters differ from each other in the features of correlation relations between morphometric traits. To the greatest extent, the allometric relations within each cluster differ in the features that indicate the position of the apex in the shell and the degree of asymmetry of each valve. It should also be noted the important role of the number of ribs in the specificity of the correlation relationships for each cluster. The number of mollusk valve ribs is genetically determined (Kraeuter et al. 1984). This suggests that the selected clusters are of genetic nature.
The relationship of sexual dimorphism with data on morphometric characteristics, as well as with sexual maturity, is of interest. This issue will be considered in our subsequent studies.
Conclusion
An intensive growth of animals indicates more favorable conditions for this species in the Sea of Azov. The different quality of trophic resources may be the leading factor that determines the diversity of size and shape of shells of Anadara kagoshimensis in the Black Sea and Sea of Azov populations under conditions of the same type of soil composition in the habitats. The features of the shell shape allow to distinguish four ecomorphotypes of A. kagoshimensis, which are available in both habitats. The differences between the habitats are in the proportions between the ecomorphotypes represented in them. Each ecomorphotype is distinguished by peculiarities of form and a specific relationship between morphometric traits. To the greatest extent, the differences are due to the variability in the asymmetry of each valve. Among the ecomorphotypes, the position of the pallial line and the width of the adductor scars are the most invariant. The significant development of pallial organs and adductor muscles in mollusks from the Black Sea, which is reflected in the features of shell shape, can compensate for the low food quality in this habitat. The ecomorphotypes differ in the external features of the shell, the adaptive significance of which is not obvious. At the same time, the features of the shell, which are associated with the functional activity of internal organs, significantly differ between mollusks from different habitats. Ecomorphotypes are most likely to be genetic in nature.
References
Abele LG (1976) Comparative species richness in fluctuating and constant environments: coral-associated decapod crustaceans. Science 192:461–463. https://doi.org/10.1126/science.192.4238.461
Anistratenko VV, Haliman IA (2006) Bivalve mollusc Anadara inaequivalvis (Bivalvia, Arcidae) in the northern part of the Sea of Azov: Completion of colonization of the Azov-Black Sea basin. Vestn Zool 40:505–511
Anistratenko VV, Anistratenko OY, Khaliman IA (2014) Conchological variability of Anadara inaequivalvis (Bivalvia, Arcidae) in the Black–Azov Sea basin. Vestn Zool 48:457–466. https://doi.org/10.2478/vzoo-2014-0054
Aydin M, Karadurmuş U, Tunca E (2014) Morphometric aspects and growth modeling of exotic bivalve blood cockle Scapharca inaequivalvis from the Black Sea, Turkey. Biologia 69:1707–1715. https://doi.org/10.2478/s11756-014-0476-3
Bañón R, Fernández J, Trigo JE et al (2015) Range expansion, biometric features and molecular identification of the exotic ark shell Anadara kagoshimensis from Galician waters, NW Spain. J Mar Biol Assoc UK 95:545–550. https://doi.org/10.1017/S0025315414002045
Barillé L, Haure J, Cognie B, Leroy A (2000) Variations in pallial organs and eulatero-frontal cirri in response to high particulate matter concentrations in the oyster Crassostrea gigas. Can J Fish Aquat Sci 57:837–843. https://doi.org/10.1139/cjfas-57-4-837
