Abstract
Lipogenesis is the process by which fatty acids are synthesized. In metabolic syndrome, an insulin resistant state along with high plasma levels of free fatty acids (FFA) and hyperglycemia may contribute to the lipogenic process. The aim of the present study was to investigate the effects of oral administration of metformin on the expression of lipogenic genes and glycemic profile in mice fed with low-carbohydrate high-fat diet by evaluating their metabolic profile. SWISS male mice were divided into 4 groups (N = 7) that were fed with standard (ST), standard plus metformin (ST + MET), low-carbohydrate high-fat diet (LCHFD) and low-carbohydrate high-fat diet plus metformin (LCHFD + MET) (100 mg kg−1 diet) diets respectively. Food intake, body weight and blood parameters, such as glucose tolerance, insulin sensitivity, glucose, HDL-c, total cholesterol, triglycerides, ASL and ALT levels were assessed. Histological analyses were performed on hematoxylin and eosin-stained epididymal adipose tissue histological specimens. The expression levels of peroxisome proliferator-activated receptor (PPARγ), sterol regulatory element-binding protein 1 (SREBP1), fatty acid synthase (FAS) and acetyl-CoA carboxylase (ACC), were assessed by RT-PCR. This study showed that metformin decreased adipocyte area, body weight and food consumption in obese animals when compared to the standard group. Furthermore, the expression of lipogenic markers in adipose tissue were diminished in obese animals treated with metformin. This data showed that oral administration of metformin improved glucose and lipid metabolic parameters in white adipose tissue by reducing the expression of lipogenesis markers, suggesting an important clinical application of MET in treating obesity-related diseases in metabolic syndrome.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Metabolic syndrome (MetS) is a cluster of several metabolic abnormalities, including central (intra-abdominal) obesity, insulin resistance, hypertension, dyslipidemia and hyperglycemia, and has become one of the major public-health challenges worldwide [1]. Insulin resistance contributes in many ways to the pathogenesis of MetS. One of the main factors associated with the genesis of insulin resistance is a high amount of free fatty acids commonly associated with expanded adipose tissue mass, which reduces glucose uptake, decreasing insulin sensitivity in muscle and adipocytes. Additionally, high circulating glucose and FFA levels lead to an increased pancreatic secretion of insulin, resulting in hyperinsulinemia, which may result in higher rates of sodium reabsorption and enhance sympathetic nervous system activity, finally contributing to the development of hypertension and increasing levels of FFA [2].
Along with insulin resistance, obesity is known to play a causative role in the pathogenesis of MetS [3]. Obesity is characterized by an excessive deposition of body fat, especially intra-abdominal fat [4], and its prevalence is rapidly increasing all over the world [5, 6]. Obesity is also known to trigger many diseases, such as type 2 diabetes, atherosclerosis, hypertension, fatty liver disease and osteoarthritis [7, 8]. The metabolic role of adipose tissue can be seen through its contribution to body mass, which may reach up to 40% in obese subjects [9]. Patients with MetS exhibit many manifestations of accelerated aging, such as cardiovascular disease, cancer, inflammatory and metabolic disorders, all of which reducing lifespan [10].
In this sense, identifying the molecular mechanisms underlying obesity has been described as one of the most critical endeavors in modern medicine [11]. Along with that, the fact that metformin treatment has been associated with reduced risk of cancer and cardiovascular disease raises the prospect of a beneficial role of this compound in the treatment of age-related diseases [12].
Metformin is a biguanide used since the 1960s in the treatment of type 2 diabetes and MetS. It enhances insulin sensitivity, induces glycolysis and suppresses gluconeogenesis in the liver [13–15]. Metformin treatment induces weight loss by reducing gluconeogenesis and also affects the hepatic lipid metabolism. These effects are also mediated by AMP-activated protein kinase (AMPK) activation. In this way, metformin may decrease the expression of genes involved in lipogenesis, such as fatty acid synthase (FAS) and acetyl CoA carboxylase (ACC), thus reducing the synthesis of fatty acids and triglycerides while increasing the rate of oxidation of fatty acids. The aforementioned effects seem to be promoted the by down-regulation of SREBP-1 (protein-1 sterol regulatory element binding protein) [16].
