Abstract
Surfactant adsorption onto solid surfaces is a major issue during surfactant flooding in enhanced oil recovery applications; it decreases the effectiveness of the chemical injection making the process uneconomical. Therefore, it was hypothesized that the adsorption of surfactant onto solid surfaces could be inhibited using a surfactant delivery system based on the complexation between the hydrophobic tail of anionic surfactants and β-cyclodextrin (β-CD). Proton nuclear magnetic resonance spectroscopy was used to confirm the complexation of sodium dodecyl sulfate (SDS)/β-CD. Surface tension analysis was used to establish the stoichiometry of the complexation and the binding constant (K a). Static adsorption testing was applied to determine the adsorption of surfactant onto different solids (sandstone, shale, and kaolinite). The release of the surfactant from the β-CD cavity was qualitatively evaluated through bottle testing. The formation of the inclusion complex SDS/β-CD with a 1:1 stoichiometry was confirmed. The K a of the complexations increases as salinity and hardness concentration increases. The encapsulation of the surfactant into the β-CD cavity decreases the adsorption of surfactant onto solid surfaces up to 79 %. Qualitative observations indicate that in the presence of solid adsorbents partially saturated with crude oil, the β-CD cavity releases surfactant molecules, which migrate towards the oil–water interface.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Surfactant flooding is an efficient method to recover residual oil after secondary recovery processes (i.e. waterflooding). On a pore scale, surfactants reduce the interfacial tension between oil and water to ultralow values, which decreases capillary forces and releases oil from the rock into the driving fluid. Therefore, under the condition of a high capillary number, oil droplets can be efficiently mobilized [1–3].
Surfactants are easily adsorbed onto reservoir rock surfaces, hence the higher the adsorption, the lower the flooding process efficiency. Several variables affect the adsorption of surfactants at the solid–liquid interface including the nature of the solid surface (hydrophobic or hydrophilic), surface charge, type of surfactant, concentration of surfactant, temperature, electrolyte concentration in the aqueous media, and others [4–13]. In general, the adsorption of anionic and nonionic surfactants increases sharply as the concentration increases and levels off to a nearly constant value at the critical micelle concentration (CMC). Adsorption increases as the molecular weight of the surfactant increases [6, 13]. The adsorption of anionic surfactants onto a negatively charged solid surface (i.e. sandstone) increases as the electrolyte concentration increases [6].
Adsorption mechanisms of anionic surfactant onto solid substrates include ion exchange between a monovalent anionic surfactant and chloride ions or hydroxyl ions, electrostatic adsorption or ion pairing, covalent bonding, hydrophobic bonding or non-polar interaction between the adsorbed species, lateral associative interactions, solvation and desolvation, adsorption by polarization of π electrons, and adsorption by dispersion forces [5, 6, 8].
The adsorption of anionic surfactants onto oppositely charged surfaces follows the “Somasundaran-Fuerstenau” isotherm, which is characterized by four regions. At low surfactant concentrations or Region I, the main adsorption mechanism is electrostatic interactions between surfactant monomers and the oppositely charged solid surface. At the onset of Region II, two adsorption mechanisms are active: electrostatic attractions and lateral associations among hydrocarbon chains already adsorbed. Hence, surfactant species begin to form aggregates and a sharp increase in adsorption takes place until the charge of the solid surface is neutralized. At this point, which corresponds to the onset of Region III, the adsorption rate slightly decreases and the main adsorption mechanism is lateral attraction among hydrophobic tails until the CMC of the surfactant is reached. At this stage, adsorption levels off (Region IV) and any further increase in surfactant concentration contributes only to micellization [5].
Several actions have been applied in the field to prevent surfactant adsorption such as the addition of alkali, sacrificial agents, chelating agents, and adsorption inhibitors to the driving fluid (e.g. brine) [13–18]. Furthermore, the use of a negative salinity gradient during surfactant flooding has been recommended because a decrease in salinity concentration reverses surfactant adsorption [19].
This exploratory research evaluates the encapsulation of an anionic surfactant into the β-cyclodextrin cavity as an approach to inhibit the adsorption of the anionic surfactant onto solid surfaces.
