Abstract
Lipid metabolism, inflammation, oxidative stress and endothelial function play important roles in the pathogenesis of cardiovascular disease (CVD), which may be affected by an imbalance in the n-6/n-3 polyunsaturated fatty acid (PUFA) ratio. This study aimed to investigate the effects of the n-6/n-3 PUFA ratio on these cardiovascular risk factors in rats fed a high-fat diet using plant oils as the main n-3 PUFA source. The 1:1 and 5:1 ratio groups had significantly decreased serum levels of total cholesterol, low-density lipoprotein cholesterol, and proinflammatory cytokines compared with the 20:1 group (p < 0.05). Additionally, the 20:1 group had significantly increased serum levels of E-Selectin, von Willebrand factor (vWF), and numerous markers of oxidative stress compared with the other groups (p < 0.05). The 1:1 group had a significantly decreased lipid peroxide level compared with the other groups (p < 0.05). Serum levels of malondialdehyde, reactive oxygen species and vWF tended to increase with n-6/n-3 PUFA ratios increasing from 5:1 to 20:1. We demonstrated that low n-6/n-3 PUFA ratio (1:1 and 5:1) had a beneficial effect on cardiovascular risk factors by enhancing favorable lipid profiles, having anti-inflammatory and anti-oxidative stress effects, and improving endothelial function. A high n-6/n-3 PUFA ratio (20:1) had adverse effects. Our results indicated that low n-6/n-3 PUFA ratios exerted beneficial cardiovascular effects, suggesting that plant oils could be used as a source of n-3 fatty acids to prevent CVD. They also suggested that we should be aware of possible adverse effects from excessive n-3 PUFA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dietary changes have critical effects on cardiovascular risk factors [1]. Dietary modification has led to an imbalance in the n-6/n-3 polyunsaturated fatty acid (PUFA) ratio with an increase in n-6 fatty acids along with a marked reduction in n-3 fatty acids in diets, resulting in a high n-6/n-3 PUFA ratio [2]. The n-6/n-3 PUFA ratio of 20–30:1 in today’s typical Western diets is quite different from the n-6/n-3 PUFA ratio of 1:1 that was found in human diets many years ago [2, 3]. The n-6 and n-3 PUFA interact with each other in biological functions and coordinate to regulate biological processes. They also compete with each other sharing the same enzymes for conversion [4]. Most of the eicosanoids formed from n-3 fatty acids are anti-inflammatory, whereas those derived from n-6 fatty acids are pro-inflammatory [5]. A balanced n-6/n-3 PUFA ratio may be more important than n-3 PUFA intake levels for preventing inflammation-related diseases, including cardiovascular disease (CVD) and other chronic disorders [6]. Thus, the n-6/n-3 PUFA ratio should be considerably improved regarding its potential role in CVD.
Marine animals such as fish were used as sources of n-3 PUFA by providing eicosapentaenoic (EPA) and docosahexaenoic (DHA) in most of the previous studies [7]. Many studies have indicated that high intakes of EPA and DHA (mainly obtained from fish) could provide cardiovascular benefits. In addition to the consumer’s concern about contaminants in fish, it may be difficult to increase fish consumption for its health benefits because of their scarcity and high cost [8]. Some plant seeds have a high content of α-linolenic acid (ALA, C18:3n-3) [9]. It would be easier to increase n-3 PUFA intake to lower the dietary n-6/n-3 PUFA ratio because of the abundant plant sources rich in ALA than by marine sources [10]. Vegetable oil blends rich in ALA have been developed to modulate lipid metabolism by improving the n-6/n-3 PUFA ratio in edible oils [11, 12]. Our previous study showed that the n-6/n-3 PUFA ratio did not acutely influence postprandial metabolism, the inflammatory response, or endothelial function. However, a meal in which dietary linseed oil was reduced, resulting in a high n-6/n-3 PUFA ratio, strengthened the difference between healthy controls and a hypertriglyceridemia group [13]. Despite that finding, the effects of the dietary n-6/n-3 PUFA ratio on risk factors for CVD using plant oils as the n-3 PUFA source have not been extensively investigated [9].
It is well known that lipid metabolism, inflammation, oxidative stress, and endothelial function play important roles in the pathogenesis of CVD, which could be affected by the dietary n-6/n-3 PUFA ratio. Hence, the present study aimed to investigate the effects of the dietary n-6/n-3 PUFA ratio on cardiovascular risk factors in rats using plant oils as the main n-3 PUFA source.
Materials and Methods
Animals and Diets
Male Sprague–Dawley rats, purchased from Zhejiang Medical Experimental Animal Center (Hangzhou, China), had an average weight of 140–160 g. The rats were caged individually in a temperature-controlled room (at 22 ± 2 °C) with 50–60 % humidity and a 12-h/12-h light–dark cycle. Rats were acclimatized for 8 days prior to the experiment.