Lipogenesis is the process by which the fatty acids are synthesized using the glucose derived from the metabolism of dietary carbohydrates as the primary substrate [17]. PPARγ (peroxisome proliferator-activated receptor) plays an important role in lipid metabolism as it promotes the upregulation of enzymes responsible for adipogenesis and fatty acid uptake [18–20]. The PPARγ, after crosstalk with other substances, suppresses the transcription factor of SREBP-1 (protein-1 sterol regulatory element binding), thereby reducing the expression of the enzymes ACC and FAS, which are involved in the fatty acids synthesis. It is suggested that these effects occur through the activation of AMPK [21].
Therefore, this study aimed to investigate the effects of oral administration of metformin on glucose and lipid metabolism in mice fed with low-carbohydrate high-fat diet, evaluating lipogenic genes in white adipose tissue of mice.
Materials and Methods
Experimental Design
The experiment was conducted with 28 SWISS male mice (4 weeks old) from the Universidade Federal de Minas Gerais (Belo Horizonte, Minas Gerais, Brazil). The mice were individually housed under a 12-h light–dark cycle, at a temperature of 22 ± 2 °C and had free access to water and food. After the adaptation period, the mice were randomly divided into four groups (n = 7) and fed with the following experimental diets for 4 months: standard diet (ST); low-carbohydrate high-fat diet (LCHFD), standard diet plus metformin (ST + MET) and low-carbohydrate high-fat diet plus metformin (LCHFD + MET) (Table 1). First, obesity was induced by feeding the SWISS male mice with low-carbohydrate high-fat diet (LCHFD) for 8 weeks. Low-carbohydrate high-fat diet was prepared as described previously [22], with 24.55% carbohydrate, 14.47% protein and 60.98% fat, representing 5.28 kcal/1 g of diet. All of the low-carbohydrate high-fat diet components were purchased from Rhoster® LTDA (São Paulo, SP, Brazil). The standard diet (Purina-Labina®, USA), for the regular maintenance of the rats, is composed of 50.30% of carbohydrate, 41.90% of protein and 7.80% of fat, with a total of 2.18 kcal/1 g of diet [23]. The metformin (Cloridrato de Metformina, Medley Farmacêutica Ltda., SP, Brazil) was orally administered 100 mg kg−1 diet, according to previous studies [24], for 2 months following the 8-week induction of obesity period. Food intake and body weight were evaluated three times a week during the treatment period. The animals were maintained according to the ethical guidelines of the Universidade Estadual de Montes Claros, where the experimental protocol was approved (Ethical Committee in Animals Experimentation/protocol 051/2013).
Measurements of Body Weight, Food Intake, Glucose Tolerance and Insulin Sensitivity Tests
The mice were individually housed and the food intake was measured twice per week during the treatment in order to obtain food efficiency (food intake/body weight). For the glucose tolerance test, d-glucose (2 mg/g body weight) was intraperitoneally injected into overnight fasted mice. Glucose levels from tail blood samples were monitored at 0, 15, 30, 60, and 120 min after injection using an Accu-Check glucometer (Roche Diagnostics®, Indianapolis, USA). An insulin sensitivity test was performed on overnight-fed mice, after intraperitoneal injection of insulin (0.75 units/kg body weight; Sigma-Aldrich®, St. Louis, 48 USA). Tail blood samples were taken at the times 0, 15, 30, and 60 min after injection for measurement of blood glucose levels [25].
Tissue Collection
At the end of the experimental period, overnight fasted mice were euthanized by decapitation and samples of blood, epididymal, retroperitoneal and mesenteric adipose tissue were collected, weighed, immediately frozen in liquid nitrogen and stored at −80 °C for subsequent analysis.