β-Cyclodextrin (β-CD) consists of glucose monomers arranged in a donut shaped ring with a hydrophobic inner cavity and a hydrophilic exterior. β-CD allows the inclusion of hydrophobic molecules in its cavity through nonbonding associations; which could be solid, liquid, or gaseous compounds such as aromatics, surfactants, alcohols, halides, fatty acids, and esters, among others. The driving force for complex formation is the released of enthalpy-rich water molecules from the cavity [20].
The most common stoichiometric ratio for the guest:host inclusion complexes is 1:1, however other ratios have been reported [21–24]. The ability of β-CD to form inclusion complexes makes it suitable for numerous applications; for instance in biomedical drug delivery, β-CD inclusion complexes are used as stabilizers and solubilizers [20, 25, 26].
Several analytical techniques are available to confirm the formation of inclusion complexes including surface tension [22], conductivity [23, 27], NMR spectroscopy [28], and UV–visible spectroscopy, and others. The analytical method to be used depends on the properties of the guest molecule; for instance, if the guest molecule is a surfactant, then the surface tension analytical procedure is suitable.
This proof of concept research evaluates the use of surfactant/β-CD complexation as an approach to curtail the adsorption of surfactant onto solid surfaces. It is expected that the bulky structure of the inclusion complex hinders electrostatic attractions of the surfactant towards potential adsorption sites and prevents lateral interactions among the hydrophobic tails of the adsorbed surfactant, hence restricting surfactant adsorption. The first part of this paper deals with the confirmation of the inclusion complex formation through association equilibrium and 1H-NMR spectroscopy. The second part of the paper focuses on static adsorption testing and the final section presents the qualitative analysis through bottle testing to establish the release of the surfactant from the β-CD cavity in the presence of oil.
Materials and Experimental Procedures
Materials
Sodium dodecyl sulfate, SDS, (C12H25NaSO4, assay ≥99 %, molecular weight: 288.38 g/mol) was purchased from Sigma-Aldrich. β-Cyclodextrin (assay ≥98.4 %, molecular weight: 1135 g/mol) was purchased from Cyclodextrin Technologies Development Inc. (Gainesville, FL, USA). All chemicals were used as received without further purification. Distilled water with a conductivity of 20 μS cm−1 was used to prepare all the aqueous solutions. Sand was obtained from Shaw Resources Company, (Nova Scotia, Canada). Kaolin was acquired from Matheson Coleman & Bell Company, (California, USA) and the oil shale was provided by the New Brunswick Department of Natural Resources. The samples of sand, kaolin, and shale were used without further purification. Synthetic soft brine (NaCl = 3 wt%) and different synthetic hard brine compositions (Table 1) were used in this work. Crude oil samples (°API = 36) from the Stoney Creek oil field (New Brunswick, Canada) were provided by Contact Exploration Inc.
Experimental Procedures
Adsorbent Surface Area
The surface areas of the solid adsorbents (sand, kaolinite, and shale) were determined using the conventional Brunauer-Emmett-Teller (BET) technique using the BET Surface Area Analyzer Autosorb-1 manufactured by Quantachrome Instruments (FL, USA).
Critical Micelle Concentration (CMC)
The CMC was determined by applying the Du Noüy ring surface tension method using a tensiometer model TensioCaD, manufactured by CAD Instruments (Les Essarts Le Roi, France) equipped with a temperature controller. The temperature was set at 25 ± 1 °C for all the experiments.
Solutions of SDS/β-CD were prepared by mixing predetermined amounts of surfactant with distilled water, followed by the addition of β-CD from a stock concentrated solution according to the desired concentration of the final solution.
