Abstract
The oxidative stability of diacylglycerol (DAG)-enriched soybean oil and palm olein produced by partial hydrolysis using phospholipase A1 (Lecitase Ultra) and molecular distillation was investigated at 110 °C by the Rancimat method with and without addition of synthetic antioxidants. Compared with triacylglycerol oils, the DAG-enriched oils displayed lower oxidative stability due to a higher content of unsaturated fatty acids and a lower level of tocopherols. With the addition (50–200 mg/kg) of tert-butylhydroquinone (TBHQ) or ascorbyl palmitate (AP), the oxidative stability indicated by induction period (IP) of these DAG-enriched oils under the Rancimat conditions was improved. The IP of the diacylglycerol-enriched soybean oil increased from 4.21 ± 0.09 to 12.64 ± 0.42 h when 200 mg/kg of TBHQ was added, whereas the IP of the diacylglycerol-enriched palm olein increased from 5.35 ± 0.21 to 16.24 ± 0.55 h when the same level of AP was added. Addition of TBHQ, alone and in combination with AP resulted in a significant (p ≤ 0.05) increase in oxidative stability of diacylglycerol-enriched soybean oil. AP had a positive synergistic effect when used with TBHQ.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Diacylglycerol (DAG)-enriched oil has been introduced as a functional cooking oil containing approximately 80 wt% DAG and 20 wt% TAG in the United States as well as on the Japanese market recently. DAGs are present as three different stereoisomers: sn-1,2-DAG, sn-2,3-DAG and sn-1,3-DAG. They occur as a natural component of the acylglycerol fraction in various fats and oils at levels up to 10% (w/w) [1]. Studies in both animals and humans have shown the beneficial health effects of DAGs. Although DAGs have a similar energy value as triacylglycerols (TAGs), they have the ability to decrease postprandial lipid levels [2, 3]. Consumption of DAGs has also been associated with both a reduction in body weight and a reduced accumulation of visceral abdominal fat [4, 5].
The oxidative stability of cooking oils is a critical issue in considering the safety and the shelf life of products. The autoxidation and thermal oxidation stability of DAG cooking oil have been found to be similar or even slightly better than those of conventional TAG oil at about 170 °C [6, 7]. However, the hydrolytic stability was found to be lower for DAG cooking oil, indicating that DAG cooking oil is more susceptible to hydrolysis when used for deep-frying under severe conditions (about 180 °C) [8]. DAG oil has been found to be less stable to oxidation at ambient temperatures (5–38 °C) when compared to the corresponding TAG oil [9]. However, the butter blends containing 40 wt% of DAG oil were more stable against oxidation than those containing oils from sunflower seeds [9].
Determining oxidative stability is tedious and time consuming when analyzed at room temperature, thus it is necessary to use an accelerated test to determine the oxidative stability. Several accelerated methods have been described: Schaal oven test, active oxygen method (AOM) and Rancimat method. Diacylglycerol-enriched oils are functional dietary oils with applications in replacing fats in fried potato chips, French fries and chicken. The evaluation of oxidative stability by the elevated temperature conditions of the Rancimat procedure seemed appropriate to those frying applications [10].
Evaluation of oxidative stability of diacylglycerol-enriched oil in the presence of antioxidants under the Rancimat accelerated oxidation conditions has not yet been reported. The objective of this study was to investigate the oxidative stability of the DAG-enriched soybean oil and palm olein with and without addition of antioxidants, tert-butylhydroquinone (TBHQ) or ascorbyl palmitate (AP), with the Rancimat method. As controls, the corresponding soybean oil and palm olein were also tested by this method.
Materials and Methods
Materials
tert-Butylhydroquinone (99.9%) and AP (99.9%) were provided by Dongguan Guangyi Food Additives Co. Ltd. (Dongguan, China). Standard α- and γ-tocopherol was purchased from Sigma (St. Louis, MO, USA). Soybean oil was purchased from the South Ocean Oil Industry Co. Ltd (Shenzhen, China). Palm olein was obtained from the Zhongshen Cereal and Oil Industry Co. Ltd (Dongguan, China). Commercial phospholipase A1 (Lecitase Ultra) (PLA1) was obtained from Novozymes A/S (Bagsvaerd, Denmark). The PLA1 enzyme activity was claimed to be 10,000 U/ml for acyl group hydrolysis.