Determination of Plasma Parameters
Serum was obtained after blood centrifugation (1000×g for 10 min at 4 °C). Total serum cholesterol, high-density lipoprotein (HDL), very low-density lipoprotein (VLDL), low-density lipoprotein (LDL) and triglycerides were assayed using enzymatic kits (Wiener®, Argentina). Serum insulin was measured by chemiluminescence using a Rat/Mouse Insulin Kit (Millipore®, Billerica, USA) and ADVIA-Centaur equipment.
Histology
Hydrated, 5.0-μm sections of paraformaldehyde-fixed and paraffin-embedded epididymal adipose tissue fragments were stained with hematoxylin and eosin (H&E), and evaluated under a conventional light microscope (Axioskop40). Images of fat tissue areas (×10 ocular and ×40 objective lenses) were captured with an Axio Cam MRc Zeis camera.
Reverse Transcription and Real Time PCR (qRT-PCR)
Total RNA from epididymal adipose tissue was extracted using TRIzol reagent (Invitrogen Corp.®, San Diego, California, USA) followed by treatment with DNAse. The cDNA generated by M-MLV (Invitrogen Corp®) using random hexamer primers was analyzed by Q-PCR using SYBR green reagent (Applied Biosystems®, USA) in a PlusOne platform (Applied Biosystems®). Specific primers for the endogenous glyceraldehyde 3- phosphate dehydrogenase (GAPDH) (internal control), FAS, ACC, SREBP1 and PPARγ cDNA were used as detailed in Table 2. The Livak and Schmittgen relative comparative CT method was applied to compare gene expression levels between groups, using the 2−ΔΔCT method [26].
Statistical Analysis
All data have been presented as mean ± SEM values and analyzed using the software GraphPad Prism 5.0® (San Diego, USA) and submitted to specific tests with a statistic confidence of 95% (p < 0.05). The statistical significance of differences in mean values between mice groups were assessed by one-way ANOVA and the Bonferroni post hoc test.
Results
First we evaluated the body profile. The results showed that metformin administered along with a LCHFD diet was able to decrease the body weight of the animals as compared to LCHFD fed mice. Body weight was also decreased in mice fed ST and ST + MET as compared to LCHFD (Fig. 1a). The ST + MET and LCHFD + MET groups of mice had lower levels of overall adiposity as compared to ST and LCHFD respectively (Fig. 1b).
Body weight and adipose tissue formation mice-fed standard (ST), ST plus metformin (ST + MET), Low-carbohydrate high-fat diet (LCHFD), LCHFD plus metformin (LCHFD + MET). a Body weight (b) Adiposity overall (kcal/body weight) (c) Epididymal adipose tissue (g/body weight), d Mesenteric adipose tissue (g/body weight), e Retroperitoneal adipose tissue (g/body weight). *p < 0.05 vs ST, **p < 0.05 vs ST + MET, ***p < 0.05 vs ST and ST + MET, + p < 0.05 vs LCHFD. Test one way-ANOVA following of Bonferroni test
The weight of the main sources of visceral adipose tissue was also evaluated in order to measure the overall adiposity of the animals. It was observed that mice fed a LCHFD diet presented higher epididymal, mesenteric and retroperitoneal adipose pads mass, as compared to the other treatment groups (Fig. 1c–e). Thus, we might say that metformin was able to decrease the weight of the aforementioned adipose tissues to levels similar to those from mice fed a ST and ST + MET.
Next, we analyzed the food and caloric consumption to correlate with the body profile results. Mice fed a LCHFD diet and those fed a LCHFD + MET consumed lower amounts of food as compared to ST and ST + MET mice (ST: 0.174 ± 0.005; ST + MET: 0.166 ± 0.005; LCHFD: 0.095 ± 0.004; LCHFD + MET: 0.100 ± 0.003), although no statistically significant differences were observed between groups of mice fed the same diet. On the other hand, a lower number of calories was consumed by the animals fed a ST diet and treated with Metformin as compared to the control. No statistically significant differences were observed between LCHFD and LCHFD + MET as compared to ST (ST: 0.489 ± 0.093; ST + MET: 0.362 ± 0.066; LCHFD: 0.500 ± 0.129; LCHFD + MET: 0.529 ± 0.090).