Complexation Equilibrium
The association constant and the stoichiometric molar ratio of the complexation were determined using the approach presented by Lu et al. [22] and Okubo et al. [29]; who demonstrated that due to the fact that β-CD is not surface active, once the surfactant is caged into the β-CD cavity, the resultant inclusion complex does not affect the surface tension of the system. Therefore, any change to the surface tension comes from the concentration of free monomeric surfactant in the bulk of the solution in the pre-micellar region (below the CMC) according to the following association equilibrium.
where K a is the association or binding constant, [SCD] is the inclusion complex concentration, and [S] and [CD] are the free surfactant monomer and free β-CD concentration, respectively. In these equations, brackets signify molar concentrations and the association constant has the unit of M−1. From Eq. 2, it is expected that for a complexation having a stoichiometric molar ratio of 1:1, [S][CD] must vary linearly with [SCD] with a slope of \(\frac{1}{{K_{\text{a}} }}\). Therefore, the association constant for the inclusion complex of SDS/β-CD can be determined from the initial concentrations of surfactant and β-CD and from the concentration of free surfactant monomers in the solution, which can be indirectly determined from the calibration curve of surface tension versus surfactant concentration.
These experiments were conducted using brines containing different salinity and hardness concentrations at a fixed concentration of β-CD of 14 mM; while increasing concentrations of surfactant were added. The detailed experimental procedure can be found in Lu et al. [22], Okubo et al. [29], and Kittisrisawaia [30].
1H-Nuclear Magnetic Resonance (1H-NMR) Spectroscopy
The complexation SDS/β-CD was confirmed using 1H-NMR spectroscopic analysis. An Agilent 400 MR NMR spectrometer operating at a frequency of 399.938 MHz, equipped with an Automation Triple Broadband probe, was used with the following parameters: number of transients: 16; pulse width: 45°; acquisition time 3.7 s, spectral width 6410.3 Hz; temperature: 25 °C. The samples were prepared in deuterated water (D2O) with the following concentrations: [SDS] solution: 0.07 molar (40 mg/2 ml D2O), [β-CD] solution: 0.018 molar (40 mg/2 ml D2O), and a [SDS/β-CD] inclusion complex solution of 0.07 molar at 1:1 molar ratio (40 mg:160 mg in 2 ml D2O of SDS and β-CD respectively).
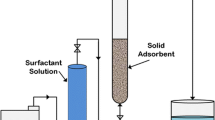
Static Adsorption Tests
The adsorption of β-CD, surfactant in free-state, and surfactant in complex-state onto solid surfaces was determined by applying the batch equilibrium adsorption procedure presented by Muherei and Junin [31] at 25 °C. The adsorption of any given compound was determined using Eq. 3 as follows.
where C i and C e are the initial and equilibrium liquid phase concentrations of the adsorbate solutions (g/l), respectively; V is the volume of the adsorbate solution (l); and W is the mass of dry adsorbent (g).
The adsorbents evaluated in this work were sand, kaolin, and shale. Batch adsorption tests were conducted in triplicate for each adsorbent. Therefore, three tests were conducted for each adsorbent using the surfactant in free-state (without β-CD) and three tests using the surfactant in complex-state (with β-CD), which gives six (6) adsorption tests per adsorbent. Furthermore, β-CD was also subjected to batch equilibrium adsorption (triplicate) using sand as the adsorbent. Thus, a total of twenty-one (21) batch equilibrium adsorption tests were conducted.
In the case of sand, 82 g of sandstone (surface area of sand = 0.2398 m2/g) was added to a 494 ml surfactant solutions contained in glass volumetric flasks. In the case of kaolin and shale, 0.2 g of crushed samples was added to 12 ml of the surfactant solutions contained in glass tubes. The amount of sand used was intentionally higher than the amount of the other materials (kaolin and shale) with the purpose of reproducing in the sand test a total surface area for adsorption similar to the total surface area provided by the kaolin and shale powder.
Preliminary testing (data not presented here) indicated that the adsorption equilibrium time was 24 h. Therefore, all the surfactant-porous media systems were left for 48 h to ensure enough time for the systems to reach equilibrium. After the equilibration time was reached, aliquots were taken for chemical analysis.
In the case of β-CD, total organic carbon (TOC) analysis was applied to determine the concentration of β-CD before and after adsorption; while in the case of sodium dodecyl sulfate (SDS), surface tension was used to indirectly determine the concentration of SDS after adsorption using a calibration curve of surface tension versus surfactant concentration. Furthermore, the batch static adsorption tests were conducted in soft brine of NaCl at a concentration of 3 wt% to eliminate any possible interactions between the surfactant and divalent ions (Ca2+, Mg2+, etc.), such as surfactant precipitation in hard brine; which could make ambiguous the interpretation of the surfactant adsorption results.
