Abstract
Surface tension, fluorescence, and dynamic light scattering were used to investigate the properties of a binary surfactant system comprising an anionic gemini surfactant (DLMC) and cationic gemini surfactant (II-12-EO2). Surface tension measurements afforded the critical micelle concentration (cmc) of the mixture and the values are all lower than those of pure constituent surfactants. For the mixtures of II-12-EO2/DLMC, the micelle aggregation number decreases with the increase of II-12-EO2, and the micropolarity of the micelle is lowest when the molar fraction of II-12-EO2 is 0.5; the hydrodynamic radius (R h) of the mixed micelle first increases and then decreases with the addition of II-12-EO2, and larger micelles are obtained when the molar fraction of II-12-EO2 is 0.5 or 0.7.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Gemini surfactants are composed of two hydrophilic and two hydrophobic groups connected through a spacer group at or near the headgroup. Owing to their special molecule structure, gemini surfactants present many excellent properties and advantages compared with conventional surfactants; these features help in the construction of aggregates at the molecular level. Therefore, more and more attention is being paid to gemini surfactants [1–5].
In many practical applications, mixtures of surfactants are often used which present much better properties than those attainable with the individual components. There are many reports about the mixtures of traditional surfactants or the mixtures of gemini surfactants with traditional surfactants [6–8], but none of them has, to our knowledge, investigated mixtures of gemini surfactants. In this paper, we studied the mixed system of a cationic gemini surfactant [N,N′-bis(dimethyl dodecylate ethanol) glycol ether dibromide] (II-12-EO2) with an anionic gemini surfactant (N,N′-dilauryl ethylenediamine dipropionic acid) (DLMC) (Fig. 1).
Experimental Section
Materials
The cationic gemini surfactant (II-12-EO2) was synthesized and purified to greater than 98 % as previously reported [9]. The anionic gemini surfactant (DLMC) was also synthesized and purified to greater than 98 % as previously reported [10]. Sodium bromide of analytical grade from Sinopharm Chemical Reagent China was baked at 773 K for 5 h before use.
Experimental Methods
The drop volume method was used to measure the surface tension of the mixed surfactants [11]. The experiment results were accurate within ±0.1 mN m−1. All the measurements were performed in 0.1 M NaBr solution and at 313 ± 0.1 K. The critical micelle concentration (cmc) values can be obtained from the break point of the surface tension versus concentration curves.
Micelle aggregation number (N m) of surfactants can be measured by the steady-state fluorescence quenching measurements. Pyrene (Py) is used as a fluorescent probe (P) and diphenylmethane (DPK) is used as the quencher (Q) [12, 13]. Each spectrum has one to five vibronic peaks and those at 373 nm and 384 nm can be used to evaluate the micropolarity of the micelle in which the quenching process is going on. The higher the values of I 1/I 3, the more hydrophobic environment of the micelle inner core.
Dynamic light scattering measurements were carried out for the mixture of II-12-EO2/DLMC at 313 ± 0.1 K, using a laser light scattering spectrometer (ALV/SP-125) with a multiple digital tau correlator (ALV-5000). The instrument is equipped with a cylindrical UNIPHASE He–Ne laser (λ = 632.8 nm) [14].
Results and Discussion
Surface Chemical Properties of the Mixed Systems
The cmc and surface tension (γ cmc) under the cmc are two major parameters to estimate the surface activity of surfactant. The surface tension values (γ) of the single and II-12-EO2/DLMC mixtures were measured, and the surface tension curves are shown in Fig. 2.
As show in Table 1, it is clear that all the cmc and γ cmc values of II-12-EO2/DLMC mixed systems are smaller than those of the two single components, indicating a synergistic behavior of these two gemini surfactants.
The surface maximum absorption (Γmax) and the average minimum area per molecule (A min) can be computed by the Gibbs Eqs. (1) and (2) [11]:
where γ is the surface tension (mN/m), T is absolute temperature (K), R is 8.314 J mol−1 K−1, N A is the Avogadro constant, and the value of n (the number of species whose concentration changes with change in c) is taken as 1 in the presence of excessive counterions.