In order to evaluate the effects of the treatment with metformin at a biochemical level, we analyzed the lipid and glycemic profiles. We observed that treatment with metformin did not alter the HDL-cholesterol levels. In contrast, the triglycerides levels were lower in mice treated with metformin as compared to the respective control groups. Mice fed a standard diet demonstrated higher levels of VLDL as compared to the other groups. Finally, we observed that mice treated with metformin under LCHFD diet had lower levels of total cholesterol as compared to the mice fed a standard diet and treated with metformin, as well as in comparison with the mice fed a LCHFD diet (Table 3).
Concerning the levels of fasting glucose, treatment with metformin was observed to have lower levels of fasting plasma glucose compared to the respective controls. The administration of metformin in mice fed a standard diet was also associated with decreased glucose levels as compared to ST (Fig. 2a).
Insulin sensitivity and glucose tolerance tests in mice-fed standard (ST), ST plus metformin (ST + MET), Low-carbohydrate high-fat diet (LCHFD), LCHFD plus metformin (LCHFD + MET). a Fasting glucose levels (b) Intraperitoneal glucose tolerance test (IPGTT) and IPGTT glucose area under the curve. c Intraperitoneal insulin sensitivity test (IPIST) and IPIST insulin area under the curve. *p < 0.05 vs ST, **p < 0.05 vs ST, + p < 0.05 vs LCHFD
The glucose tolerance test showed higher glucose intolerance in mice fed a LCHFD as compared to the other treatment groups. The insulin sensitivity test showed similar results, but showed that metformin treatment of mice fed LCHFD was not associated with improvement in insulin sensitivity (Fig. 2b, c).
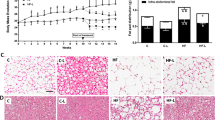
To confirm the effects of metformin at a tissue level, the histological analysis showed that metformin reduced the adipocyte diameter of LCHFD + MET mice compared to LCHFD, ST and ST + MET, demonstrating the benefits of metformin’s treatment to rescue the adipocyte hypertrophy found in obesity and even normal weight (Fig. 3).
Epididymal adipose tissue of mice-fed standard (ST), ST plus metformin (ST + MET), Low-carbohydrate high-fat diet (LCHFD), LCHFD plus metformin (LCHFD + MET). a Mean diameter of adipocytes (b) Hematoxylin and eosin (HE) staining of epididymal adipose tissue samples. Inlet HE staining. Scale bar ×40. + p < 0.05 vs LCHFD ***p < 0.05 vs ST and ST + MET (one-way ANOVA and Bonferroni post-test)
Finally, the expression of selected genes that are related to adipogenesis was measured. We observed that PPARγ (lipid storage and glucose metabolism regulator), SREBP (involved in cholesterol biosynthesis), FAS (involved in fatty acid synthesis) and ACC (fatty acid metabolism regulator) had their expression significantly suppressed in the epididymal adipose tissue from animals fed LCHFD and treated with metformin. Furthermore, there was a reduction in the expression of PPARγ and SREBP1 genes in ST + MET group compared to the ST group, demonstrating the action of metformin in standards groups as well (Fig. 4a–d).
Lipogenic genes expression by RT-PCR in epididymal adipose tissue of mice-fed standard (ST), ST plus metformin (ST + MET), Low-carbohydrate high-fat diet (LCHFD), LCHFD plus metformin (LCHFD + MET). a Peroxisome proliferator-activated receptor (PPARγ) (Arbitrary Unit) (b) sterol regulatory element-binding protein 1 (SREBP1-c) (Arbitrary Unit) (c) fatty acid synthase (FAS) (Arbitrary Unit) (d) acetyl-CoA carboxylase (ACC) (Arbitrary Unit). *p < 0.05 vs ST, **p < 0.05 vs ST + MET, ***p < 0.05 vs ST and ST + MET, + p < 0.05 vs LCHFD
Discussion
The main findings of this study and its originality refer to the mRNA expression of the genes PPARγ, SREBP1-c, FAS and ACC that showed statistic significant differences among LCHFD-fed and metformin-treated groups. These data were corroborated by Li et al., who showed that metformin exhibits an anti-lipogenic effect by suppressing hepatic expression of lipogenic enzymes including ACC and FAS [27]. In our study, SREBP1 enzyme was increased in mice fed with low-carbohydrate high-fat diet. As a consequence of elevated insulin levels, which is common in cases of insulin resistance, increased levels of SREBP1 led to an increased expression of the lipogenic enzymes ACC and FAS, thus acting directly on the lipogenesis process. In a study from Liang et al. mice lacking SREBP-1c expression also had a marked decrease in FAS mRNA expression, even in the presence of insulin [28].