The adsorption data corresponding to each adsorbent was statistically analyzed through the application of the t statistic for related samples.
Bottle Testing: Surfactant Release from the β-CD Cavity
The driving force for the formation of inclusion complexes using β-CD is the replacement of water molecules that are thermodynamically unfavored by the partial or total inclusion of hydrophobic molecules into its cavity, which lowers the energy of the system [21]. Furthermore, β-CD is able to dissolve oils in its hydrophobic core [32].
Bottle testing was conducted to qualitatively evaluate the release of surfactant from the core of the β-CD in the presence of crude oil. It was reasoned that a competitive process could be initiated in this environment; in which surfactant could be released from the β-CD core to migrate towards the water–oil interface; while simultaneously oil could be dissolved into the β-CD cavity. The release and migration of surfactant towards the oil–water interface would promote the dispersion and/or emulsification of the oil within the aqueous phase.
In these tests, the respective solid adsorbents (sand, kaolin, and shale) were mixed with crude oil to produce a solid/crude oil blend with an oil saturation and/or concentration of 70 wt%. Three sets of experiments were conducted as follows.
-
1.
Blank Tests/Set # 1: Complexation SDS/β-CD Two (2) grams of solid adsorbents (sand, kaolin, and shale) were placed in three different glass tubes; followed by the addition of 12 ml of brine (NaCl 3 wt%) containing a concentration of 14 mM SDS + 14 mM β-CD. Experiments were conducted in triplicate at 25 °C and the mixtures were left to equilibrate for a period of 24 h.
-
2.
Blank Tests/Set # 2: β-CD in Free-State Two (2) grams of the corresponding solid adsorbents (sand, kaolin, and shale) containing 70 wt% of crude oil were placed in three different glass tubes; followed by the addition of 12 ml of brine (NaCl 3 wt%) containing only β-CD at a concentration of 14 mM. Experiments were conducted in triplicate at 25 °C and the mixtures were left to equilibrate for a period of 24 h.
-
3.
SDS Release Test/Set # 3: Complexation SDS/β-CD Two (2) grams of the corresponding solid adsorbents (sand, kaolin, and shale) containing 70 wt% of crude oil were placed in three different glass tubes; followed by the addition of 12 ml of brine (NaCl 3 wt%) containing a concentration of 14 mM SDS + 14 mM β-CD. Experiments were conducted in triplicate at 25 °C and the mixtures were left to equilibrate for 24 h.
After equilibration, the glass tubes from each set of experiments were subjected to visual observations (qualitative interpretations) to detect changes in the aqueous and oil phases.
Statistical Analysis
The experimental results were processed using Microsoft Excel 2010 software and expressed as mean ± standard deviation of the mean of n separate experiments. The statistical analysis of the surfactant adsorption data was performed using the t statistic for related samples. All graphs were plotted using Microsoft Excel 2010 software.
Results and Discussion
Surface Area Measurements
Table 2 summarizes the corresponding surface areas of sand, kaolin, and shale. Kaolin had the highest surface area, followed by oil shale, and sandstone.
Critical Micelle Concentration (CMC)
Table 3 presents the critical micelle concentrations of the surfactant solutions in free- and in complex-state in various hard brine concentrations. The CMC values for both systems decrease as salinity concentration increases. This trend suggests that the increased concentration of electrolyte ions minimizes the electro-repulsive forces among surfactant head groups and micellization takes place at lower surfactant concentrations.
SDS solution in complex-state shows higher values of CMC when compared with the critical micelle concentration of the system in free-state. During the complexation process, free surfactant monomers in the bulk of the solution are rapidly included into the hydrophobic core of the β-CD until the molar stoichiometry of the complexation is achieved; after which additional surfactant is required for micellization to take place [33].