Standards of the diacylglycerols of soybean oil and palm olein were prepared by column chromatography separation after molecular distillation as described below. Soybean oil (or palm olein) (50.0 g) were partially hydrolyzed by phospholipase A1 (Lecitase Ultra) at a reaction temperature of 45 °C, an enzyme load of 30 U/g (of the oil mass) and a reaction time of 8 h. After the reaction, the mixture was allowed to settle for 60 min to separate into two layers, the upper oil layer and the lower aqueous layer. The upper oil layer was molecularly distilled at 160 °C to remove the released fatty acids. The residue of the first distillation was molecularly distilled at 215 °C again, and the distillate was collected as diacylglycerol oil (DO). DO (1.0 g) was dissolved in 20 ml of hexane, and then the solution was loaded into a silica column (φ1.5 × 30 cm). TAGs were eluted with 250 ml of hexane, and DAGs were eluted with 400 ml of ethyl ether/hexane (3:7, v/v). A DAGs standard was collected after the solvents were removed by a rotary evaporator under vacuum. The purity of the standard was analyzed by thin layer chromatography (TLC) and HPLC/ESI/MS. The standard was developed by thin TLC on silica gel plates (SIL GF254, 20 × 20 cm × 0.25 mm) provided by Qingdao Haiyang Chemical Co., Ltd. (Qingdao, China), using benzene: diethyl ether: acetic ether: acetic acid (80:10:10:0.2, v/v/v/v) as the mobile phase. After visualized by iodine vapor, only diacylglycerols fractions were seen.
Production of Diacylglycerol-Enriched Oil and DAG Oil
The diacylglycerol-enriched soybean oil (DESO) and the diacylglycerol-enriched palm olein (DEPO) were produced by partial hydrolysis of soybean oil and palm olein by phospholipase A1 (PLA1) (Lectiase Ultra,) supplied by Novozymes A/S (Bagsvaerd, Denmark) and followed by molecular distillation to remove the newly released free fatty acids (FFAs). The PLA1 enzyme activity was reported as 10,000 U/mL for acyl group hydrolysis and postulated to possess sn-1,3 specificity for TAGs [11]. The partial hydrolysis was conducted at reaction temperature of 35 and 45 °C, enzyme load of 26 and 40 U/g (of the oil mass), water content of 40 wt% (of the oil mass) and reaction time of 8 h under neutral condition for soybean oil and palm olein, respectively. The advantage of production of diacylglycerol-enriched oil by partial hydrolysis catalyzed by PLA1 (Lectiase Ultra) included the lower enzyme load and the shorter reaction time compared with lipase catalyzed processes including glycerolysis, esterification and partial hydrolysis.
The soybean DAG oil (SDO) and the palm DAG oil were produced by molecular distillation of DESO and DEPO at 225 °C. The distillate of diacylglycerol with a high purity was defined as the DAG oil.
Determination of Oxidative Stability
TBHQ and AP were added in the oils with the levels varying from 50–200 mg/kg, respectively, to investigate the effect of the type and level of antioxidant on the oxidative stability on DAG-enriched oils. Mixtures of TBHQ and AP with different ratios but the same total level of 200 mg/kg was added to the DAG-enriched oils to study the interaction of the antioxidants. Oxidative stability of the oils was analyzed by the Rancimat method using a Metrohm 743 Rancimat (Herisau, Switzerland) instrument. Samples of 3.0 g were analyzed under a heating block of a given selected temperature and a constant airflow of 10 L/h.
Tocopherols
Tocopherols were evaluated following the AOCS method Ce 8–89. A solution of oil in n-hexane was analyzed in a Shimadzu HPLC instrument (LC20 AT) on a silica gel LiChrosorb Si-60 column (particle size 5 μm, 250 × 4.6 mm i.d.; Grace Division, IL, USA), which was eluted with n-hexane/2-propanol (98.5:1.5, v/v) at a flow rate of 1 ml/min. A fluorescence detector (Shimadzu RF-10AXL) was used with excitation and the emission wavelengths set at 290 and 330 nm, respectively. Levels of α and γ-tocopherol in oils were quantified using calibration curves for α and γ-tocopherol.