The present results also showed that metformin was capable of controlling the weight gain of mice fed with low-carbohydrate high-fat diet, which may assist in the treatment of obesity and MetS. These data are consistent with several reports that show that obese patients with type 2 diabetes treated with metformin do not gain body weight; indeed, many patients under treatment with this agent lose body weight by decreasing food consumption [29]. Additionally, some studies suggest that metformin may be effective in reducing body weight in obese subjects who develop MetS by consuming too much dietary fat and in preventing or treating type 2 diabetes [30].
The main results from the biochemical analysis showed decreased levels of total cholesterol and triglycerides in animals treated with metformin and fed LCHFD. This might be explained by the fact that the LCHFD group had higher amounts of saturated fat, which is directly associated with increased total cholesterol levels corroborating with a study where the replacement of saturated fat for monounsaturated and polyunsaturated fatty acids reduces total cholesterol [31]. In addition, it has been demonstrated in other studies that in an insulin resistant state, metformin is capable of modulating lipid metabolism by reducing plasma levels of free fatty acids and their tissue oxidation, hepatic VLDL secretion, and consequently total cholesterol and triglycerides levels with an increase in HDL [32–34]. The levels and type of free fatty acids also directly affects the action of insulin, probably by affecting the glucose-fatty acid cycle [35].
We observed that metformin decreased blood glucose levels in groups fed with high-fat and standard diets, unlike the results reported by Cheng et al. [36]. Our results also corroborate those of Tang and Reed [37] and Souza et al. [38], which identified a significant decrease in the levels of glucose in obese mice and rats, respectively, treated with metformin, although no significant difference was found for the glucose tolerance and insulin sensitivity tests in our study. However, the comparison among the mentioned studies is limited due to the different methodological designs adopted by each of them. These differences are mainly attributed to the different animal models (rats and mice) and lineages used. Additionally, the metformin concentration and form of administration applied differ, which confers to our study a lower realistic dose.
In addition, metformin was able to decrease the average area of adipocytes in all groups when compared to the LCHFD group, contributing to an improvement in overall adiposity. This contributes to the studies that show the potential of metformin to inhibit lipolysis and the availability of free fatty acids in adipocytes. In addition, the drug increases the number of insulin receptors and improves their affinity in adipocytes and muscle [39].
The main findings of this study are controversial regarding the effects of LCHF diets on humans. This diet also functions as a part of several therapeutic approaches to ameliorate obesity effects by causing loss of weight and improvements in glycemic and lipid parameters [40, 41]. It should be noted that there are some papers that correlate increased risk of developing glucose intolerance and non-insulin-dependent diabetes mellitus with the LCHF diet [42]. On the other hand, in mice it is already shown in the literature that the LCHF diet is found to increase weight gain and does not improve the glycemic profile [43]. These differences might correlate with the differences of metabolism present in different species.
Additionally, the dose of metformin chosen to be applied to our animals in this study is higher than the human dose equivalent. However, this difference in the doses applied might be annulled based on the basal metabolic rates in mice, which is many times greater than in humans, thus doses given to mice will not necessarily be similar to humans [44, 45].