Complexation Equilibrium
Figure 1 show the experimental data (open symbols) and the linear regression fittings (dashed lines) of the experimental data of [S][CD] as a function of [SCD] obtained in the premicellar region during the formation of inclusion complexes for some of the salinity concentrations (1.05, 2.1, and 4.2 wt%) used. The experimental data shows that the relationships between [S][CD] and [SCD] do not follow perfect linear fits, however the coefficients of determination (R2) for each case demonstrates that greater than 90 % of the differences between the concentration of the reactants [S][CD] are accounted for by differences in the concentration of the product [SCD] as the inclusion complex is formed. The high values of R 2 (>0.9) indicate that linear regression fits well the correlation between these two variables, which according to complexation equilibrium principles, supports a stoichiometric molar ratio of 1:1 for all the inclusion complexes of SDS/β-CD formed within the range of salinity and hardness concentrations used in this work.
Table 4 shows the association constants (K a) obtained for the SDS/β-CD inclusion complexes in different brine concentrations at 25 °C. These results indicate that K a increases with salinity until it reaches a concentration of 2.1 wt%. This observation is in agreement with previous research [34], in which the binding constant of the complexation increases with the polarity of the medium.
At higher salinities, such is the case of 4.2 wt%, the association constant decreases. This effect might be explained by the fact that at high salinity and/or hardness concentrations, precipitation of the surfactant (SDS) takes place due to interactions with the divalent cations in the brine, and as a result less SDS is available for association with β-CD, which is demonstrated by the lower value of the calculated K a (325.26 M−1).
1H-Nuclear Magnetic Resonance (1H-NMR) Spectroscopy
1H NMR is commonly used to confirm the complexation between cyclodextrins and guests molecules [21, 34]. Figure 2 illustrates the 1H-NMR spectra for β-CD in free-state, SDS, and inclusion complex SDS/β-CD (no to scale); while Table 5 presents the actual 1H-NMR chemical shifts of β-CD in free- and in complex-state.
Protons H3 and H5 located inside the β-CD cavity in the spectrum of the complexation show up-field chemical shifts (Δδ < 0) when compared with the spectrum of the β-CD in free-state. These chemical shifts indicate the formation of the inclusion complex, in which the hydrophobic tail of the SDS is inserted into the β-CD cavity [35–37]. Protons H1, H2, H4, and H6 also show chemical shifts suggesting that the polar end of the surfactant molecule, which sticks out of the core of the cavity folds over the exterior of the cavity causing the observed chemical shifts.
Furthermore, the complexation induced a chemical shift of the SDS methylene group from approximately 4.05 ppm to around 3.92 ppm; this up-field chemical shift (Δδ = −0.13 ppm) also suggests the inclusion of the SDS into the hydrophobic core of the β-CD.
Static Adsorption Tests
β-CD Adsorption
Figure 3 presents the Total Organic Carbon (TOC) in mg/L as a function of β-CD solution concentration in g/L before (square-solid symbol) and after (diamond-solid symbol) contacting the solid adsorbent. This experimental data indicates that there is no difference in the concentration of β-CD in the solutions before and after the static adsorption test, which suggest that β-CD does not adsorb onto the solid surface.
Surfactant Adsorption in Free- and in Complex-State
Table 6 summarizes the adsorption data obtained from a total of 18 batch adsorption tests. The adsorption of SDS is decreased by 19 % onto sand, 78 % onto kaolin, and 79 % onto shale, when compared with the adsorption of SDS in free-state. Overall, these results suggest that the complexation SDS/β-CD significantly reduces surfactant adsorption.
Figure 4 plots surfactant adsorption behavior as a function of solid adsorbent and surfactant system (in free- or in complex-state). The results of this exploratory research indicate that the complexation of SDS and β-CD is highly effective in reducing and/or inhibiting the adsorption of surfactant onto solid surfaces.
Bottle Testing: Surfactant release from the β-CD cavity
-
1.
Blank Tests/Set # 1: Complexation SDS/β-CD After equilibration (24 h), the glass tubes from each set of experiments were directly observed to detect changes in the aqueous and oil phases. Figure 5 shows a picture of the Blank Tests/Set # 1: Complexation SDS/β-CD in the absence of oil. All the systems (kaolin, sand, and shale) show transparent and yellowish aqueous phases, which suggest the leaching of mineral and/or organic matter from the solid materials.
-
2.