The rats were then randomly divided into four groups, with 10 rats in each group, according to the n-6/n-3 PUFA ratio (1:1, 5:1, 10:1, 20:1) in their upcoming diets. The diets contained 47.1 % carbohydrate, 19.4 % protein, and 33.5 % fat (energy % kcal). The food composition of the diets is shown in Table 1. Blended Oils differing to meet the conditions of the n-6/n-3 PUFA ratio were obtained by mixing appropriate proportions of commercial corn oil, linseed oil, olive oil, and butter (Table 2). The compositions of the four diets were the same except for the different n-6/n-3 PUFA ratios while maintaining a PUFA/monounsaturated fatty acid (MUFA)/saturated fatty acid (SFA) ratio of approximately 1:1:1. The fatty acid compositions of the blended oils in the diets are summarized in Table 3. All diets had a similar fat and energy content (4.02 kcal/g). All diets contained all-rac-α-tocopheryl acetate 50 IU/kg as a composition of the AIN-76 vitamin mix. The α-tocopherol content of the four blended oils was measured. Commercial α-tocopherol was then added to the four blended oils to maintain the all-rac-α-tocopheryl acetate level at 200 IU/kg in all diets to prevent fatty acid oxidation. The diets were prepared once every 2 weeks and stored at 4 °C under nitrogen during the experimental period. They were provided fresh to the rats every 2 days.
There was no difference in body weight or serum lipid profile among the four groups at baseline. They were given the assigned experimental diets and free access to water. Body weight was monitored weekly, and food intake was recorded every other day.
The experimental protocol was reviewed and approved by the Institutional Animal Care and Use Committee of Southeast University (approval number: 20130218) on February 26, 2013. All animal care and experimental procedures were carried out in accordance with the principles of European Directive 2010/63/EU and the protocol for animal study of the Animal Management Committee of Jiangsu Province, China.
Blood Processing
At the end of week 12, the rats were fasted overnight (about 12 h, with water ad libitum) to reduce the impact of feeding. All animals were anesthetized with sodium pentothal, blood was collected, and the rats were sacrificed by decapitation. Blood samples were put into clean test tubes. The serum was separated by centrifugation at 3000g for 10 min at 4 °C and then stored at −80 °C until analyzed.
Serum Biochemical Analyses
At the end of the experiment, we measured the serum levels of triacylglycerol (TAG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), insulin, glucose, apolipoprotein C3 (ApoC3), C-reactive protein (CRP), tumor necrosis factor α (TNF-α), interleukin 6 (IL-6), lipid peroxide (LPO), NADPH oxidase (NADPH-OX), oxidized low-density lipoprotein (ox-LDL), 8-iso-prostaglandin (8-iso-PG), myeloperoxidase (MPO), malondialdehyde (MDA), Protein Carbonyl (PC), reactive oxygen species (ROS), E-Selectin (ES), and the von Willebrand factor (vWF). Serum TAG, TC and LDL-C and HDL-C levels were measured by enzymatic assays. The glucose level was determined by the glucose oxidase method. Insulin resistance was estimated by the homeostasis model assessment of insulin resistance (HOMA-IR) score as follows: HOMA-IR = fasting insulin (μU/mL) × fasting glucose (mmol/L)/22.5. The levels of TNF-α and IL-6 were measured using enzyme-linked immunosorbent assay (ELISA) kits (Science Biotechnology, Yantai, China). Levels of ApoC3, CRP, LPO, NADPH, ox-LDL, 8-iso-PG, MPO, MDA, PC, ROS, INS, ES and vWF were measured using ELISA kits (JRDUN BIOTECH, Shanghai, China). All the procedures and conditions were consistent with the instructions of these kits.
Statistical Analyses
Data are given as means ± standard deviations (SD) using GraphPad Prism, version 5 (GraphPad software, San Diego, CA, USA). The data were analyzed by SPSS 17.0 statistics software (SPSS, Chicago, IL, USA). Body weight was analyzed using a repeated measures two-way analysis of variance (ANOVA). All other data were analyzed using one-way ANOVA. The Student–Newman–Keuls test was used for all post hoc analyses. Statistical significance was defined as p < 0.05.
Results
Effects of the Dietary n-6/n-3 PUFA Ratio on Growth Curves
Food intakes (g) during the 12-week period were similar among the four groups. There were no significant differences in body weight among the four groups (p > 0.05, data not shown).
Effects of the Dietary n-6/n-3 PUFA Ratio on Serum Lipid Metabolism
As shown in Table 4, the serum level of TAG was lower in the 1:1 group than in the 10:1 group (p < 0.05). The 1:1 group had significantly decreased serum levels of TC and LDL compared with the 10:1 and 20:1 groups (p < 0.05). Additionally, the levels of TC and LDL-C were lower in the 5:1 group than in the 20:1 group (p < 0.05). The 1:1 and 5:1 groups showed significantly lower non-HDL-C levels than the 20:1 group (p < 0.05). In contrast, the 20:1 group had a significantly increased ApoC3 level compared with the 1:1 group (p < 0.05). Among the four groups, however, the serum levels of HDL-C in the 1:1 group were lowest (p < 0.05).
Effects of the Dietary n-6/n-3 PUFA Ratio on Serum Glucose and Insulin Sensitivity
The levels of glucose and insulin were not different among the four groups (p > 0.05). There were also no significant differences in the HOMA-IR among the groups (p > 0.05) (Table 4).
Effects of the Dietary n-6/n-3 PUFA Ratio on Serum Inflammatory Cytokines
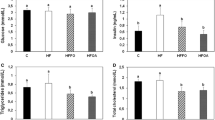
The serum levels of IL-6 were lower in the 1:1 group than in the 10:1 and 20:1 groups (p < 0.05) (Fig. 1a). Additionally, the 5:1 group had a significantly decreased IL-6 level compared with the 20:1 group (p < 0.05) (Fig. 1a). The 10:1 and 20:1 groups exhibited significantly higher TNF-α levels than the 1:1 and 5:1 groups (p < 0.05) (Fig. 1a). Among the four groups, the CRP level was the highest in the 20:1 group (p < 0.05) (Fig. 1b).