Our choice to treat the animals with a low-carbohydrate high-fat diet is based on several studies published by our group that successfully induced an obesity state and some of its associated complications in animal models [22, 46–49]. However, it is worth mentioning that the diet’s effect is more associated with its content, the types of molecules, such as unsaturated vs saturated fatty acids or carbohydrates with a high GI or low GI, than the diet’s composition in percentage of lipids and carbohydrates. The production of fatty acids is part of key processes (lipogenesis and adipogenesis) that occur in the adipose tissue. Fatty acids stimulate the production of TAG and adipogenesis processes in the white adipose tissue, by activating genes such as PPARγ, which modulates CCAAT/enhancer-binding protein α, and consequently activating adipogenic genes [50]. Additionally, the pathway of fatty acids synthesis accounts with the increase of acetyl-CoA carboxylase (ACC), responsible to convert acetyl-CoA to malonyl-CoA. Increase in fatty acids release is discussed to be caused by SREBP-1c, induced by high insulin levels. SREBP-1c increases fatty acid levels in obese (ob/ob) mice with insulin resistance and hyperinsulinemia caused by leptin deficiency [51]. Thus, increased levels of SREBP-1c augments lipogenic gene expression, fatty acid synthesis, and triglyceride accumulation [52].
In summary, oral administration of metformin offers a protective effect against lipogenesis in the white adipose tissue of an obese mice model induced by a low-carbohydrate high-fat diet and improves glucose and lipid metabolism as a consequence of a decrease in the expression of lipogenic genes, which appear to be critical regulators of metabolic homeostasis. These effects were associated with beneficial regulation of reduced body, food intake, adipocyte diameter and total cholesterol. These results support the hypothesis of the use of metformin as a novel therapeutic agent for the prevention and treatment of obesity-related disorders. Future studies should be performed to evaluate complementary molecular mechanisms and interactions involved in the present findings.
This study also has potential limitations. The current study used Swiss mice as an animal model of obesity induced by low-carbohydrate/high-fat diet, which have metabolic and physiological differences that may have distinct effects that differ from how humans would present.
Conclusion
In conclusion, the present study indicates that oral treatment with metformin in Swiss mice induces important alterations in the adipose tissue, evidenced by the decreased diameter of the adipocytes, overall adiposity, body weight and food consumption, which might be a consequence of the decreased expression of lipogenic genes.
Abbreviations
- ACC:
-
Acetyl CoA carboxylase
- AMPK:
-
AMP-activated protein kinase
- FAS:
-
Fatty acid synthase
- FFA:
-
Free fat acids
- GAPDH:
-
Glyceraldehyde 3- phosphate dehydrogenase
- HDL:
-
High-density lipoprotein
- LCHFD:
-
Low-carbohydrate high-fat diet
- LCHFD + MET:
-
Low-carbohydrate high-fat diet plus metformin
- H&E:
-
Hematoxylin and eosin
- LDL:
-
Low-density lipoprotein
- MetS:
-
Metabolic syndrome
- PPARγ:
-
Peroxisome proliferator-activated receptor
- qRT-PCR:
-
Real time PCR
- SREBP-1:
-
Protein-1 sterol regulatory element binding
- ST:
-
Standard diet
- VLDL:
-
Very low-density lipoprotein
References
Alberti KG, Zimmet P, Shaw J (2005) The metabolic syndrome—a new worldwide definition. Lancet 366:1059–1062
Robert HE, Scott MG, Paul ZZ (2005) The metabolic syndrome. Lancet 365:1415–1428
Bjorntorp P (1991) Metabolic implications of body fat distribution. Diabetes Care 14:1132–1143
Tchernof A, Després J (2013) Pathophysiology of human visceral obesity: an update. Physiol Rev 93:359–404