Blank Tests/Set # 2: β-CD in Free-State Figure 6 displays the bottle testing corresponding to the Blank Tests/Set # 2:β-CD in free-state containing solid-oil blends, in which the concentration of crude oil is 70 wt%. In these tests, the aqueous phase is slightly darker if compared with the bottle testing presented in Fig. 5. The darker color of the aqueous phase suggests the segregation of small volume of crude oil from the solid material. However, the bulk of the crude oil concentration remains in the solid phases at the bottom of the tubes.
-
3.
SDS Release Test/Set # 3: Complexation SDS/β-CD Figure 7 shows crude oil dispersed into the bulk of the aqueous phase. Particularly, for the case of kaolin and sand, where drops of dispersed crude are clearly visible in the corresponding solutions. It is speculated, that the hydrophobic core of the β-CD releases the surfactant, which migrates towards the oil–water interface stabilizing the dispersed oil beads. At the bottom of the glass tubes, kaolin and sand, display lighter colorations when compared with their equivalent test tubes in Fig. 6. This also indicates drifting of the crude oil from the solid phase, which might be driven by a lower interfacial tension in the system due to the release of the surfactant from the β-CD cavity.
Figure 7 also indicates that in the case of oil shale, a redistribution of the saturation of the dense organic matter (kerogen) takes place in the presence of surfactant, which renders a more homogeneous distribution of the heavy organic material within the solid phase. In this case, a fine dispersion of oil is also visibly in the upper aqueous phase.
Furthermore, liquid samples were carefully removed from the tubes to measure the corresponding surface tensions. Table 7 presents the surface tension data for each set of experiments. Surface tension measurements were repeated 9 times per sample, thus the arithmetic mean and the standard deviation of the surface tension (ST) measurements are also presented.
The surface tension of the baseline solution (brine) is 64 mN/m (First row in Table 7). The addition of the complexation SDS/β-CD at a concentration of 14 mM β-CD and 14 mM SDS (Blank Tests/Set # 1) decreases the surface tension of the original brine to approximately 29 mN/mm due to the presence of surfactant monomers in free-state.
The surface tension analysis of the Blank Tests/Set # 2, in which only β-CD was added to the brine, shows marginal variations of surface tension. β-CD does not have surface active properties; consequently the surface tension of the brine should not be affected by the addition of β-CD. According to Dharmawardana and coworkers [38], the surface tension of β-CD aqueous solution tends to equal the surface tension of pure water. This lack of surface activity is qualitative confirmed in Fig. 6, where separation and dispersion of oil from the porous media into the aqueous phase was not observed.
Surface tension measurements for experiments in Set # 3 (column 6 in Table 7) show a decrease in the surface tension values. In the presence of crude oil, the equilibrium of the SDS/β-CD complexation given by the reversible reaction S + CD ↔ SCD is broken; therefore the released surfactant monomers migrate towards the oil–water interface rendering the separation and dispersion of oil into the aqueous phase as observed in Fig. 7.
Conclusions
This proof of concept research confirmed the complexation of SDS/β-CD with 1:1 molar stoichiometry through complexation equilibrium principles and 1H NMR. The strength of the inclusion complex formation (association constant, K a) increases as the electrolyte concentration in the aqueous media increases.
The surfactant delivery system inhibits the adsorption of SDS onto sand, kaolin, and shale by 19, 78, and 79 % respectively which supports the hypothesis of this research. Therefore, this approach shows itself to be efficient at preventing surfactant adsorption onto rock surfaces.
The released of surfactant from the hydrophobic core of the β-CD in the presence of solid-crude oil blends was qualitatively observed, however significantly more research is necessary to fully establish the potential of this approach for enhanced oil recovery applications.
