Abstract
The effect of bis-(2-ethylhexyl)phosphoric acid (DEHPA) on the region of existence, conductivity and structure of sodium bis-(2-ethylhexyl)phosphate (NaDEHP) microemulsion has a dual nature and depends on DEHPA concentration. In the system NaDEHP–DEHPA–kerosene–water, the narrowing of the microemulsion region is observed with DEHPA concentration in the organic phase growth from 0.1 to 0.5 mol/L. The increase of DEHPA concentration in the organic phase from 0.1 to 0.4 mol/L leads to the reduction of electrical conductivity of the microemulsions. Based on the conductivity and viscosity measurements, we suppose the transition from reverse microemulsion with isolated droplets to percolate microemulsion at volume fraction of water 0.18 ( \(W = C_{{H_{2} O}} /C_{\text{NaDEHP}}\) = 8). Droplet size of the microemulsions increases linearly with W growth. The rise of DEHPA concentration in the organic phase from 0.1 to 0.3 mol/L causes the growth of the coefficient at W in the equation d = kW + b from 0.038 to 0.249, i.e., it increases the slope of the lines. In contrast, DEHPA introduction at the concentration 0.1 mol/L (in the organic phase) leads to the expansion of the microemulsion region, does not affect the conductivity and decreases the coefficient at W. The rate of copper recovery into the microemulsion increases considerably with the rise of DEHPA concentration from 0.0 to 0.3 mol/L; no dual effect is observed. The following composition of the microemulsion for non-ferrous metals leaching is recommended: C NaDEHP = 1.6 mol/L, C DEHPA = 0.3 mol/L (in the organic phase); W = 8–32.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
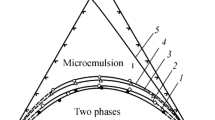
Microemulsions are thermodynamically stable, macroscopically homogeneous mixtures of at least three components: polar and nonpolar liquid phases (usually water and oil) and a surfactant that, on a microscopic level, forms a film separating the two incompatible liquids into two sub-phases. A microemulsion phase may be in equilibrium with excess oil (Winsor I), or water (Winsor II), or both oil and water (Winsor III). Phase diagrams of amphiphiles may also contain a single-phase microemulsion region (Winsor IV), which is a subject of our study.
Microstructure of Winsor IV type microemulsion usually changes with increasing water content from water-in-oil microemulsion droplets to bicontinuous structure and then to oil-in-water. The structure of microemulsion may be described by a concept of spontaneous curvature of the surfactant layer. At markedly positive spontaneous curvatures we have discrete oil-swollen micelles or oil droplets; at significant negative spontaneous curvatures there are discrete water-swollen reversed micelles or water droplets. At a zero spontaneous curvature we have a structure of minimal surface aggregates, which are disordered over larger distances. So, a bicontinuous microemulsion consists of a disordered, connected surfactant film separating the water and oil domains. The film has a spontaneous curvature that is close to zero, i.e., it has no tendency to curve toward either oil or water [1].
Microemulsions are promising systems for the technology due to solubilization of both polar and apolar substances. Microemulsions are used for cleaning of solid surfaces, for enhanced oil recovery, as water-repellents in construction industry, and for substance separation in analytical chemistry. Microemulsions are perspective for enzymatic reactions, for polymerization, for the synthesis of inorganic nanoparticles and nanocomposites [1–7]. Microemulsions are applied for the solvent extraction of organic and inorganic substances [8, 9].
Microemulsions for metal recovery may contain extractants—the substances that are used for separating by a solvent extraction technique. Reverse (W/O) extractant-containing microemulsions in equilibrium with water phase (Winsor II) are the most commonly used microemulsion systems for the solvent extraction of metals. Winsor II system forms upon contact of the organic solution of a surfactant with an extractant with the water solution of metal salts. Reverse microemulsions are able to solubilize hydrophilic metal ions. This leads to the increase of distribution coefficients of metal ions [10]. For example, extraction of Al and Zn into W/O microemulsion with bis-(2-ethylhexyl)phosphoric acid was shown [11]. Co and Ni were recovered into W/O microemulsion of cetyltrimethylammonium bromide and pentanol [12], Cd extraction into W/O microemulsion was studied in the system tri-n-octylamine–secondary octanol–kerosene–HCl–water [13].
We suggested using extractant-containing microemulsions for recovery of metals directly from a solid phase (microemulsion leaching) [14]. This method allows combining leaching and extraction processes. In microemulsions, it is possible to use different extractants of various classes, and also their mixtures. During the leaching, valuable metals are selectively extracted into the microemulsion, and components which are not or poorly extractable remain as a part of a solid phase and have to be separated at a filtration stage. The extracted metals in the microemulsion, after the re-extraction (stripping) remain in a water phase, and are subject to additional separation and purification. The organic phase after re-extraction is returned to the microemulsion preparation.