Bolker B, Holyoak M, Křivan V et al (2003) Connecting theoretical and empirical studies of trait-mediated interactions. Ecology 84:1101–1114. https://doi.org/10.1890/0012-9658(2003)084[1101:CTAESO]2.0.CO;2
Bondarev IP (2013) Ecomorphological analyses of marine mollusks’ shell thickness of Rapana venosa (Valenciennes, 1846) (Gastropoda: Muricidae). Int J Mar Sci. https://doi.org/10.5376/ijms.2013.03.0045
Bondarev IP (2014) Biological basis for structuring the benthic zone facies of the Black sea shelf. Geol Miner Resour World Ocean 4:72–90
Bondarev IP (2020) Features of biocenotic relations of Anadara kagoshimensis (Bivalvia, Arcidae) in the Kazachya Bay of the Black Sea. Russ J Biol Invas 11:198–207. https://doi.org/10.1134/S2075111720030030
Bookstein FL (1996) Biometrics, biomathematics and the morphometric synthesis. Bull Math Biol 58:313–365. https://doi.org/10.1007/BF02458311
Borodina AV, Soldatov AA (2019) The effect of anoxia on the content and composition of carotenoids in the tissues of the bivalve invader Anadara kagoshimensis (Tokunaga, 1906). Russ J Biol Invas 10:307–314. https://doi.org/10.1134/S2075111719040027
Broom M (1982) Analysis of the growth of Anadara granosa (Bivalvia: Arcidae) in natural, artificially seeded and experimental populations. Mar Ecol Prog Ser 9:69–79. https://doi.org/10.3354/meps009069
Broom MJ (1985) The biology and culture of marine bivalve mollusks of the genus Anadara, Manila. Int Center Living Aquat Resour, Manila
Brown JH, Gillooly JF, Allen AP et al (2004) Toward a metabolic theory of ecology. Ecology 85:1771–1789. https://doi.org/10.1890/03-9000
Chikhachev AS, Frolenko LN, Rekov YI (1994) New alien invasive species in the Sea of Azov. Fish Ind 3:40–45
Chikina MV (2009) Macrozoobenthos of loose grounds of North Caucasian coast of the Black Sea: spatial structure and long-term dynamics. Extended Abstract of Cand. Sci (Biol) Dissertation. Moscow
Chikina MV, Kolyuchkina GA, Kucheruk NV (2003) Biological reproduction of Scapharca inaequivalvis (Bruguiere) (Bivalvia, Arcidae) in the Black Sea. Sea Ecol 64:72–77
Clarke AH (1965) The scallop superspecies Aequipecten irradians (Lamarck). Malacologia 2:161–188
Crocetta F (2011) Marine alien Mollusca in the Gulf of Trieste and neighbouring areas: a critical review and state of knowledge (updated in 2011). Acta Adriat 52:247–260
Dahl J, Peckarsky BL (2002) Induced morphological defenses in the wild: Predator effects on a mayfly, Drunella coloradensis. Ecology 83:1620–1634. https://doi.org/10.1890/0012-9658(2002)083[1620:IMDITW]2.0.CO;2
de Zwaan A, Isani G, Cattani O, Cortesi P (1995) Long-term anaerobic metabolism of erythrocytes of the arcid clam Scapharca inaequivalvis. J Exp Mar Bio Ecol 187:27–37. https://doi.org/10.1016/0022-0981(94)00168-D
Diniz-Filho JAF, Von Zuben CJ, Fowler HG et al (1994) Multivariate morphometrics and allometry in a polymorphic ant. Insect Soc 41:153–163. https://doi.org/10.1007/BF01240475
Finogenova NL (2016) Features of formation of shells in bivalve mollusc Anadara kagoshimensis (Bivalvia, Arcidae) Tendrovsky and Kinburn Spit. Sci Bull Uzhgorod Univ (Ser Biol) 40:104–107
Finogenova NL, Kurakin AP, Kovtun OA (2013) Morphological differentiation of Anadara inaequivalves (Bivalvia, Arcidae) in the Black Sea. Hydrobiol J 49:3–11. https://doi.org/10.1615/HydrobJ.v49.i1.10
García-Berthou E (2001) On the misuse of residuals in ecology: testing regression residuals vs. the analysis of covariance. J Anim Ecol 70:708–711. https://doi.org/10.1046/j.1365-2656.2001.00524.x
Gardner J (1995) Developmental stability is not disrupted by extensive hybridization and introgression among populations of the marine bivalve molluscs Mytilus edulis (L.) and M. galloprovincialis (Lmk.) from south-west England. Biol J Linn Soc 54:71–86. https://doi.org/10.1016/0024-4066(95)90037-3