Abbreviations
- CD:
-
Cyclodextrin
- CMC:
-
Critical micelle concentration
- CMC*:
-
Apparent critical micelle concentration
- DW:
-
Distilled water
- EOR:
-
Enhanced oil recovery
- 1H NMR:
-
Proton nuclear magnetic resonance
- SDS:
-
Sodium dodecyl sulfate
- ST:
-
Surface tension
- STDEVA:
-
Standard deviation
- TDS:
-
Total dissolved solids
- TOC:
-
Total organic carbon
- α-CD:
-
Alpha-cyclodextrin
- β-CD:
-
Beta-cyclodextrin
- γ-CD:
-
Gamma-cyclodextrin
- δ :
-
Chemical shift
References
Salager JL, Forgiarini AM, Bullón J (2013) How to attain ultralow interfacial tension and three-phase behavior with surfactant formulation for enhanced oil recovery: a review. Part 1. Optimum formulation for simple surfactant–oil–water ternary systems. J Surfactants Deterg 16:449–472
Salager JL, Forgiarini AM, Márquez L, Manchego L, Bullón J (2013) How to attain an ultralow interfacial tension and a three-phase behavior with a surfactant formulation for enhanced oil recovery: a review. Part 2. performance improvement trends from Winsor’s premise to currently proposed inter-and intra-molecular mixture. J Surfactants Deterg 16:631–663
Salager JL, Manchego L, Márquez L, Bullón J, Forgiarini A (2014) Trends to attain a lower interfacial tension in a revisited pure alkyl polyethyleneglycol surfactant–alkane–water ternary system. Basic concepts and straightforward guidelines for improving performance in enhanced oil recovery formulations. J Surfactants Deterg 15:199–213
Muherei MA, Junin R, Bin Merdhah AB (2009) Adsorption of sodium dodecyl sulfate, Triton X100 and their mixtures to shale and sandstone: a comparative study. J Pet Sci Eng 67:149–154
Zhang R, Somasundaran P (2006) Advances in adsorption of surfactants and their mixtures at solid/solution interfaces. Adv Colloid Interface Sci 123:213–229
Paria S, Khilar KC (2004) A review on experimental studies of surfactant adsorption at the hydrophilic solid–water interface. Adv Colloid Interface Sci 110:75–95
Xu Q, Vasudevan TV, Somasundaran P (1991) Adsorption of anionic—nonionic and cationic—nonionic surfactant mixtures on kaolinite. J Colloid Interface Sci 142:528–534
Scamehorn JF, Schechter RS, Wade WH (1982) Adsorption of surfactants on mineral oxide surfaces from aqueous solutions. I: Isomerically pure anionic surfactants. J Colloid Interface Sci 85:463–478
Scamehorn JF, Schechter RS, Wade WH (1982) Adsorption of surfactants on mineral oxide surfaces from aqueous solutions. II: Binary mixtures of anionic surfactants. J Colloid Interface Sci 85:479–493
Scamehorn JF, Schechter RS, Wade WH (1982) Adsorption of surfactants on mineral oxide surfaces from aqueous solutions: III. Binary mixtures of anionic and nonionic surfactants. J Colloid Interface Sci 85:494–501
Somasundaran P, Krishnakumar S (1997) Adsorption of surfactants and polymers at the solid-liquid interface. Colloids Surf A 123:491–513
Somasundaran P, Huang L (2000) Adsorption/aggregation of surfactants and their mixtures at solid–liquid interfaces. Adv Colloid Interface Sci 88:179–208
Trogus FJ, Sophany T, Schechter RS, Wade WH (1977) Static and dynamic adsorption of anionic and nonionic surfactants. SPE J 17:337–344
Flaaten A, Nguyen QP, Pope GA, Zhang J (2009) A systematic laboratory approach to low-cost high-performance chemical flooding. SPE Reservoir Eval Eng 12:713–723
Hirasaki G, Miller CA, Puerto M (2011) Recent advances in surfactant EOR. SPE J 16:889–907
Levitt D, Jackson A, Heinson C, Britton LN, Malik T, Dwarakanath V, Pope GA (2009) Identification and evaluation of high-performance EOR surfactants. SPE Reservoir Eval Eng 12:243–253
Puerto M, Hirasaki GJ, Miller CA, Barnes JR (2012) Surfactant systems for EOR in high-temperature high-salinity environments. SPE J 17:11–19
Zhang J, Nguyen QP, Flaaten A, Pope GA (2009) Mechanisms of enhanced natural imbibition with novel chemicals. SPE Reservoir Eval Eng 12:912–920