Sodium bis-(2-ethylhexyl)phosphate (NaDEHP) microemulsion is a promising system for the microemulsion leaching of non-ferrous metals from ores and secondary raw materials. NaDEHP is a sodium salt of bis-(2-ethylhexyl)phosphoric acid (DEHPA)—a well-known industrial extractant that is used for separation and purification of substances. Application of NaDEHP microemulsion allows selective extraction of non-ferrous metals with very low iron extraction. Selectivity of extraction of non-ferrous metals (nickel, cobalt, copper) and iron from a sample of cobalt-copper concentrate in NaDEHP microemulsion corresponds to selectivity of the extractant—bis-(2-ethylhexyl)phosphoric acid [14].
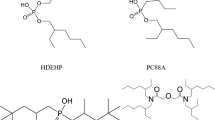
NaDEHP structure is analogous to sodium bis-(2-ethylhexyl) sulfosuccinate (AOT), which is a well-known surfactant (Fig. 1). The area per head group of NaDEHP molecule was calculated to be 0.64 nm2 for spherical reverse micelles in benzene, close to that of AOT (0.60 nm2) [15]. Both AOT and NaDEHP can form microemulsions in ternary systems with water and aliphatic hydrocarbon solvents without a co-surfactant. For example, the formation of bicontinuous and direct microemulsion in the system NaDEHP–heptane–water is described [16]. Reverse microemulsion is shown in the system of NaDEHP–hexane–water solution of NaCl or CaCl2 [17]. The formation of microemulsion is studied in the system NaDEHP–decane–water [18].
DEHPA will affect the properties of NaDEHP microemulsion. DEHPA is a surfactant; for example, it essentially decreases the interfacial tension in the system DEHPA–n-hexane–aqueous acid (HCl, HNO3, H2SO4, HClO4) solution. The area per head group of DEHPA molecule in this system was in the range 1.82–1.86 nm2 at pH value 1.0–5.0 [19]. It is shown that the replacement of 10–30 mol% NaDEHP by DEHPA leads to an increase of water solubilization capacity in the system NaDEHP–DEHPA–n-heptane–water [20].
The effect of DEHPA on the region of existence, physicochemical properties and nanostructure of microemulsions formed by its sodium salt (NaDEHP) is practically unexplored. The purpose of this work was to study the effect of DEHPA on the region of existence, structural transitions, and droplet size of NaDEHP microemulsions, as well as the ability of the microemulsions to recover copper from a solid phase.
Experimental Procedures
Materials
DEHPA (Acros Organics, Belgium) with purity of more than 95 %, NaOH of pure grade were used without further purification. NaDEHP was obtained in situ from DEHPA and NaOH during the microemulsion preparation. Distilled water was used for sample preparation. Kerosene KO-25 (illuminating kerosene, mixture of hydrocarbons C 8–C 15, density at 20 °C was 785 kg/m3, received from Expert-Oil, Russia) was used as an organic solvent. Kerosene was chosen because of its practical importance; kerosene is a commonly used organic solvent in the process of solvent extraction of metals. Microemulsions in the system NaDEHP–DEHPA–kerosene–water were proposed previously for microemulsion leaching of metals [14]. CuO of pure grade was used as a powder, with particle size 5–75 μm, average particle size 23 μm.
Methods
Sample Preparation and Determination of the Region of Existence
The samples of NaDEHP microemulsions were prepared by mixing the required amounts of an aqueous NaOH solution and a DEHPA solution in kerosene. During the mixing, the neutralization reaction of NaOH and bis-(2-ethylhexyl)phosphoric acid took place and the formation of optically clear microemulsion was observed. DEHPA, introduced over the stoichiometric ratio DEHPA:NaOH = 1:1, can act as a co-surfactant, which affects the microemulsion properties, and as an extractant for metal recovery. Water formed in the neutralization reaction was taken into account in the calculation of \(W = C_{{H_{2} O}} /C_{\text{NaDEHP}}\) values.
Determination of the region of existence of the microemulsion was carried out in the temperature-controlled test tubes at a temperature of 20.0 ± 0.2 °C, by increasing the water concentration while keeping the NaDEHP-to-kerosene ratio fixed. Water was added into a sample of the microemulsion with an interval ΔW = 0.5. After water addition, the sample was stirred and allowed to reach equilibrium for 5 min; the equilibrium phase state was detected visually. Water introduction into the microemulsion was continued before the appearance of turbidity and the subsequent phase separation. Microemulsion in equilibrium with excess organic phase (Winsor I) at low NaDEHP concentration and microemulsion in equilibrium with water-rich liquid crystalline phase at high NaDEHP concentration were observed at water content higher than the boundary of the microemulsion existence. The boundary was confirmed by obtaining samples with water content slightly less than the boundary value and slightly exceeding the maximum water solubilization.