TBHQ in Soybean Oil
Soybean oil (1.0 g) was extracted with 10 ml (3.33 ml × 3) of methanol. The solution of TBHQ in methanol was filtered through a 0.45 μm nylon membrane filter and then 20.0 μL of the sample was injected into a Diamonsil C18 5 μm column (150 × 4.6 mm i.d.) (Dikma Technologies Inc., Tianjin, China) with a photo diode array (PDA) detector at 280 nm, which was eluted with methanol in a Shimadzu HPLC system (LC20 AT) at a flow rate of 1.0 ml/min and temperature of 37 °C. Level of TBHQ was quantified using a calibration curve for TBHQ.
Analysis of Acylglycerol by HPLC and HPLC/ESI/MS
The acylglycerol composition of oils was determined by reverse-phase high performance liquid chromatography (RP-HPLC) using an Agilent 1100 series HPLC instrument (Agilent Technologies Inc., Palo Alto, CA, USA). Samples were dissolved in a mobile phase (acetonitrile/isopropanol, 56:44, v/v) at a concentration of 10.0 mg/ml, filtered through a 0.45 μm nylon membrane filter and then 20.0 μL of the sample was injected into a Diamonsil C18 5 μm column (150 × 4.6 mm i.d.) (Dikma Technologies Inc., Tianjin, China) with photo diode array (PDA) detector at 210 nm. The flow rate was set at 1 ml/min, and the column temperature was 40 °C. A calibration curve of diacylglycerols was constructed from the standards described above, and the results are reported as the weight percentage of total acylglycerol.
The composition of acylglycerol was identified by HPLC/ESI/MS. Samples including DAGs standard, palm olein and DAG oil were diluted to a concentration of 1.0 mg/l in the solvent (acetonitrile/isopropanol, 56:44, v/v), and were then injected onto a Pinnacle II C18 5 μm column (150 × 2.1 mm i.d.) (Restek Corp., Bellefonte, PA, USA) in a 4000 QTRAP HPLC tandem mass spectrometer (Applied Biosystems Inc., Foster City, CA, USA) with electrospray ionization under positive ion mode. Formic acid (0.5%) in acetonitrile/isoproanol (55:45, v/v) was added to improve the ionization of acylglycerols. The mass spectra, between 300 and 1,200 amu were obtained at an ion scan rate of 5,500 amu/s.
Diacylglycerol Fraction Analysis
The sn-1,3-DAG and sn-1,2(2,3)-DAG components of oils were isolated by TLC on silica gel plates (SIL GF254, 20 × 20 cm, 0.25 mm), using benzene/diethyl ether/acetic ether/acetic acid (80:10:10:0.2, v/v/v/v) as the mobile phase. The sn-1,3-DAG fraction (RF = 0.87) and sn-1,2(2,3)-DAG fraction (RF = 0.78) were scraped off after visualized by iodine vapor and extracted with diethyl ether (2 ml × 3).
Samples of DAGs were then filtered through a 0.45 μm nylon membrane filter to remove impurities, and 20.0 μL of sample were injected onto a Lichrosorb Si-60 5 μm column (250 × 4.6 mm i.d., Grace Division, IL, USA) and separated by HPLC (LC20 AT, Shimadzu Inc., Kyoto, Japan) with UV detection at 210 nm. The mobile phase was n-hexane/98% aqueous isopropanol (99.6:0.4, v/v) with a flow rate of 1.0 ml/min. The ratio of sn-1, 3 DAG and sn-1,2(2,3)-DAG were calculated using peak area data.