For the mixtures of II-12-EO2/DLMC, if there is no interaction between the two components, then the ideal cross-sectional area per molecule can be calculated using Eq. (3) [11]:
where A min,1 and A min,2 are the cross-sectional areas per molecule of the first individual surfactant and the second surfactant, respectively.
According to the ideal mixture theory, the cmc values of the mixture can be computed using Clint Eq. (4):
where α is the molar fraction of II-12-EO2 in the mixture system; \(C_{1}^{M}\),\(C_{2}^{M}\) and \(C_{12}^{M}\) are the cmc values of II-12-EO2, DLMC, and their mixture, respectively. Ideal mixture theory is only suitable for synergistic surfactant mixtures which consist of the same hydrophilic group or non-synergistic mixtures based on similar hydrophilic groups. However, there will be large deviations for non-syngergistic mixtures which have different hydrophilic groups. In such cases, non-ideal mixed micelle theory should be applied.
According to non-ideal mixed micelle theory, the cmc values of the mixture can be computed by Eq. (5):
where \(f_{1}^{M}\) and \(f_{2}^{M}\) are the activity coefficient of each single surfactant. When \(f_{1}^{M}\) = \(f_{2}^{M}\) = 1, the Eq. (5) is equivalent to Eq. (4), and the mixture is regarded as an ideal mixture system.
The activity coefficient of a surfactant can be calculated using Eqs. (6) and (7):
where \(x_{1}^{M}\) is the molar fraction of II-12-EO2 in the micelle, \(\beta^{M}\) is the interaction parameter of surfactants in the micelle and reflects the degree of interactions among the surfactants.
As shown in Table 1, the A min values of the mixed system are smaller than those of A ideal,min, and this suggests a strong interaction between the two surfactant molecules. This is because each gemini surfactant has two ionic heads and there are therefore strong electrostatic attractions among the mixed surfactants. This phenomenon is also confirmed by the values of β σ and β m.
In Fig. 3, the dashed line plots the cmc values which were calculated from Eq. (4) through the ideal mixture theory; the full line plots the cmc values which were computed from Eq. (5) according to the non-ideal mixture theory; the points are the measurement values of the experiments. It is clear that all the experimental values are always lower than the corresponding cmcideal values and all the cmc values of the mixed systems are lower than those of the single surfactants, which indicates the existence of interactions that result in non-ideality of the mixture systems. The values of the solid line are basically consistent with the experimental values, which shows that the non-ideal mixture theory is applicable in this system.
Molecular Interactions
The nature and strength of the interaction in the mixed system can be determined by the calculation of parameters β. The molar fraction x σ1 and interaction parameter β σ at the air/water interface can be calculated using Eqs. (8) and (9) [11]:
where x σ1 is the molar fraction of II-12-EO2 in the surface layer, c 12 is the total concentration of surfactant at the given surface tension. c 01 and c 02 are the concentrations of a single surfactant at the same surface tension, α 1 is the molar fraction of II-12-EO2 in the mixed system, and β σ is the interaction parameter of surfactants in the surface phase.
The micellar interaction parameter β m in the mixed system can also be obtained from Eqs. (10) and (11) [2]:
The term x m1 is the molar fraction of II-12-EO2 in the micelle and c m12 is the cmc value of the mixture system. c m1 and c m2 are the cmc values of the pure component and β m is defined as the interaction parameter of surfactants in the mixed micelles. When the value of β (β σ and βm) is negative, it means the existence of attraction between the two components. And the more negative the value is, the stronger the mutual attraction; when the value of β is positive, it indicates mutual repulsion between the two components; when the value of β is 0, it suggests an ideal mixture of the surfactants in the micelle or surface layer. For II-12-EO2/DLMC mixtures, the values of x σ1 , x m1 , β σ, and β m are listed in Tables 1 and 2.