Conductance Measurements
The conductance measurements were taken by the conductivity meter Hanna 8733 (Hanna Instruments, Germany), and temperature was controlled with the accuracy ±0.2 °C. Before each measurement, the microemulsion was kept at a given temperature for 30 min.
Viscosity Measurements
The dynamic viscosity was measured with a rotation viscometer Rheotest 2 (RHEOTEST, Germany) with coaxial cylinders at 20.0 ± 0.2 °C in the shear rate range 27–1,312 s−1. Before each measurement, the microemulsion was kept at T = 20 °C for 30 min.
Dynamic Light Scattering
The average hydrodynamic diameter of the microemulsion droplets was determined by dynamic light scattering by Zetasizer Nano ZS (Malvern Instruments, UK) equipped with a He–Ne laser operating at 532 nm at temperature 20 °C. To obtain statistically reliable results, each measurement was performed at least five times.
Copper Leaching
Copper was chosen as an example of non-ferrous metal for the leaching study, because it is well extracted by DEHPA. Previous leaching experiments demonstrated that recovery factor of copper from cobalt-copper concentrate into NaDEHP microemulsion was higher than recovery factors of cobalt and nickel [14]. The leaching process was performed in the closed flasks at a ratio of solid and liquid phase S:L = 1:50 at a temperature 80 °C and mechanical stirring (rotational motion with an amplitude of 4 mm and frequency of 200 turn/min) in an ELPAN 457 water bath shaker (Poland). This mechanical stirring corresponds to the regime in which the leaching rate is independent of the rate of mixing a suspension of the solid phase in the microemulsion. During the leaching process, the microemulsion was stable and transparent.
To remove the suspended particles of the solid phase, samples of the microemulsion were centrifuged for 10 min at 5,000 g. Metals were re-extracted from the microemulsion by mixing for 1 min with a threefold volume of 10 % sulfuric acid. For the re-extraction process and phase separation, the samples were kept for at least 1 day at room temperature. The copper content was then determined by the photometric method by coloring with cuprizone using a KFK-2 photoelectric colorimeter (Russia) at 590 nm in cuvettes with a thickness of 1 cm.
Results and Discussion
Region of Existence
The region of existence of NaDEHP microemulsion in kerosene was determined in the presence of various amounts of extractant DEHPA. The effect of “extra” DEHPA (i.e., introduced over the stoichiometric ratio DEHPA:NaOH = 1:1) on the region of existence was investigated at DEHPA concentration in the organic phase 0.0–0.5 mol/L. DEHPA in this system plays a dual role: it is used as a reactant for surfactant NaDEHP synthesis, and the “additional” DEHPA can act as a co-surfactant and as an extractant for metal recovery. The results obtained are shown in Fig. 2 as a dependence of maximum water content in the microemulsion \(W_{\text{cr}} = C_{{H_{2} O}} /C_{\text{NaDEHP}}\) on NaDEHP concentration at different “additional” DEHPA concentrations.
The presented data (Fig. 2) show that the shape of the microemulsion region is similar for the systems with DEHPA concentrations 0.0–0.4 mol/L: the maximum value of W cr is observed at NaDEHP concentrations 1.6 mol/L. A similar shape of the microemulsion region with maximum value of W cr at medium NaDEHP concentration is shown for the systems NaDEHP–heptane–water [11] and NaDEHP–decane–water [17].
DEHPA introduction into NaDEHP microemulsion changes the boundary of the region of existence of the microemulsion at 20 °C; the change depends on the DEHPA concentration. Introduction of DEHPA in the organic phase at concentration 0.1 mol/L extends the region of the microemulsion. When DEHPA concentration increases from C DEHPA = 0.1 to 0.5 mol/l, W cr decreases and the narrowing of the microemulsion region is observed (curves 3–6 in Fig. 2). A similar result was shown for NaDEHP microemulsion in heptane: water solubilization capacity of the microemulsion increased from W cr = 20 to W cr = 140 when 10 mol% of NaDEHP was changed into DEHPA, and then decreased to W cr = 20 with DEHPA proportion in the mixture NaDEHP+DEHPA growing to 40 mol% [20].
Electrical Conductivity
Co-surfactant introduction in microemulsion can change the region of existence of microemulsion and other properties: electrical conductivity, droplet size and microemulsion structure. The effect of DEHPA on such properties of NaDEHP microemulsion has not investigated in detail.
Electrical conductivity of samples with NaDEHP concentration in the organic phase equal to 1.6 mol/L was studied at 20 °C in the range of water content from \(W = C_{{H_{2} O}} /C_{\text{NaDEHP}}\) = 4.0 to the boundary of microemulsion existence. The results of the conductivity measurements for the samples with different amounts of DEHPA are shown in Fig. 3 as conductivity logarithm dependencies on volume fraction of water (Φ).