Fatty Acid Composition
The fatty acid composition of oils were analyzed by GC (GC 900A, Shanghai Kechuang Chromatograph Instrument Co., Ltd., Shanghai, China) equipped with a capillary column (HP-5, 30 m × 0.32 mm × 0.25 μm; Agilent Technologies Inc., Palo Alto, CA, USA), a flame ionization detector (FID) and N2 as carrier gas. The injection was performed in split mode with a split ratio of 80:1. Samples (4.0 g) were dissolved in 40.0 ml of methanol and then 0.5 ml of 1.0 M methanolic KOH was added, after 10 min reaction at the boiling point of methanol, n-hexane (20 ml) and water (40 ml) were added, and the mixture was then transferred to a separatory funnel. The upper organic phase was dried over anhydrous Na2SO4, and then concentrated under a steam of nitrogen. The fatty acid methyl ester (FAME) solution (1 μL) was injected at an injector temperature of 240 °C, column temperature of 195 °C, FID temperature of 240 °C and carrier gas (N2) flow of 60 ml/min. The fatty acid composition reported was based on the area response using a flame ion detector (FID).
Statistical Analysis
Each analysis of contents of acylglycerols, fatty acids profiles and levels of antioxidants was done in triplicate with data reported as means ± standard deviations. All experiments of Rancimat test were carried out in four replicates with data reported as means ± standard deviations. One-way ANOVA was carried out using SPSS 14 statistical software (SPSS Inc., Chicago, IL). Differences were considered to be significant at p ≤ 0.05 using Duncan’s multiple range test. Differences were considered to be significant for p ≤ 0.05.
Results and Discussion
Acylglycerol and Antioxidant Composition and FA Profiles in the Oils
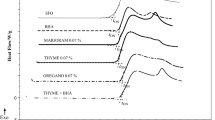
The acylglycerol and FA compositions of six oils used in this study are shown in Table 1. The HPLC chromatogram of four diacylglycerol-enriched oils with the acylglycerol peaks identified by mass spectrometry is shown in Fig. 1. Since the phospholipase A1 (Lecitase Ultra) displayed some extent of the sn-1,3 selectivity towards triacylglycerol, the resulting diacylglycerol-enriched oil from the partial hydrolysis had a lower content of saturated FAs compared to the corresponding TAG oil because the saturated FAs often take the sn-1 position in vegetable oils. The DAG oils had the highest iodine numbers (SDO, 140.60 ± 0.84 mg I2/100 g; PDO, 87.61 ± 0.48 mg I2/100 g) among the oils, which was in accordance with the FA profiles of the oils.
HPLC chromatogram of diacylglycerol-enriched oils using reversed phase column (C 18 5 μm × 150 mm × 4.6 mm) and a PDA detector at 210 nm with a mobile phase of acetonitrile/isopropanol (56:44, v/v). Linoleic (L), linolenic (Ln), oleic (O), palmitic (P), stearic (S), monoacylglycerol (MAG), diacylglycerol (DAG), triacylglycerol (TAG). a diacylglycerol-enriched soybean oil (DESO), b soybean diacylglycerol oil (SDO), c diacylglycerol-enriched palm olein (DEPO) d palm diacylglycerol oil (PDO)
The content of the monoacylglycerols (MAGs) in palm olein was much higher than that in soybean oil (Fig. 1). The contents of MAGs according to the peak areas using the PDA detector at 210 nm were 0.40% and 3.43% in soybean oil and palm olein, respectively. Due to a lack of suitable standards of MAGs, the mass contents of MAGs in the oils were not determined. Most of the MAGs were molecularly distilled in the DAG oils as a main component especially in PDO because of the small difference in vapor pressure between DAGs and MAGs. From Fig. 1b, d TAGs were found to be entrained into the distillate (DAG oils) during the molecular distillation for purification of DAGs. The acid values of all the oils determined by titration with 0.1 M KOH were less than 0.20 mg KOH/g, which eliminated the effect of free fatty acids in the oils on the oxidative stability of the oils.