Ghisotti F (1973) Scapharca cornea (Reeve), ospite nuova del Mediterraneo. Conchiglie 9:3–4
Ghisotti F, Rinaldi E (1976) Osservazioni sulla popolazione di Scapharca insediatasi in questi ultimi anni su un tratto del litorale romagnolo. Conchiglie 12:183–195
Gomoiu MT (1984) Scapharca inaequivalvis (Bruguière) – a new species in the Black Sea. Cercet Mar – Rech Mar 17:131–141
Honkoop PJ, Bayne B, Drent J (2003) Flexibility of size of gills and palps in the Sydney rock oyster Saccostrea glomerata (Gould, 1850) and the Pacific oyster Crassostrea gigas (Thunberg, 1793). J Exp Mar Biol Ecol 282:113–133. https://doi.org/10.1016/S0022-0981(02)00463-X
Iglesias JIP, Navarro E, Alvarez Jorna P, Armentina I (1992) Feeding, particle selection and absorption in cockles Cerastoderma edule (L.) exposed to variable conditions of food concentration and quality. J Exp Mar Biol Ecol 162:177–198. https://doi.org/10.1016/0022-0981(92)90200-T
Jolicoeur P (1963) The multivamriate generalization of the allometry equation. Biometrics 19:497–499. https://doi.org/10.2307/2527939
Kauffman EG (1965) Form, function and evolution. In: Moore RC (ed) Treatise on invertebrate paleontology, Part N, Mollusca 6, vol 1. Geological Society of America and University of Kansas, Lawrence, pp 129–205
Klompmaker AA, Kelley PH (2015) Shell ornamentation as a likely exaptation: evidence from predatory drilling on Cenozoic bivalves. Paleobiology 41:187–201. https://doi.org/10.1017/pab.2014.12
Kolyuchkina GA, Miljutin DM (2013) Application of the morpho-functional analysis of hydrobionts (Anadara sp. cf. Anadara inaequivalvis Bivalvia) to environmental monitoring. Oceanology 53:169–175. https://doi.org/10.1134/S0001437013010050
Kolyuchkina GA, Syomin VL, Spiridonov VA et al (2019) The resilience of macrozoobenthos of boreal coastal lagoons to non-indigenous species invasion: A case study of Taman Bay (the Sea of Azov). Reg Stud Mar Sci 28:100573. https://doi.org/10.1016/j.rsma.2019.100573
Kraeuter J, Adamkewicz L, Castagna M et al (1984) Rib number and shell color in hybridized subspecies of the Atlantic bay scallop, Argopecten irradians. Nautilus (Philadelphia) 98:17–20
Longepierre S, Grenot C, Hailey A (2003) Individual, local and subspecific variation in female Hermann’s tortoise (Testudo hermanni) reproductive characters. Contrib Zool 72:221–226. https://doi.org/10.1163/18759866-07204003
Lutaenko KA (2006) On the fauna of bivalves of the subfamily Anadarinae (Arcidae) from southern India. Bull Russ Far East Malacol Soc 10:102–121
Mandic O, Piller WE (2001) Pectinid coquinas and their palaeoenvironmental implications – examples from the early Miocene of northeastern Egypt. Paleogeogr Paleoclimatol Paleoecol 173:171–191
Marinov TM (1990) The zoobenthos from the Bulgarian sector of the Black Sea. Bulgarian Academy of Science Publications, Sofia
McCoy MW, Bolker BM, Osenberg CW et al (2006) Size correction: comparing morphological traits among populations and environments. Oecologia 148:547–554. https://doi.org/10.1007/s00442-006-0403-6
McDonald JH, Seed R, Koehn RK (1991) Allozymes and morphometric characters of three species of Mytilus in the Northern and Southern Hemispheres. Mar Biol 111:323–333. https://doi.org/10.1007/BF01319403
Oksanen J, Blanchet FG, Kindt R et al (2018) Community ecology package. R package version 2.5-2. https://CRAN.R-project.org/package=vegan. Accessed 13 Nov 2020
Payne BS, Miller AC, Hubertz ED, Lei J (1995) Adaptive variation in palp and gill size of the zebra mussel (Dreissena polymorpha) and Asian clam (Corbicula fluminea). Can J Fish Aquat Sci 52:1130–1134. https://doi.org/10.1139/f95-109