Effects of the Dietary n-6/n-3 PUFA Ratio on Serum Oxidative Stress
Among the four groups, the serum LPO level was lowest in the 1:1 group (p < 0.05) (Fig. 2a), whereas the 20:1 group showed significantly higher serum levels of PC (Fig. 2g), MPO (Fig. 2e), MDA (Fig. 2f), NADPH-OX (Fig. 2b) and ROS (Fig. 2h) than the other three groups (p < 0.05). The serum level of NADPH-OX was lower in the 10:1 group than the 1:1 group (p < 0.05) (Fig. 2b). In addition, the 20:1 group had a significantly increased serum level of ox-LDL compared with the 1:1 and 5:1 groups (p < 0.05) (Fig. 2c). The 20:1 group also showed significantly higher serum 8-iso-PG levels than the 1:1 group (p < 0.05) (Fig. 2d). Although no significant differences were observed, the serum MDA (Fig. 2f) and ROS (Fig. 2h) levels increased slightly in the 1:1 group compared with the 5:1 group. The serum MDA and ROS levels also tended to increase with the increasing n-6/n-3 PUFA ratio, from 5:1 to 20:1.
Effects of the Dietary n-6/n-3 PUFA Ratio on Endothelial Function
The serum levels of VWF (Fig. 3a) and ES (Fig. 3b) were higher in the 20:1 group than in the other groups (p < 0.05). Although no significant difference was observed, the serum vWF levels increased slightly in the 1:1 group compared with the 5:1 group. The serum vWF levels also tended to increase with the increasing n-6/n-3 PUFA ratio (from 5:1 to 20:1) (Fig. 3a).
Discussion
Lipid abnormalities, inflammation, oxidative stress and endothelial dysfunction have been considered important risk factors in the development of atherosclerosis [14–16]. The effects of dietary fat on diseases such as CVD have attracted wide attention. Both the amount and profile of fatty acids in the diet have been shown to play an important role in regard to health benefits [7, 17]. The relatively high intake of n-6 PUFA versus n-3 PUFA in typical Western diets may increase the risks of CVD. Conversely, reducing the intake of n-6 PUFA and/or increasing the intake of n-3 PUFA have been postulated as being beneficial for preventing CVD. Although possible health effects of n-3 PUFA are well known, there are still some concerns about lipid oxidation of n-3 PUFA, which are highly prone to oxidation [18]. Many previous studies used fish oil as their n-3 PUFA source, although it is unsuitable for long-term use. Few studies, however, have investigated the effects of changing the dietary n-6/n-3 PUFA ratio on risk factors of CVD using plant oils as the n-3 PUFA source.
The present study was carried out to investigate the effect of the dietary n-6/n-3 PUFA ratio from plant sources on CVD risk factors, such as lipid metabolism, inflammation, oxidative stress and endothelial dysfunction. The four diets fed to the rats contained similar amounts of total fat, but the n-6/n-3 PUFA ratios ranged from 1:1 to 20:1. The highest ratio (20:1) was chosen because it approximates the upper extreme of the n-6/n-3 PUFA ratio found in Western societies and the lowest ratio (1:1) because it is similar to the n-6/n-3 PUFA ratio in the diet on which humans evolved [2, 3]. The 10:1 ratio was chosen to approximate the n-6/n-3 PUFA ratio of the average human diet [19, 20] and the 5:1 ratio because it is close to the commonly recommended n-6/n-3 PUFA ratio [20–22]. Four differently blended oils were created by changing the percentages of corn oil, linseed oil, olive oil and butter in the newly prepared oils. The butter content, which is not a plant oil, was similar in the four blended oils. The n-6 and n-3 fatty acids are present in butter in trace amounts. Butter contains more than 70 % SFA and therefore was used to provide SFA in the four high-fat diets.
Large amounts of SFA in diets have an adverse effect on cardiovascular health [23]. The four high-fat diets, with their high levels of SFA, were similar to the Western-type high-fat diet, which could increase cardiovascular risk factors [24, 25]. We hypothesized that the process could be affected by the dietary n-6/n-3 PUFA ratio. The four diets in this study had n-6 and n-3 fatty acids provided mainly as 18-carbon fatty acids from plant oils, whereas many previous studies evaluated the effects of 20- to 22-carbon n-3 fatty acids on CVD risk factors. In regards to the effects of SFA, MUFA, and PUFA on health, the SFA:MUFA:PUFA ratio was fixed in this study.