It was claimed that soybean oil purchased from the market had added synthetic antioxidant, namely, TBHQ, to improve the oxidative stability. Therefore, the contents of residual TBHQ in the soybean oil-based diacylglycerol-enriched oils in this study were determined. The content of synthetic antioxidant in palm olein, which was taken directly from the factory without addition of any antioxidant, was not analyzed. Nevertheless, the main natural antioxidants in vegetable oils, namely, tocopherols, were determined in all the kinds of oils. The content of TBHQ in soybean oil was 74.68 ± 1.05 mg/kg. However, no TBHQ was detected in the DESO and the SDO. The synthetic antioxidant, which had a lower molecular weight and boiling point than fatty acids, was completely distilled into the distillate, the FFAs (the content of TBHQ in the FFAs not shown), at a temperature of 160 °C during the molecular distillation. Levels of tocopherols in the diacylglycerol-enriched oils were much lower than that in the corresponding TAG oils, especially the level of γ-tocopherol, whose molecular weight is lower than α-tocopherol, indicating that γ-tocopherol was more easily distilled into the distillate. At a temperature of 160 °C at a low feed rate, tocopherols in the palm fatty acids distillate of palm oil would be totally molecularly distilled into the distillate [12]. However, the contents of α-tocopherols in the DAG oils were significantly (p ≤ 0.05) higher than that in the diacylglycerol-enriched oils, since tocopherols were molecularly distilled into the DAG oils at temperature 225 °C.
Selection of Temperature for the Rancimat Test
Stability values of the tested oils at different temperatures obtained by the Rancimat method expressed as IP are shown in Table 2. The IP of palm olein at a temperature of 110 °C was 21.53 ± 0.22 h; however, that of PDO decreased to 1.10 ± 0.02 h. Results showed that each 10 °C rise in temperature decreased the IP by a temperature coefficient of nearly 2.0, which was similar to those previously reported for edible oils [13, 14]. The oxidative stability of the DAG-enriched oils was significantly (p ≤ 0.05) lower than the corresponding TAG oils, especially the oils with the high content of DAGs. The same results were obtained by Kristensen et al. [9] when comparing the oxidative stability of DAG oils to TAG oils. DAGs were more susceptible to oxidation due to the simpler molecular structure and less steric hindrance compared to TAGs. A similar conclusion was drawn when the palm olein was converted into palm-based biodiesel (fatty acid methyl esters, FAME) which was more easily oxidized [15]. A higher content of DAGs in the diacylglycerol-enriched oils with shorter IPs also confirmed this observation. The second reason for the lower oxidative stability of these diacylglycerol-enriched oils was the significantly (p ≤ 0.05) lower levels of natural antioxidants, especially γ-tocopherol which displayed higher antioxidative stability than α-tocopherol, than that in the corresponding TAG oils. Antioxidants played a very important role in the oxidative stability of acylglycerols and fatty acid derivatives with less steric hindrance compared to TAGs. The IP of the biodiesel produced from rapeseed oil increased nearly four times (from 9.2 to 36 h) when 400 mg/kg of TBHQ was added [15]. The third reason for less oxidative stability for these diacylglycerol-enriched oils was the enrichment of unsaturated fatty acids in the these oils due to the partial hydrolysis by PLA1 which possessed sn-1,3-selectivity towards TAGs, since unsaturated fatty acids had a higher probability to occupy the sn-2 position of the natural vegetable oils. The last reason the authors postulated was the initialization of oxidation during the partial hydrolysis by agitation and the high temperature treatment during the second-step molecular distillation which was also reported to be responsible for the lower oxidative stability of DAG oils [9].
The results of the oxidative stability of the DAG-enriched oils in this study were different from the work conducted by Shimizu et al. [7]. In their report, the autoxidation and thermal oxidation stability of DAG cooking oil were found to be similar or even slightly better than those of conventional TAG oil at about 170 °C. The fatty acids profile of the TAG oil (a mixture of rapeseed oil, perilla seed oil, and safflower oil) used in their study was similar to that of the DAG oil and the OSI (IP) of these oils at 120 °C was nearly the same. However, in this study, the corresponding TAG oils had a higher content of saturated fatty acids and a lower content of unsaturated fatty acids, and their IPs were about two to three times longer when compared to the DAG oils. Furthermore, the processes for production of DAG oils were also different. In this study, the DAG-enriched oils were produced by partial hydrolysis and purified by molecular distillation twice for a high purity at a high temperature. Nevertheless, the DAG oils reported by Shimizu et al. were prepared by esterification and purified by molecular distillation only once at a relatively low temperature, which also caused the different results of the oxidative stability of the DAG-enriched oils.