Peacor SD, Werner EE (2001) The contribution of trait-mediated indirect effects to the net effects of a predator. Proc Natl Acad Sci USA 98:3904–3908. https://doi.org/10.1073/pnas.071061998
Peres-Neto PR, Jackson DA (2001) How well do multivariate data sets match? The advantages of a Procrustean superimposition approach over the Mantel test. Oecologia 129:169–178. https://doi.org/10.1007/s004420100720
Peters RH (1983) The ecological implications of body size. Cambridge University Press, Cambridge
Poutiers JM (1998) Bivalves (Acephala, Lamellibranchia, Pelecypoda). In: Carpenter KE, Niem VH (eds) FAO species identification guide for fishery purposes. The living marine resources of the Western Central Pacific. 1. Seaweeds, corals, bivalves and gastropods. FAO, Rome, pp 123–362
R Core Team (2020) A language and environment for statistical computing. R A Lang. Environ Stat Comput R Found Stat Comput, Vienna. https://www.R-project.org. Accessed 13 Nov 2020
Revkov NK (2016) Colonization’s features of the Black Sea basin by recent invader Anadara kagoshimensis (Bivalvia: Arcidae). Mar Biol J 1:3–17. https://doi.org/10.21072/mbj.2016.01.2.01
Revkov NK, Shcherban SA (2017) Biology features of the bivalve mollusk Anadara kagoshimensis in the Black Sea. Ecosystems 9:47–56
Rinaldi E (1985) Alcuni dati significativi sulla proliferazione di Scapharca inaequivalvis (Bruguière, 1789) in Adriatico lungo la costa Romagnola. Boll Malacol 21:41–42
Sahin C, Düzgüneş IE, Okumuş I (2006) Seasonal variations in condition index and gonadal development of the introduced blood cockle Anadara inaequivalvis (Bruguiere, 1789) in the southeastern Black Sea coast. Turk J Fish Aquat Sci 6:155–163
Santhanam R (2018) Biology and ecology of edible marine bivalve Molluscs. Series: Biology and ecology of marine life. Apple Academic Press, Toronto
Shiganova T (2008) Introduced species. In: Kostianoy AG, Kosarev AN (eds) The Black Sea environment. The Handbook of Environmental Chemistry. Springer-Verlag, Berlin, pp 375–406
Shiganova TA, Musaeva EI, Lukasheva TA et al (2012) Increase in findings of Mediterranean nonnative species in the Black Sea. Russ J Biol Invas 3:255–280. https://doi.org/10.1134/s2075111712040042
Somers KM (1986) Multivariate allometry and removal of size with principal components analysis. Syst Biol 35:359–368. https://doi.org/10.1093/sysbio/35.3.359
Sousa R, Gutiérrez JL, Aldridge DC (2009) Non-indigenous invasive bivalves as ecosystem engineers. Biol Invas 11:2367–2385. https://doi.org/10.1007/s10530-009-9422-7
Sşahin C, Emiral H, Okumuş I et al (2009) The benthic exotic species of the Black Sea: Blood Cockle (Anadara inaequivalvis, Bruguiere, 1789: Bivalve) and RapaWhelk (Rapana thomasiana, Crosse, 1861: Mollusca). J Anim Vet Adv 8:240–245
Strafella P, Ferrari A, Fabi G et al (2017) Anadara kagoshimensis (Mollusca: Bivalvia: Arcidae) in Adriatic Sea: morphological analysis, molecular taxonomy, spatial distribution, and prediction. Mediterr Mar Sci 443. https://doi.org/10.12681/mms.1933
Streftaris N, Zenetos A (2006) Alien marine species in the Mediterranean – the 100 ‘worst invasives’ and their impact. Mediterr Mar Sci 7:87–118
Suzuki H, Yamaguchi K, Seto K (2012) Effect of hypoxia and low salinity on growth and survival of the ark shell Scapharca kagoshimensis through the field experiment in Lake Nakaumi, southwest Japan. Aquac Sci 60:261–268. https://doi.org/10.11233/aquaculturesci.60.261
Theisen BF (1982) Variation in size of gills, labial palps, and adductor muscle in Mytilus edulis L. (Bivalvia) from Danish waters. Ophelia 21:49–63. https://doi.org/10.1080/00785236.1982.10426576