The cardioprotective effects of n-3 PUFA could partly result from their beneficial effects on the lipid profile. As expected, the present study demonstrated that a low n-6/n-3 PUFA ratio of 1:1 decreased serum TC, non-HDL-C and LDL-C levels compared with those on a diet with a high n-6/n-3 PUFA ratio (20:1). The 1:1 group had a lower serum level of TAG than the 10:1 group. Our results are in accordance with an earlier report that blended oil rich in ALA oil decreased serum levels of TAG, TC, and LDL-C [11]. One previous study had showed that flaxseed and fish oil diets both lowered TC and non-HDL compared with levels achieved with safflower oil. The fish oil group, however, had a higher HDL-C level than the flaxseed group [26]. Another study demonstrated that a high-oleic rapeseed oil diet blended with flaxseed oil reduced TC, LDL-C and HDL-C compared with a typical Western diet [27]. In the present study, the 1:1 group had a significantly lower HDL-C level than the other groups. Supporting our results, Zhao et al. reported that an ALA diet with an LA: ALA ratio of 1.6:1 decreased HDL-C in hypercholesterolemic subjects compared with the average American diet with an LA: ALA ratio of 9.6:1 [28]. Duan et al. [29] observed that n-6/n-3 PUFA ratios of 1:1 and 5:1 exerted beneficial effects on lipid metabolism in pigs. In a recent study, Li et al. [30] reported that dietary n-6/n-3 PUFA ratios of 1:1 and 5:1 facilitated the absorption and use of fatty acids. A 2-year ALA-rich oil supplementation study showed that the ALA group had lower HDL-C levels than the LA group in a human population at risk for CVD [31]. We speculated that a very low n-6/n-3 PUFA ratio using plant oils as the n-3 PUFA source could reduce both LDL-C and HDL-C.
Elevated plasma ApoC3 protein has several pro-atherogenic properties, such as inhibiting the lipolysis of TAG-rich lipoproteins [32] and increasing the formation of atherogenic small dense LDL [33]. There is also evidence that ApoC3 promotes inflammation and endothelial cell dysfunction [34]. Up to now, however, few studies have evaluated ApoC3 levels in serum following administration of diets with different n-6/n-3 ratios. In the present study, the 1:1 group showed lower serum ApoC3 levels than the 20:1 group. This is consistent with prior findings of reduced ApoC3 after ingestion of EPA and DHA [35]. The results led us to hypothesize that the serum level of ApoC3 was affected by the different n-6/n-3 PUFA ratios.
Inflammation has been shown to play an important role in the development of many chronic diseases, including atherosclerotic diseases. The proinflammatory cytokines TNF-α and IL-6 have been associated with CVD risk. Specifically, IL-6 stimulates the production of CRP, which has been shown to be a stronger predictor of CVD than LDL-C [36]. The n-6 and n-3 PUFA have opposing effects on inflammation. The n-3 PUFA have strong anti-inflammatory effects, whereas n-6 PUFA tend to be proinflammatory. Thus, for regulating the production of proinflammatory and anti-inflammatory mediators, it is important to maintain a balance between n-6 and n-3 PUFA.
Many previous studies have reported that n-3 PUFA decrease the production of these inflammatory cytokines [29, 37, 38]. Lin et al. [38] reported that mammary inflammation around the time of parturition appeared to be attenuated by consumption of a diet rich in n-3 PUFA. The anti-inflammatory effects of n-3 PUFA may contribute their protective actions to avoid atherosclerosis and CVD-related mortality [37]. Our results indicated that a high n-6/n-3 PUFA ratio increased the serum TNF-α and IL-6 levels compared with a low n-6/n-3 PUFA ratio. High n-6/n-3 PUFA ratios (10:1 and 20:1) resulted in higher TNF-α levels than low n-6/n-3 PUFA ratios (1:1 and 5:1). The 1:1 group exhibited a lower serum IL-6 level than were seen in the 10:1 and 20:1 groups, and the 5:1 group showed lower IL-6 levels than the 20:1 group. Our study also showed that the serum CRP level was higher in the 20:1 group than in the other groups. In accord with our studies, one report suggested that a high n-6/n-3 PUFA ratio was associated with higher serum CRP levels in long-term hemodialysis patients [39]. Another study demonstrated that a diet with an LA: ALA ratio of 1.6:1 decreased the CRP level in hypercholesterolemic subjects compared with a diet with an LA: ALA ratio of 9.6:1 [28]. Papadopoulos et al. [40] observed that sow’s lactation feed with a low n-6/n-3 PUFA ratio administered beginning 8 days before farrowing was associated with a better inflammatory profile during the periparturient period. Duan et al. [29] reported that the dietary n-6/n-3 PUFA ratios of 1:1 and 5:1 suppressed expression of the inflammatory cytokines TNF-α and IL-6. Our study indicated that a low n-6/n-3 PUFA ratio using plant ALA as the main n-3 PUFA source had the effect of inhibiting inflammation compared with a diet with a high n-6/n-3 PUFA ratio.
Oxidative stress plays a key role in the development of CVD. Despite many known health benefits of n-3 PUFA, there is concern that their high degree of unsaturation may increase oxidative stress and lipid peroxidation (LPO). Given the different effects of SFA, MUFA, and PUFA on oxidative stress, the diet in the present study maintained a constant SFA:MUFA:PUFA ratio throughout. To our knowledge, few studies have evaluated oxidative stress in serum following diets with different n-6/n-3 ratios but a constant SFA:MUFA:PUFA ratio. In our study, the 1:1 group had a decreased serum LPO level than the other groups. In agreement with our findings, it had also been reported that the LPO level was significantly high with sunflower oil (n-6 rich) diets and less so with mustard oil (n-3 rich) diets in rats under stress conditions [41]. The 8-iso-PG, a reliable marker of oxidative stress [42], is a stable isoprostane derived from oxidized arachidonate by ROS [43]. In this study, the 20:1 group had an increased serum 8-iso-PG level compared with the 1:1 group. Our study also found that the diet with an n-6/n-3 PUFA ratio of 20:1 increased the serum protein carbonylation compared with that in the other groups. Our results concurred with the protective effects of EPA/DHA supplementation on the carbonylation of proteins [44].