The oxidative stability of the DAG oils of palm olein was better than that of soybean oil due to the higher content of saturated fatty acids. The temperature of 110 °C was selected as the tested temperature for the Rancimat method, since IPs for DAG-enriched oils were quite short at temperature over 110 °C. The previous report showed that airflow had no effect on the IP of the tested oils [16]. According to Rancimat practice tips and tricks, the airflow is usually set according to the requirements of the standard or the customer. As long as the cooling effect deriving from the gas flow is compensated it does not show an influence on the determination of the induction time, and airflow recommended for stability test in the standards varies from 7 to 20 L/h for different materials, so the airflow in this study was fixed at 10 L/h.
Effects of Antioxidants on the Oxidative Stability
The effects of freshly added TBHQ and AP on IP of the DESO and the DEPO are shown in Fig. 2. TBHQ and AP increased IP of these two diacylglycerol-enriched oils with increasing of concentration. The IP of the DESO increased from 4.21 ± 0.09 to 12.64 ± 0.42 h when 200 mg/kg of TBHQ was added, whereas the IP of the DEPO increased from 5.35 ± 0.21 to 16.24 ± 0.55 h when the same amount of AP was added. TBHQ was more effective than AP at improving the oxidative stability of the DAG-enriched oils from soybean oil. However, AP showed better performance than TBHQ in the DAG-enriched oils from palm olein. The reason for the better performance of AP than TBHQ, the authors postulate, was that AP may have a synergistic effect with the existing natural antioxidants in palm oil at a high temperature. AP was observed to have a better performance than TBHQ for improving of oxidative stability of palm-based biodiesel under the Rancimat conditions in our previous work [17]. It is possible for an antioxidant with lower antioxidant activity at low temperatures to have better performance at high temperature, such as tocopherols, the antioxidant activity of tocopherols is dependent on temperature and is in the order of δ > γ > β > α tocopherol [18]. However, the order was found to be reversed at a low temperature (30 °C) [19]. TBHQ displayed a better performance of antioxidative ability for high unsaturated vegetable oils, such as soybean oil and sunflower oil, over other synthetic antioxidants under the Rancimat conditions [20], and consequently was widely used in these vegetable oils commercially.
The DAG oils from soybean oil and palm olein without addition of synthetic antioxidants had very short IPs, usually less than 1 h at 110 °C. The reason for these oils with less oxidative stability might be that the processing conditions (high temperatures) during production enhanced oxidation of these DAG oils. Furthermore, the levels of antioxidants and the enrichment of unsaturated fatty acids in these oils were negative to the oxidative stability. After addition of TBHQ or AP, the IPs of these oils increased very rapidly (see Fig. 3). With the addition of 200 mg/kg of TBHQ, the IP of the SDO and the PDO increased to 6.20 ± 0.18 and 3.90 ± 0.12 h, respectively. The same results that TBHQ was better for soybean diacylglycerol oil, but AP was better for palm diacylglycerol oil, were observed. With the addition of a suitable amount and type of antioxidant, the IPs of the DAG oils were over 6 h at the temperature of 110 °C.
The Rancimat test determines the end point by using conductance based on stable secondary reaction products which are from the decomposition of the primary peroxy species in the autoxidation [16]. AP, known as a secondary antioxidant, scavenges oxygen and can react with peroxy species using a redox mechanism, thus converting the peroxy species back into the acyl group to prevent the decomposition of the primary peroxy species and to prolong the induction period under the Rancimat conditions. AP forms chelates with metals which promote the production of free radicals and prolongs the induction period of oils for autoxidation [21]. TBHQ is a primary antioxidant, which reacts with lipid and peroxy radicals and converts them to more stable, nonradical products. TBHQ is capable of donating a hydrogen atom to lipid radicals and produces lipid derivatives and antioxidant radicals that are more stable and less readily available to participate in propagation reactions [22].