Flegal KM et al (2010) Prevalence and trends in obesity among US adults, 1999–2008. JAMA 303:235–241
Bae NK, Kwon IS, Cho YC (2009) Ten year change of body mass index in Korean: 1997–2007. Korean J Obes 18:24–30
Mokdad AH et al (2003) Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA 289:76–79
Grundy SM (2004) Obesity, metabolic syndrome, and cardiovascular disease. J Clin Endocrinol Metab 89:2595–2600
Trayhurn P (2013) Hipoxia and adipose tissue function and dysfunction in obesity. Physiol Rev 93:1–21
Libby G et al (2009) New users of metformin are at low risk of incident cancer: a cohort study among people with type 2 diabetes. Diabetes Care 32:1620–1625
Taubes G (2009) Insulin resistance. Prosperity’s plague. Science 325:256–260
Giovannucci E et al (2010) Diabetes and cancer: a consensus report. Diabetes Care 33:1674–1685
He L et al (2009) Metformin and insulin suppress hepatic gluconeogenesis through phosphorylation of CREB binding protein. Cell 137:635–646
Correia S et al (2008) Mechanisms of action of metformin in type 2 diabetes and associated complications: an overview. Mini Rev Med Chem 8:1343–1354
Pollak MN (2012) Investigating metformin for cancer prevention and treatment: the end of the beginning. Cancer Discov 9:778–790
Viollet B et al (2012) Cellular and molecular mechanisms of metformin: an overview. Clin Sci 122:253–270
Girard J, Foifelle F, Ferré P (1997) Mechanisms by which carbohydrates regulate expression of genes for glycolytic and lipogenic enzymes. Annu Rev Nutr 17:325
Calder PC (2012) Mechanisms of action of (n-3) fatty acids. J Nutr 142:592–599
Puglisi MJ, Hasty AH, Saraswathi V (2011) The role of adipose tissue in mediating the beneficial effects of dietary fish oil. J Nutr Biochem 22:101–108
Lima TM et al (2007) Mechanisms by which fatty acids regulate leucocyte function. Clin Sci 113:65–77
Suchankova G et al (2005) Dietary polyunsaturated fatty acids enhance hepatic AMP-activated protein kinase activity in rats. Biochem Biophys Res Commun 326:851–858
Santos SHS et al (2013) Oral Angiotensin-(1-7) prevented obesity and hepatic inflammation by inhibition of resistin/TLR4/MAPK/NF-kappaB in rats fed with high-fat diet. Peptides 46:47–52
Andrade JMO et al (2014) Resveratrol increases brown adipose tissue thermogenesis markers by increasing SIRT1 and energy expenditure and decreasing fat accumulation in adipose tissue of mice fed a standard diet. Eur J Nutr 53:1503–1510
Montalvo AM (2012) Metformin improves healthspan and lifespan in mice. Nat Commun 4:2192
Santos SHS et al (2012) Increased circulating angiotensin-(1-7) protects white adipose tissue against development of a proinflammatory state stimulated by a high-fat diet. Regul Pept 178:64–70
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−∆∆CT method. Methods 25:402–408
Li Y et al (2011) AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab 13:376–388
Liang G et al (2002) Diminished hepatic response to fasting/refeeding and liver X receptor agonists in mice with selective deficiency of sterol regulatory element-binding protein-1c. J Biol Chem 277:9520–9528
Desilets AR, Dhakal-Karki S, Dunican KC (2008) Role of metformin for weight management in patients without type 2 diabetes. Ann Pharmacother 42:817–826
Yukari M et al (2010) Metformin reduces body weight gain and improves glucose intolerance in high-fat diet-fed C57BL/6J mice. Biol Pharm Bull 33:963–970
Kuipers RS et al (2011) Saturated fat, carbohydrates and cardiovascular disease. Neth J Med 69:372–378
Augusto CSJ, Michelle RU, Ana TS (2008) Meformina e AMPK: um antigo fármaco e uma nova enzima no contexto da síndrome metabólica. Arquivos Brasileiros de Endocrinologia e Metabolismo 52:120–125
SBD-Sociedade Brasileira de Diabetes (2003) Consenso Brasileiro sobre Diabetes: Diagnóstico e classificação do Diabetes Mellitus e tratamento do Diabetes Mellitus Tipo 2. Diagraphic, Rio de Janeiro