The serum MPO level is an independent risk factor for atherosclerosis [45]. In this study, consumption of the 20:1 diet led to higher serum MPO levels than that in the other groups. MPO-derived oxidants can cause oxidative damage during inflammation [46]. For example, the nitration and chlorination of HDL catalyzed by MPO can transform it into dysfunctional HDL [47]. In the past, HDL has been generally believed to exert protective activity that helps avoid atherosclerosis. However, recent studies indicated that dysfunctional HDL can lose its beneficial function. Inflammation was also associated with dysfunctional HDL that has lost its ability to protect against atherosclerosis [48]. We speculated that lipid metabolism, inflammation and oxidative stress might interact with each other in the atherosclerotic process.
LDL lipoproteins are easily oxidized because of their high PUFA content. Ox-LDL is considered the key factor in the formation and development of atherosclerosis because it is involved in endothelial function damage, foam cell formation, and inflammation, among other activities. Furthermore, overproduction of ROS contributes to the pathophysiologic process in CVD [14]. In our study, the serum level of ox-LDL in the 20:1 group was markedly higher than in the 1:1 and 5:1 groups. Our study also showed that the levels of NADPH-OX and ROS were higher in the 20:1 group than in the other groups. Furthermore, the 10:1 group had an increased NADPH-OX level compared with that in the 1:1 group. Our results were consistent with the hypothesis that activation of NADPH-OX could generate excessive ROS which oxidizes LDL. The ox-LDL could then promote NADPH-OX activation—thus creating a vicious circle that promotes the development of atherosclerosis [49, 50]. A previous study showed that a dietary supplement with linseed oil lowered the ox-LDL level compared with a supplement containing EPA and DHA. This might be because of the less unsaturated bonding of ALA than that of EPA and DHA, making it less susceptible to oxidative attack [51].
MDA is an oxidative end-product of PUFA. High circulating levels of ox-LDL and MDA-LDL are strongly associated with atherosclerotic disease. Up to now, few studies have studied the effect of different n-6/n-3 PUFA ratios on circulating MDA levels. A previous study indicated that there was a significant difference in MDA in old dogs given 5:1 and 25:1 diets [52]. In our study, the diet with an n-6/n-3 PUFA ratio of 20:1 significantly increased serum MDA compared with that in the other groups. Another study suggested that ox-LDL disrupted the growth and survival of human coronary artery endothelial cells through an MDA-dependent pathway [53].
Impaired vascular function contributes to the pathogenesis of atherosclerosis. There is growing evidence that increased generation of ROS and oxidative stress participates in pro-atherogenic mechanisms of vascular dysfunction and atherothrombosis [54]. To our knowledge, few studies have reported the effect of different n-6/n-3 PUFA ratios on the serum vWF level in rats. We also assayed soluble E-selectin, another circulating endothelial dysfunction biomarker. In the present experiment, both vWF and ES were significantly higher in the diet with the highest n-6/n-3 ratio (20:1) compared with that in the other groups. Supporting our results, a previous study reported that dietary intake of n-3 PUFA showed negative associations with vWF in a population-based sample consuming a Western diet [55]. A previous study demonstrated that a diet with high-oleic rapeseed oil blended with flaxseed oil decreased the E-selectin level compared with a typical Western diet [27]. Collectively, a high n-6/n-3 PUFA ratio of 20:1 in the diet probably damages endothelial function.
The present study demonstrated that a low n-6/n-3 PUFA ratio had beneficial effects on serum lipid metabolism, inflammation cytokines, oxidative stress, and endothelial function. However, further research is necessary to illustrate the underlying metabolic pathways, such as the AMPK and ERK signal pathways [56, 57]. In addition, more research is needed to investigate lipid metabolism in adipose tissue, skeletal muscle, and liver, which play important metabolic roles to modulate lipid metabolism and inflammation [58, 59]. It should be pointed out that our results were obtained for blood indexes in rats fed high-fat diets with the constant PUFA: MUFA: SFA ratio of approximately 1:1:1.
It is known that different fatty acids have different health effects. Both the amount and the proportion of different fatty acids in the diet are important for human health. Further studies might determine whether there is an interaction between the dietary composition of fatty acids and the n-6/n-3 PUFA ratio. For example, is the risk of CVD affected by the n-6/n-3 PUFA ratio in low-fat diets or in diets with low SFA levels?
Conclusion
The results of this study demonstrated that the n-6/n-3 PUFA ratio could regulate lipid metabolism, inflammation, oxidative stress, and endothelial function. The optimal n-6/n-3 PUFA ratios were 1:1 and 5:1. Our results support a beneficial effect of a low n-6/n-3 PUFA ratio on risk factors for CVD, including favorable lipid profiles, anti-inflammation activity, actions against anti-oxidative stress, and improving endothelial function. On the contrary, the high n-6/n-3 PUFA ratio of 20:1 produced adverse effects, including dyslipidemia, a proinflammatory state, oxidative damage, and endothelial dysfunction. We should be aware of the possible adverse effects caused by excessive n-3 PUFA. These results may offer a way to design diets that help to prevent CVD. On the whole, our study suggested that plant-derived oil rich in n-3 PUFA was able to reduce the n-6/n-3 ratio in the diet. Additionally, blended oils resulting in a low n-6/n-3 PUFA ratio resulted in lowering the risk of CVD.