A synergistic interaction was observed between TBHQ and AP when the total level of antioxidant in the diacylglycerol-enriched soybean oil and palm olein was 200 mg/kg (see Fig. 4). The results showed that the antioxidant activity of TBHQ in combination with AP was better that of TBHQ used alone in the DEPO. A 1:1 ratio of TBHQ to AP resulted in the greatest increase of oxidative stability of diacylglycerol-enriched oils as indexed by IP. AP acted as an oxygen scavenger and also as a reducing agent, which regenerated TBHQ radical intermediate to its reduced form [22]. The best synergism of antioxidants was found at a 1:1 ratio of TBHQ to AP. At this ratio, together with the inherent tocopherols from the oils, the primary antioxidants and the secondary antioxidants produced the strongest enhanced activity in this system by Rancimat method. A positive synergistic effect of TBHQ in combination with AP was also observed for improvement of antioxidation stability of high-oleic vegetable oils [20]. Furthermore, the natural tocopherol antioxidants will also regenerate the oxidized AP and TBHQ into their reduced state. The regeneration of carotenoids by tocopherols was observed in the palm oil during frying [23], which caused a synergistic antioxidant effect on the improvement of oxidative stability of those oils.
Effects of addition of an antioxidant (with a total level of 200 mg/kg) with different ratios of TBHQ to AP on the Rancimat induction period (IP) (h) of a the diacylglycerol-enriched soybean oil (DESO) and b the diacylglycerol-enriched palm olein (DEPO). tert-Butylhydroquinone (TBHQ), ascorbyl palmitate (AP)
Conclusions
The oils with a higher content of DAGs showed a lower oxidative stability compared to their corresponding TAG oils. The oxidative stability of the DESO and the DEPO indicated by IP at 110 °C was 4.21 ± 0.06 and 5.40 ± 0.21 h, where the IP of their corresponding triacylglycerol (TAG) oils was 9.46 ± 0.08 and 21.53 ± 0.22 h, respectively. The oxidative stability of the DAG-enriched oils was increased by the addition of antioxidants. Addition of TBHQ, alone and in combination with AP, resulted in a significant (p ≤ 0.05) increase in the oxidative stability of diacylglycerol-enriched soybean oil.
References
Watanabe T, Sugiura M, Sato M, Yamada N, Nakanishi K (2005) Diacylglycerol production in a packed bed bioreactor. Process Biochem 40:637–643
Ai M, Tanaka A, Shoji K, Ogita K, Hase T, Tokimitsu I, Shimokado K (2007) Suppressive effects of diacylglycerol oil on postprandial hyperlipidemia in insulin resistance and glucose intolerance. Atherosclerosis 195:398–403
Tomonobu K, Hase T, Tokimitsu I (2006) Dietary diacylglycerol in a typical meal suppresses postprandial increases in serum lipid levels compared with dietary triacylglycerol. Nutrition 22:128–135
Nagao T, Watanabe H, Goto N, Onizawa K, Taguchi H, Matsuo N, Yasukawa T, Tsushima R, Shimasaki H, Itakura H (2000) Dietary diacylglycerol suppresses accumulation of body fat compared to triacylglycerol in men in a double-blind controlled trial. J Nutr 130:792–797
Umeda T, Bauer JE, Otsuji K (2006) Weight loss effect of dietary diacylglycerol in obese dogs. J Anim Physiol Anim Nutr 90:208–215
Nishide T, Shimizu M, Tiffany TR, Ogawa H (2004) Cooking oil: cooking properties and sensory evaluation. In: Katsuragi Y, Yasukawa T, Matsuo N, Flickinger BD, Tokimitsu I, Matlock MG (eds) Diacylglycerol oil. AOCS Press, Champaign, IL, pp 197–207
Shimizu M, Moriwaki J, Nishide T, Nakajima Y (2004) Thermal deterioration of diacylglycerol and triacylglycerol oils during deep-frying. J Am Oil Chem Soc 81:571–576