Sheng J (2010) Modern chemical enhanced oil recovery: theory and practice. Gulf Professional Publishing Burlington. MA, USA
Szejtli J (2004) Past, present and future of cyclodextrin research. Pure Appl Chem 76:1825–1845
Connors KA (1997) The stability of cyclodextrin complexes in solution. Chem Rev 5:1325–1358
Lu R, Hao J, Wang H, Tong L (1997) Determination of association constants for cyclodextrin–surfactant inclusion complexes: a numerical method based on surface tension measurements. J Colloid Interface Sci 192:37–42
Satake I, Yoshida S, Hayakawa K, Maeda T, Kusumoto Y (1986) Conductometric determination of the association constants of β-cyclodextrin with amphiphilic ions. Bull Chem Soc Jpn 59:3991–3993
Palepu R, Reinsborough VC (1988) Surfactant-cyclodextrin interactions by conductance measurements. Can J Chem 66:325–328
Stella VJ, Rajewski RA (1997) Cyclodextrins: their future in drug formulation and delivery. Pharm Res 14:556–567
Szejtli J (1998) Introduction and general overview of cyclodextrin chemistry. Chem Rev 98:1743–1754
Satake I, Ikenoue T, Takeshita T, Hayakawa K, Maeda T (1985) Conductometric and potentiometric studies of the association of α-cyclodextrin with ionic surfactants and their homologs. Bull Chem Soc Jpn 58:2746–2750
Schneider HJ, Hacket F, Rüdiger V, Ikeda H (1998) NMR studies of cyclodextrins and cyclodextrin complexes. Chem Rev 98:1755–1786
Okubo T, Kitano H, Ise N (1976) Conductometric studies on association of cyclodextrin with colloidal electrolytes. J Phys Chem 80:2661–2664
Kittisrisawai S (2014) Novel surfactant delivery system: the solution to surfactant adsorption onto rock formations during enhanced oil recovery chemical flooding. PhD Dissertation, The University of New Brunswick, Fredericton, p 256
Muherei MA, Junin R (2009) Equilibrium adsorption isotherms of anionic, nonionic surfactants and their mixtures to shale and sandstone. Mod Appl Sci 3:158–167
Kilsdonk EP, Yancey PG, Stoudt GW, Bangerter FW, Johnson WJ, Phillips MC, Rothblat GH (1995) Cellular cholesterol efflux mediated by cyclodextrins. J Biol Chem 270:17250–17256
Georges J, Desmettre S (1987) An electrochemical study of mixed solutions of β-cyclodextrin and sodium dodecyl sulfate. J Colloid Interface Sci 118:192–200
Schneider HJ (2009) Binding mechanisms in supramolecular complexes. Angew Chem Int Ed 48:3924–3977
Chen M, Diao G, Zhang E (2006) Study of inclusion complex of β-cyclodextrin and nitrobenzene. Chemosphere 63:522–529
Yallapu MM, Jaggi M, Chauhan SC (2010) β-Cyclodextrin-curcumin self-assembly enhances curcumin delivery in prostate cancer cells. Colloids Surf B 79:113–125
Pîrnau A, Bogdan M, Floare CG (2009) NMR spectroscopic characterization of β-cyclodextrin inclusion complex with vanillin. J Phys Conf Ser 182:012013
Dharmawardana UR, Christian SD, Tucker EE, Taylor RW, Scamehorn JF (1993) A surface tension method for determining binding constants for cyclodextrin inclusion complexes of ionic surfactants. Langmuir 9:2258–2263
Acknowledgments
The authors would like to acknowledge Dr. Larry Calhoun, Chemistry Department, University of New Brunswick for his contributions with the NMR analysis. The financial support provided to this research by the Chemical Engineering Department at the University of New Brunswick, the Natural Sciences and Engineering Research Council (NSERC), and the Canadian Foundation for Innovation (CFI) is also gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Kittisrisawai, S., Romero-Zerón, L.B. Complexation of Surfactant/β-Cyclodextrin to Inhibit Surfactant Adsorption onto Sand, Kaolin, and Shale for Applications in Enhanced Oil Recovery Processes. Part I: Static Adsorption Analysis. J Surfact Deterg 18, 603–613 (2015). https://doi.org/10.1007/s11743-015-1688-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11743-015-1688-4