Abbreviations
- ALA:
-
α-Linolenic acid (18:3n-3)
- ANOVA:
-
Analysis of variance
- ApoC3:
-
Apolipoprotein C3
- CRP:
-
C-reactive protein
- CVD:
-
Cardiovascular disease
- DHA:
-
Docosahexaenoic acid (22:6n-3)
- ELISA:
-
Enzyme-linked immunosorbent assay
- EPA:
-
Eicosapentaenoic acid (20:5n-3)
- ES:
-
E-selectin
- HDL-C:
-
High-density lipoprotein cholesterol
- HOMA-IR:
-
Homeostasis model assessment insulin resistance
- IL-6:
-
Interleukin-6
- 8-Iso-PG:
-
8-Iso-prostaglandin
- LDL-C:
-
Low-density lipoprotein cholesterol
- LPO:
-
Lipid peroxide
- MDA:
-
Malondialdehyde
- MPO:
-
Myeloperoxidase
- MUFA:
-
Monounsaturated fatty acid(s)
- NADPH-OX:
-
NADPH oxidase
- Ox-LDL:
-
Oxidized low-density lipoprotein
- PC:
-
Protein carbonyl
- PUFA:
-
Polyunsaturated fatty acid(s)
- ROS:
-
Reactive oxygen species
- SFA:
-
Saturated fatty acid(s)
- TAG:
-
Triacylglycerol
- TC:
-
Total cholesterol
- TNF-α :
-
Tumor necrosis factor α
- Vwf:
-
Von Willebrand factor
References
Eilat-Adar S, Mete M, Fretts A, Fabsitz RR, Handeland V, Lee ET, Loria C, Xu J, Yeh J, Howard BV (2013) Dietary patterns and their association with cardiovascular risk factors in a population undergoing lifestyle changes: The Strong Heart Study. Nutr Metab Cardiovas 23:528–535
Simopoulos AP (2006) Evolutionary aspects of diet, the omega-6/omega-3 ratio and genetic variation: nutritional implications for chronic diseases. Biomed Pharmacother 60:502–507
Simopoulos AP (2002) The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed Pharmacother 56:365–379
Schmitz G, Ecker J (2008) The opposing effects of n-3 and n-6 fatty acids. Prog Lipid Res 47:147–155
Bagga D, Wang L, Farias-Eisner R, Glaspy JA, Reddy ST (2003) Differential effects of prostaglandin derived from omega-6 and omega-3 polyunsaturated fatty acids on COX-2 expression and IL-6 secretion. Proc Natl Acad Sci USA 100:1751–1756
Simopoulos AP (2008) The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp Biol Med 233:674–688
Holub B, Mutch DM, Pierce GN, Rodriguez-Leyva D, Aliani M, Innis S, Yan W, Lamarche B, Couture P, Ma DW (2014) Proceedings from the 2013 Canadian Nutrition Society conference on advances in dietary fats and nutrition. Appl Physiol Nutr Metab 39:754–762
Lenihan-Geels G, Bishop KS, Ferguson LR (2013) Alternative sources of omega-3 fats: can we find a sustainable substitute for fish? Nutrients 5:1301–1315
Kuhnt K, Degen C, Jaudszus A, Jahreis G (2012) Searching for health beneficial n-3 and n-6 fatty acids in plant seeds. Eur J Lipid Sci Tech 114:153–160
Williams CM, Burdge G (2006) Long-chain n-3 PUFA: plant v. marine sources. Proc Nutr Soc 65:42–50
Umesha SS, Naidu KA (2012) Vegetable oil blends with alpha-linolenic acid rich garden cress oil modulate lipid metabolism in experimental rats. Food Chem 135:2845–2851
Riediger ND, Azordegan N, Harris-Janz S, Ma DW, Suh M, Moghadasian MH (2009) ‘Designer oils’ low in n-6:n-3 fatty acid ratio beneficially modifies cardiovascular risks in mice. Eur J Nutr 48:307–314
Song Z, Yang L, Shu G, Lu H, Sun G (2013) Effects of the n-6/n-3 polyunsaturated fatty acids ratio on postprandial metabolism in hypertriacylglycerolemia patients. Lipids Health Dis. 12:181
Victor VM, Rocha M, Sola E, Banuls C, Garcia-Malpartida K, Hernandez-Mijares A (2009) Oxidative stress, endothelial dysfunction and atherosclerosis. Curr Pharm Design 15:2988–3002
Tietge UJ (2014) Hyperlipidemia and cardiovascular disease: inflammation, dyslipidemia, and atherosclerosis. Curr Opin Lipidol 25:94–95
Libby P (2002) Inflammation in atherosclerosis. Nature 420:868–874
Ooi EM, Ng TW, Watts GF, Barrett PH (2013) Dietary fatty acids and lipoprotein metabolism: new insights and updates. Curr Opin Lipidol 24:192–197
Jacobsen C (2010) Enrichment of foods with omega-3 fatty acids: a multidisciplinary challenge. Ann N Y Acad Sci 1190:141–150
Lee SP, Dart AM, Walker KZ, O’Dea K, Chin-Dusting JP, Skilton MR (2012) Effect of altering dietary n-6:n-3 PUFA ratio on cardiovascular risk measures in patients treated with statins: a pilot study. Br J Nutr 108:1280–1285
Kris-Etherton PM, Taylor DS, Yu-Poth S, Huth P, Moriarty K, Fishell V, Hargrove RL, Zhao G, Etherton TD (2000) Polyunsaturated fatty acids in the food chain in the United States. Am J Clin Nutr 71:179S–188S