Li CM, Kimura F, Endo Y, Maruyama C, Fujimoto K (2005) Deterioration of diacylglycerol- and triacylglycerol-rich oils during frying of potatoes. Eur J Lipid Sci Tech 107:173–179
Kristensen JB, Nielsen NS, Jacobsen C, Mu HL (2006) Oxidative stability of diacylglycerol oil and butter blends containing diacylglycerols. Eur J Lipid Sci Tech 108:336–350
Farhoosh R, Moosavi SMR (2007) Rancimat test for the assessment of used frying oils quality. J Food Lipids 3:263–271
Wang Y, Zhao MM, Ou SY, Xie LY, Tang SZ (2009) Preparation of a diacylglycerol-enriched soybean oil by phospholipase A1 catalyzed hydrolysis. J Mol Catal B Enzym 56:165–172
Posada LR, Shi J, Kakuda Y, Xue SJ (2007) Extraction of tocotrienols from palm fatty acid distillates using molecular distillation. Sep Purif Technol 57:220–229
Farhoosh R (2007) The effect of operational parameters of the Rancimat method on the determination of the oxidative stability measures and shelf-life prediction of soybean oil. J Am Oil Chem Soc 84:205–209
Farhoosh R, Niazmand R, Rezaei M, Sarabi M (2008) Kinetic parameter determination of vegetable oil oxidation under Rancimat test conditions. Eur J Lipid Sci Tech 110:587–592
Dunn RO (2008) Antioxidants for improving storage stability of biodiesel. Biofuels Bioprod Bioref 2:304–318
Mateos R, Uceda M, Aguilera MP, Escuderos ME, Maza GB (2006) Relationship of Rancimat method values at varying temperatures for virgin olive oils. Eur Food Res Technol 223:246–252
Wang Y, Hua XF, Tang YL (2007) Effects of antioxidants on oxidative stability of biodiesel (in Chinese). J Chin Cereals Oils Assoc 23:153–156
Shahidi F, Zhang Y (2005) Antioxidants: regulatory status. In: Shahidi F (ed) Bailery’s industrial oil and fat products, v1: edible oil and fat products: chemistry, properties, and health effects, 6th edn. Wiley, Hoboken, NJ, pp 491–512
Kulas E, Ackman RG (2001) Properties of α-, β-, and γ-tocopherol in purified fish oil triacylglycerols. J Am Oil Chem Soc 78:361–367
Merrill LI, Pike OA, Ogden LV, Dunn ML (2008) Oxidative stability of conventional and high-oleic vegetable oils with added antioxidants. J Am Oil Chem Soc 85:771–776
Beddows CG, Jagait C, Kelly MJ (2001) Effect of ascorbyl palmitate on the preservation of alpha-tocopherol in sunflower oil, alone and with herbs and spices. Food Chem 73:255–261
Shihadi F, Wanasundara PKJPD (2005) Antioxidants: science, technology, and applications. In: Shahidi F (ed) Bailery’s industrial oil and fat products, v1: edible oil and fat products: chemistry, properties, and health effects, 6th edn. Wiley, Hoboken, NJ, pp 431–489
Schroeder MT, Becker EM, Skibsted LH (2006) Molecular mechanism of antioxidant synergism of tocotrienols and carotenoids in palm oil. J Agr Food Chem 54:3445–3453
Acknowledgments
The financial support from the Science and Technology Council of Guangdong under grant 2008A01090003 and the Ministry of Science and Technology of People’s Republic of China under grant 2006BAD27B04 is gratefully acknowledged. Furthermore, the authors acknowledge the assistance of Dr. Michael G. Jackson and Dr. Jiuwei Teng, Department of Food Quality and Safety, Jinan University, Guangzhou, China, in the preparation of this article.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Wang, Y., Zhao, M., Tang, S. et al. Evaluation of the Oxidative Stability of Diacylglycerol-Enriched Soybean Oil and Palm Olein Under Rancimat-Accelerated Oxidation Conditions. J Am Oil Chem Soc 87, 483–491 (2010). https://doi.org/10.1007/s11746-009-1521-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11746-009-1521-1