Urrutia MB, Iglesias JIP, Navarro E (1997) Feeding behaviour of Cerastoderma edule in a turbid environment: Physiological adaptations and derived benefit. Hydrobiologia 355:173–180. https://doi.org/10.1023/a:1003050700881
Utsumi S, Kishida O, Ohgushi T (2010) Trait-mediated indirect interactions in ecological communities. Popul Ecol 52:457–459. https://doi.org/10.1007/s10144-010-0236-3
Ward JE, Shumway SE (2004) Separating the grain from the chaff: particle selection in suspension- and deposit-feeding bivalves. J Exp Mar Bio Ecol 300:83–130. https://doi.org/10.1016/j.jembe.2004.03.002
Weber RE, Lykke-Madsen M, Bang A et al (1990) Effects of cadmium on anoxic survival, haematology, erythrocytic volume regulation and haemoglobin-oxygen affinity in the marine bivalve Scapharca inaequivalvis. J Exp Mar Biol Ecol 144:29–38. https://doi.org/10.1016/0022-0981(90)90017-7
Werner EE, Peacor SD (2003) A review of trait-mediated indirect interactions in ecological communities. Ecology 84:1083–1100. https://doi.org/10.1890/0012-9658(2003)084[1083:AROTII]2.0.CO;2
Wojczulanis-Jakubas K, Jakubas D, Chastel O, Kulaszewicz I (2015) A big storm in a small body: seasonal changes in body mass, hormone concentrations and leukocyte profile in the little auk (Alle alle). Polar Biol 38:1203–1212. https://doi.org/10.1007/s00300-015-1687-y
Woodward G, Ebenman B, Emmerson M et al (2005) Body size in ecological networks. Trends Ecol Evol 20:402–409. https://doi.org/10.1016/j.tree.2005.04.005
Yoshino K, Katano T, Hayami Y et al (2013) Morphological variation of pallial organs at sites of differing turbidity: a case study of an arcid bivalve. J Mar Biol Assoc UK 93:1009–1015. https://doi.org/10.1017/S0025315412000185
Yurimoto T, Mori Y, Ito S, Maeno Y (2008) Reproductive cycle of the subcrenated ark shell Scapharca kagoshimensis (Tokunaga, 1906) in Ariake Bay, Japan. J Shellfish Res 27:1101–1108. https://doi.org/10.2983/0730-8000-27.5.1101
Zaika VE, Valovaya NA, Povchun AS, Revkov NK (1990) Mytilids of the Black Sea. Naukova Dumka, Kiev. [in Russian]
Zaitsev Y, Ozturk B (2001) Anadara inaequivalvis (Bruguière, 1789). In: Zaitsev Y, Ozturk B (eds) Exotic species in the Aegean, Marmara, Black, Azov and Caspian Seas. Turkish Marine Research Foundation, Istanbul, pp 92–93
Zaitzev Y, Mamaev V (1997) Biodiversity in the Black Sea: A study of change and decline. In: Marine biological diversity in the Black Sea: a study of change and decline. United Nations Publications, New York, pp 1–208
Zenkovich VP (1958) Shores of the Black and Azov Seas. Geografgiz, Moscow. [in Russian]
Zhang M, Li L, Liu Y, Gao X (2019) Effects of a sudden drop in salinity on immune response mechanisms of Anadara kagoshimensis. Int J Mol Sci 20:4365. https://doi.org/10.3390/ijms20184365
Zolotarev PN, Terentyev AS (2012) Changes in the macrobenthos communities of the Gudauta oyster bank. Oceanology 52:251–257
Funding
The work was conducted at the authors’ own expense. No external funding was utilised.
Author information
Authors and Affiliations
Contributions
AM – the acquisition of data, AM, OZ – contributed substantially to the conception and design of the study, OZ – the analysis and interpretation.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical statements
The authors guarantee that all studies presented in the manuscript were conducted in an ethical and responsible manner, and in full compliance with all relevant codes of experimentation and legislation.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mirzoeva, A., Zhukov, O. Conchological variability of Anadara kagoshimensis (Bivalvia: Arcidae) in the northern part of the Black–Azov Sea basin. Biologia 76, 3671–3684 (2021). https://doi.org/10.1007/s11756-021-00844-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11756-021-00844-4