Kris-Etherton PM, Harris WS, Appel LJ (2002) Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation 106:2747–2757
Lorente-Cebrian S, Costa AG, Navas-Carretero S, Zabala M, Martinez JA, Moreno-Aliaga MJ (2013) Role of omega-3 fatty acids in obesity, metabolic syndrome, and cardiovascular diseases: a review of the evidence. J Physiol Biochem 69:633–651
Cascio G, Schiera G, Di Liegro I (2012) Dietary fatty acids in metabolic syndrome, diabetes and cardiovascular diseases. Curr Diabetes Rev 8:2–17
Fleming RM (2002) The effect of high-, moderate-, and low-fat diets on weight loss and cardiovascular disease risk factors. Prev Cardiol 5:110–118
van Bilsen M, Planavila A (2014) Fatty acids and cardiac disease: fuel carrying a message. Acta Physiol (Oxf) 211:476–490
Riediger ND, Othman R, Fitz E, Pierce GN, Suh M, Moghadasian MH (2008) Low n-6:n-3 fatty acid ratio, with fish- or flaxseed oil, in a high fat diet improves plasma lipids and beneficially alters tissue fatty acid composition in mice. Eur J Nutr 47:153–160
Gillingham LG, Gustafson JA, Han SY, Jassal DS, Jones PJ (2011) High-oleic rapeseed (canola) and flaxseed oils modulate serum lipids and inflammatory biomarkers in hypercholesterolaemic subjects. Brit J Nutr 105:417–427
Zhao G, Etherton TD, Martin KR, West SG, Gillies PJ, Kris-Etherton PM (2004) Dietary alpha-linolenic acid reduces inflammatory and lipid cardiovascular risk factors in hypercholesterolemic men and women. J Nutr 134:2991–2997
Duan Y, Li F, Li L, Fan J, Sun X, Yin Y (2014) n-6:n-3 PUFA ratio is involved in regulating lipid metabolism and inflammation in pigs. Brit J Nutr 111:445–451
Li F, Duan Y, Li Y, Tang Y, Geng M, Oladele OA, Kim SW, Yin Y (2015) Effects of dietary n-6:n-3 PUFA ratio on fatty acid composition, free amino acid profile and gene expression of transporters in finishing pigs. Br J Nutr 113:739–748
Bemelmans WJ, Broer J, Feskens EJ, Smit AJ, Muskiet FA, Lefrandt JD, Bom VJ, May JF, Meyboom-de JB (2002) Effect of an increased intake of alpha-linolenic acid and group nutritional education on cardiovascular risk factors: the Mediterranean Alpha-linolenic Enriched Groningen Dietary Intervention (MARGARIN) study. Am J Clin Nutr 75:221–227
Yao Z, Wang Y (2012) Apolipoprotein C-III and hepatic triglyceride-rich lipoprotein production. Curr Opin Lipidol 23:206–212
Zheng C, Khoo C, Furtado J, Sacks FM (2010) Apolipoprotein C-III and the metabolic basis for hypertriglyceridemia and the dense low-density lipoprotein phenotype. Circulation 121:1722–1734
Kawakami A, Aikawa M, Alcaide P, Luscinskas FW, Libby P, Sacks FM (2006) Apolipoprotein CIII induces expression of vascular cell adhesion molecule-1 in vascular endothelial cells and increases adhesion of monocytic cells. Circulation 114:681–687
Maki KC, Bays HE, Dicklin MR, Johnson SL, Shabbout M (2011) Effects of prescription omega-3-acid ethyl esters, coadministered with atorvastatin, on circulating levels of lipoprotein particles, apolipoprotein CIII, and lipoprotein-associated phospholipase A2 mass in men and women with mixed dyslipidemia. J Clin Lipidol 5:483–492
Ridker PM, Rifai N, Rose L, Buring JE, Cook NR (2002) Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med 347:1557–1565
Calder PC (2010) Omega-3 fatty acids and inflammatory processes. Nutrients 2:355–374
Lin S, Hou J, Xiang F, Zhang X, Che L, Lin Y, Xu S, Tian G, Zeng Q, Yu B, Zhang K, Chen D, Wu D, Fang Z (2013) Mammary inflammation around parturition appeared to be attenuated by consumption of fish oil rich in n-3 polyunsaturated fatty acids. Lipids Health Dis 12:190
Noori N, Dukkipati R, Kovesdy CP, Sim JJ, Feroze U, Murali SB, Bross R, Benner D, Kopple JD, Kalantar-Zadeh K (2011) Dietary omega-3 fatty acid, ratio of omega-6 to omega-3 intake, inflammation, and survival in long-term hemodialysis patients. Am J Kidney Dis 58:248–256
Papadopoulos GA, Maes DG, Van Weyenberg S, van Kempen TA, Buyse J, Janssens GP (2009) Peripartal feeding strategy with different n-6: n-3 ratios in sows: effects on sows’ performance, inflammatory and periparturient metabolic parameters. Br J Nutr 101:348–357
Benson MK, Devi K (2009) Influence of omega-6/omega-3 rich dietary oils on lipid profile and antioxidant enzymes in normal and stressed rats. Indian J Exp Biol 47:98–103