For the mixture of II-12-EO2/DLMC, the mole fraction of II-12-EO2 increases with the increase of molar fraction of II-12-EO2 both in the surface phase and micellar phase. It is quite clear that the compositions of surface layer and micelle correspond to the change of II-12-EO2. And the negative values of β σ and β m also indicate the strong interactions between the two gemini surfactants.
The existence of synergism between surfactants can also be predicted by the strength of the interaction (β) and the relevant properties of the individual surfactant components of the mixture.
Conditions for Synergism of the Mixed Systems
The conditions for synergism in the surface tension reduction efficiency are given by Eq. (12) [11]:
The degree of synergism can be judged by Eq. (13):
where \(c_{12,\;\hbox{min} }\) is the minimum concentration of the mixture to achieve a given surface tension, \(c_{1(2)}^{0}\) represents the more effective surfactant’s concentration (the smaller one) at a given surface tension. The data of \(1 - c_{12,\hbox{min} } /c_{1(2)}^{0}\) are listed in Tables 1 and 2, and the larger the value is, the stronger the synergism.
It is clear that all the values of β σ are less than zero, which means the existence of synergism in the surface tension reduction efficiency. This is also confirmed by the values of \(1 - c_{12,\hbox{min} } /c_{1(2)}^{0}\).
When the cmc values of the mixture are lower than the values of each single surfactant, it suggests the existence of synergism in mixed micelle formation. The conditions for synergism are given by Eq. (14):
The degree of synergism in the micelle formation can also be estimated by Eq. (15):
where \(c_{12,\hbox{min} }^{m}\) represents the lowest cmc values of the mixed surfactant system and \(c_{1}^{m}\) represents the smaller cmc of the two components. The data of \(1 - {\raise0.7ex\hbox{${c_{12,\hbox{min} } }$} \!\mathord{\left/ {\vphantom {{c_{12,\hbox{min} } } {c_{1(2)}^{0} }}}\right.\kern-0pt} \!\lower0.7ex\hbox{${c_{1(2)}^{0} }$}}\) are listed in Tables 1 and 2.
It is clear that all the β σ values of the mixed system are less than zero, which indicates the exsistence of synergism in the micelle formation. At the same time, the synergism is the strongest at the maximum \(1 - {\raise0.7ex\hbox{${c_{12,\hbox{min} }^{m} }$} \!\mathord{\left/ {\vphantom {{c_{12,\hbox{min} }^{m} } {c_{1(2)}^{m} }}}\right.\kern-0pt} \!\lower0.7ex\hbox{${c_{1(2)}^{m} }$}}\) value.
The conditions for synergism in the surface tension reduction are given by Eq. (16).
As shown in Table 2, the II-12-EO2/DLMC mixed system can fully meet these three conditions, and therefore the existence of synergism can also be confirmed in surface tension reduction.
Micellar Characteristics of the Mixed System [14]
As shown in Table 3, the micellar aggregation number of II-12-EO2 is relatively smaller than that of DLMC. This can be attributed to the larger ionic head and longer connector of II-12-EO2, and it also indicates a more compact micelle structure of DLMC. This phenomenon can also be confirmed by the value of I 1/I 3.
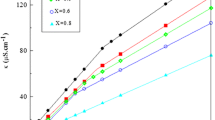
Figure 4 shows the variations of I 1/I 3 with total surfactant molar concentration c. It is apparent that these plots have the usual sigmoid shape, and the sharp decrease of the I 1/I 3 values represents the transfer of pyrene from a highly polar to less polar or more hydrophobic environment, which corresponds to the formation of micelle. The values of I 1/I 3 for II-12-EO2 are larger than that of DLMC. This phenomenon may be attributed to the existence of the hydrophilic EO2 spacer, which enhances the polarity of the microenvironment. The smaller I 1/I 3 value of DLMC also means a more hydrophobic environment of the micelle inner core.