Grosskopf I et al (1997) Metformin enhances clearance of chylomicrons and chylomicron remnants in nondiabetic mildly overweight glucose-intolerant subjects. Diabetes Care 20:1598–1602
Storlien LH et al (1991) Influence of dietary fat composition on development of insulin resistance in rats: relationship to muscle triglyceride and ω-3 fatty acids in muscle phospholipid. Diabetes 40(2):280–289
Cheng JT et al (2001) Metformin-like effects of Quei Fu Di Huang Wan, a Chinese herbal mixture, on streptozotocin-induced diabetic rat. Hormone Metab Res 33:727–732
Tang T, Reed MJ (2001) Exercise adds to metformin and acarbose efficacy in db/db mice. Metabolism 50:1049–1053
Souza CT et al (2003) Insulin secretion in monosodium glutamate (MSG) obese rats submitted to aerobic exercise training. Physiol Chem Phys Med 35:43–53
Santos RF et al (1995) Changes in insulin receptor tyrosine kinase activity associated with metformin treatment of type 2 diabetes. Diabetes Metabol 21:274–280
Elhayany A, Lustman A, Abel R, Attal-Singer J, Vinker S (2010) A low carbohydrate Mediterranean diet improves cardiovascular risk factors and diabetes control among overweight patients with type 2 diabetes mellitus: a 1-year prospective randomized intervention study. Diabetes Obes Metab 12:204–209
Alison BE (2013) Nutrition therapy recommendations for the management of adults with diabetes. Diabetes Care 36:3821–3842
Marshall JA, Hamman RF, Baxter J (1991) High-fat, low-carbohydrate diet and the etiology of non-insulin-dependent diabetes mellitus: the San Luis Valley Diabetes Study. Am J Epidemiol 134:590–603
Lamont BJ, Waters MF, Andrikopoulos S (2016) A low-carbohydrate high-fat diet increases weight gain and does not improve glucose tolerance, insulin secretion or β-cell mass in NZO mice. Nutr Diabetes 6:e194
Demetrius L (2005) Of mice and men. EMBO Rep 6:S39–S44
Kummitha CM, Kalhan SC, Saidel GM, Lai N (2014) Relating tissue/organ energy expenditure to metabolic fluxes in mouse and human: experimental data integrated with mathematical modeling. Physiol Rep 2:e12159
Feltenberger JD et al (2013) Oral formulation of angiotensin-(1-7) improves lipid metabolism and prevents high-fat diet-induced hepatic steatosis and inflammation in mice. Hypertension 62:324–330
Crespo TS et al (2016) Effects of omentectomy in addition to sleeve gastrectomy on the metabolic and inflammatory profiles of obese rats. Surg Obes Relat Dis 16:1–8
Andrade JMO et al (2014) Cross talk between angiotensin-(1-7)/mas axis and sirtuins in adipose tissue and metabolism of high-fat feed mice. Peptides 55:158–165
Andrade JMO et al (2014) Proteomic white adipose tissue analysis of obese mice fed with a high-fat diet and treated with oral angiotensin-(1-7). Peptides 60:56–62
Rosen ED et al (2002) C/EBPα induces adipogenesis through PPARγ: a unified pathway. Genes Dev 16:22–26
Shimomura I, Hammer RE, Ikemoto S, Brown MS, Goldstein JL (1999) Leptin reverses insulin resistance and diabetes mellitus in mice with congenital lipodystrophy. Nature 401:73–76
Shimomura I et al (2000) Decreased IRS-2 and increased SREBP-1c lead to mixed insulin resistance and sensitivity in livers of lipodystrophic and ob/ob mice. Mol Cell 6:77–86
Financial support
This work was partially supported by the Coordenadoria de Aperfeiçoamento do Pessoal de Nível Superior (CAPES) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação de Amparo a Pesquisa do Estado de Minas Gerais (FAPEMIG).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
About this article
Cite this article
de Oliveira Santana, K.N., Lelis, D.F., Mendes, K.L. et al. Metformin Reduces Lipogenesis Markers in Obese Mice Fed a Low-Carbohydrate and High-Fat Diet. Lipids 51, 1375–1384 (2016). https://doi.org/10.1007/s11745-016-4209-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11745-016-4209-y