Montuschi P, Barnes PJ, Roberts LN (2004) Isoprostanes: markers and mediators of oxidative stress. Faseb J 18:1791–1800
Szuldrzynski K, Zalewski J, Machnik A, Zmudka K (2010) Elevated levels of 8-iso-prostaglandin F2alpha in acute coronary syndromes are associated with systemic and local platelet activation. Pol Arch Med Wewn 120:19–24
Mendez L, Pazos M, Gallardo JM, Torres JL, Perez-Jimenez J, Nogues R, Romeu M, Medina I (2013) Reduced protein oxidation in Wistar rats supplemented with marine omega3 PUFAs. Free Radical Bio Med 55:8–20
Salonen I, Huttunen K, Hirvonen MR, Dufva J, Groundstroem K, Dufva H, Pekkanen J, Salonen RO (2012) Serum myeloperoxidase is independent of the risk factors of atherosclerosis. Coron Artery Dis 23:251–258
Nicholls SJ, Hazen SL (2005) Myeloperoxidase and cardiovascular disease. Arterioscl Throm Vas 25:1102–1111
Pan B, Yu B, Ren H, Willard B, Pan L, Zu L, Shen X, Ma Y, Li X, Niu C, Kong J, Kang S, Eugene CY, Pennathur S, Zheng L (2013) High-density lipoprotein nitration and chlorination catalyzed by myeloperoxidase impair its effect of promoting endothelial repair. Free Radical Bio Med 60:272–281
Smith JD (2010) Myeloperoxidase, inflammation, and dysfunctional high-density lipoprotein. J Clin Lipidol 4:382–388
Torrejon C, Jung UJ, Deckelbaum RJ (2007) n-3 Fatty acids and cardiovascular disease: actions and molecular mechanisms. Prostag Leukotr Ess 77:319–326
Thum T, Borlak J (2004) Mechanistic role of cytochrome P450 monooxygenases in oxidized low-density lipoprotein-induced vascular injury: therapy through LOX-1 receptor antagonism? Circ Res 94:e1–e13
Lluis L, Taltavull N, Munoz-Cortes M, Sanchez-Martos V, Romeu M, Giralt M, Molinar-Toribio E, Torres JL, Perez-Jimenez J, Pazos M, Mendez L, Gallardo JM, Medina I, Nogues MR (2013) Protective effect of the omega-3 polyunsaturated fatty acids: Eicosapentaenoic acid/Docosahexaenoic acid 1:1 ratio on cardiovascular disease risk markers in rats. Lipids Health Dis. 12:140
Kearns RJ, Hayek MG, Turek JJ, Meydani M, Burr JR, Greene RJ, Marshall CA, Adams SM, Borgert RC, Reinhart GA (1999) Effect of age, breed and dietary omega-6 (n-6): omega-3 (n-3) fatty acid ratio on immune function, eicosanoid production, and lipid peroxidation in young and aged dogs. Vet Immunol Immunop 69:165–183
Yang TC, Chen YJ, Chang SF, Chen CH, Chang PY, Lu SC (2014) Malondialdehyde mediates oxidized LDL-induced coronary toxicity through the Akt-FGF2 pathway via DNA methylation. J Biomed Sci 21:11
Kondo T, Hirose M, Kageyama K (2009) Roles of oxidative stress and redox regulation in atherosclerosis. J Atheroscler Thromb 16:532–538
Shahar E, Folsom AR, Wu KK, Dennis BH, Shimakawa T, Conlan MG, Davis CE, Williams OD (1993) Associations of fish intake and dietary n-3 polyunsaturated fatty acids with a hypocoagulable profile. The Atherosclerosis Risk in Communities (ARIC) Study. Arterioscler Thromb 13:1205–1212
Li F, Yang H, Duan Y, Yin Y (2011) Myostatin regulates preadipocyte differentiation and lipid metabolism of adipocyte via ERK1/2. Cell Biol Int 35:1141–1146
Tan B, Li X, Yin Y, Wu Z, Liu C, Tekwe CD, Wu G (2012) Regulatory roles for l-arginine in reducing white adipose tissue. Front Biosci (Landmark Ed) 17:2237–2246
Yang H, Li F, Xiong X, Kong X, Zhang B, Yuan X, Fan J, Duan Y, Geng M, Li L, Yin Y (2013) Soy isoflavones modulate adipokines and myokines to regulate lipid metabolism in adipose tissue, skeletal muscle and liver of male Huanjiang mini-pigs. Mol Cell Endocrinol 365:44–51
Tan B, Yin Y, Liu Z, Tang W, Xu H, Kong X, Li X, Yao K, Gu W, Smith SB, Wu G (2011) Dietary l-arginine supplementation differentially regulates expression of lipid-metabolic genes in porcine adipose tissue and skeletal muscle. J Nutr Biochem 22:441–445
Acknowledgments
This work was supported by the National Natural Science Foundation of China Youth Science Fund Project (No. 81001244).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
About this article
Cite this article
Yang, L.G., Song, Z.X., Yin, H. et al. Low n-6/n-3 PUFA Ratio Improves Lipid Metabolism, Inflammation, Oxidative Stress and Endothelial Function in Rats Using Plant Oils as n-3 Fatty Acid Source. Lipids 51, 49–59 (2016). https://doi.org/10.1007/s11745-015-4091-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11745-015-4091-z