Dynamic light scattering was used to determinate the average micelle hydrodynamic radius of the II-12-EO2/DLMC mixed system when the total surfactant molar concentration is fixed at 0.001 M. The results show that the R h values of micelle are larger than 100 nm and the distribution is quite narrow when the values of α (II-12-EO2) are 0.5 or 0.7, which indicates that large micelles are formed. The strong interactions of the ionic head can lead to large micelles, especially between the positive and the negative ion gemini surfactants. Normally, the surfactant micelle is spherical within 10 times the cmc, and with the increase of concentration, the micelle changes shape from a sphere to a rod and from a rod to a hexagonal phase. But for the mixed surfactants, especially for the cationic and anionic gemini surfactants, the strong interaction between surfactants can largely change the values of A min, and lead to new structures of micelle. It is clear that the mixed systems of gemini surfactants can form large micelle structures at a very lower concentration.
Conclusion
The surface chemical properties of II-12-EO2/DLMC mixed systems were studied and high surface activities were displayed. The mixed systems exhibit synergism in surface tension reduction efficiency, surface tension reduction effectiveness, and micelle formation. The micellar aggregation number and micropolarity of the II-12-EO2/DLMC mixed systems were determined. In the mixed system, the micelle aggregation number decreases with the increase of molar fraction of II-12-EO2, and the micropolarity of micelle inner core is the lowest when the value of n(DLMC)/n(II-12-EO2) is 1:1. The hydrodynamic radii of the II-12-EO2/DLMC mixed system are measured by dynamic light scattering measurements. It is evident that larger micelle structures are formed at a very low concentration when the molar fraction of II-12-EO2 is 0.5 or 0.7.
References
Menger FM, Littau CA (1991) Gemini surfactants: synthesis and properties. J Am Chem Soc 113:1451–1452
Rosen MJ (1993) A new generation of surfactants. Chem Tech 30:23–27
Menger FM, Littau CA (1993) A new class of self-assembling molecules. J Am Chem Soc 115:10083–10090
Zana R (1998) Dimeric (gemini) surfactants. In: Holmberg K (ed), Marcel Dekker, New York, p 242–278
Kunieda H, Masuda N, Tsubone K (2000) Comparison between phase behavior of anionic dimeric (gemini-type) and monomeric surfactants in water and water-oil. Langmuir 16:6438–6444
Sharma KS, Rodgers C, Palepu RM et al (2003) Studies of mixed surfactant solutions of cationic dimeric (gemini) surfactant with nonionic surfactant C12E6 in aqueous medium. J Colloid Interface Sci 268:482–488
Zana R, Levy H, Kwetkat K (1998) Mixed micellization of dimeric (gemini) surfactants and conventional surfactants. J Colloid Interface Sci 197:370–376
Liu Letian, Rosen Milton J (1996) The interaction of some novel diquaternary gemini surfactants with anionic surfactants. J Colloid Interface Sci 179:454–459
Bao XY, Xu HJ, Liang JL (2006) Synthesis and synergism of a kind of ester cationic gemini surfactant. China Surfactant Deterg Cosmet 36:1–4
Xu HJ, LÜ CX, Ye ZW (2004) Synthesis and properties of a kind of anionic gemini surfactant. J East China Univ Sci Technol 30:502–505
Zhao GX (2003) Principles of surfactant action. Light Industrial, China, pp 330–439
Huang JB, Zhao GX, Jiang YC, Wu SH (1993) Fluorescence probe study on the self-organized assemblies of the mixed catanionic surfactants. Acta Phys Chim Sin 9:577–580
Ray GB, Chakraborty I, Moulik SP (2006) Pyrene absorption can be a convenient method for probing critical micellar concentration (cmc) and indexing micellar polarity. J Colloid Interface Sci 294:248–254
Li F, Li GZ, Wang HQ, An YL (1998) Study on the aggregation and interaction of DDAPS micelles with fluorescence and light-scattering methods. Chem J Chin Univ 19:1117–1120
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Xu, H., Liu, B., Kang, P. et al. Properties of a Binary System Containing Anionic and Cationic Gemini Surfactants. J Surfact Deterg 18, 297–302 (2015). https://doi.org/10.1007/s11743-014-1655-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11743-014-1655-5