DEHPA introduction at a concentration of 0.1 mol/L practically does not affect on the electrical conductivity of NaDEHP microemulsion. DEHPA concentration growth in the organic phase of the microemulsions from 0.1 to 0.4 mol/L leads to a decrease of the conductivity both at low Φ values and at relatively high water content.
With the growth of Φ value, a sharp increase in the conductivity is observed in the range of Φ < 0.18 (W < 8), while at Φ > 0.18 (W > 8) a slight increase (Fig 3.) is observed, regardless of the DEHPA concentration in the microemulsion. This is typical for the phenomenon of volume-induced percolation, and the percolation threshold is approximately equal to 0.18 (W = 8). For example, the similar conductivity dependence on volume fraction of water in the microemulsion systems of AOT-linear aliphatic hydrocarbon–water has been shown. A percolation threshold in these systems is in the range of Φ values of 0.16 to 0.22 at 50 °C, and decreases with the growth of the hydrocarbon chain length from n-hexane to n-decane [21]. An increase of conductivity by several orders at 4 < W < 7 and a slight conductivity change at W > 7 is demonstrated for the system NaDEHP–n-heptane–water at 20 °C [16]. In the investigated system of NaDEHP–DEHPA–kerosene–water, the threshold of volume-induced percolation is observed at a water volume fraction of Φ = 0.18 (W = 8), which is comparable with the above values.
DEHPA in the concentration range of 0.0 to 0.4 mol/L (in the organic phase) does not affect on the threshold of volume-induced percolation of NaDEHP microemulsion. This differs from the action of acidic additives in AOT-based microemulsion. Partial replacement of AOT by DEHPA in AOT–heptane–water microemulsion reduces the temperature of temperature-induced percolation [20]. Introduction of n-alkyl acids, especially medium and long chain acids (C 4–C23), into AOT-based microemulsion in isooctane leads to an increase in the temperature of percolation [22].
Percolation phenomena and structural transitions in microemulsions are frequently accompanied by extreme changes of viscosity [16, 23–25]. For example, for the system NaDEHP–n-heptane–water, two maxima were observed on the dependence of viscosity versus water concentration at W = 4 (interpreted as transition from reverse to bicontinuous microemulsion) and at W = 100 (transition from bicontinuous to O/W microemulsion) [16]. Viscosity maxima of the microemulsion phase accompanied the transitions from Winsor I to Winsor III and from Winsor III to Winsor II systems [23]. In the system AOT–decane–aqueous 0.5 % NaCl solution, two viscosity maxima were observed at oil volume fraction approximately equal to 20 and 80 %, which corresponded to structural transitions from O/W microemulsion to a bicontinuous one and from bicontinuous to W/O microemulsions [25].
The viscosity of DEHPA-containing NaDEHP microemulsion with different volume fractions of water was investigated to confirm the percolation threshold. Microemulsions in the investigated system are non-Newtonian fluids; their viscosity decreases with the shear rate growth. Dynamic viscosity at shear rates 27–1,312 s−1 was measured, the data at low (27 s−1), medium (81 s−1) and high (243 s−1) shear rate are presented in Fig. 4. Maximum viscosity is observed at all shear rates at Φ = 0.19; this is at approximately the same Φ values as the threshold of conductivity percolation.
A sharp change in the properties of the microemulsion—for example, in electrical conductivity and viscosity—is related to changes in its structure. To better understand the nature of the structural transition in the system NaDEHP–DEHPA–kerosene–water at Φ = 0.18, the dependence of the conductivity from Φ value at different temperatures was studied. The investigation was carried out in the range of water volume fraction from Φ = 0.10 to the boundary of microemulsion existence. The results are shown in Fig. 5.
The region of existence of the microemulsion expands with the temperature growth. For example, W cr = 140 is reached at T = 80 °C, which corresponds to water volume fraction Φ = 0.80 (Fig. 5). When the temperature grows, the interaction of ionic surfactants with water increases, spontaneous curvature of the surfactant layer changes from negative to zero and positive; this leads to the formation of bicontinuous and direct (O/W) microemulsion [1, 7]. Therefore, the formation of bicontinuous and direct microemulsion in the system NaDEHP–DEHPA–kerosene–water at high volume fraction of water and high temperature is possible.
The regions of the microemulsions with different structure can be established based on the changes in the conductivity curves. Bending of the conductivity curves is observed at Φ = 0.18 at all temperatures studied, which is especially evident on the logarithm curve, and maximum of conductivity appears at Φ = 0.70 (at T = 80 °C). We can determine three areas on the conductivity curves: I—at Φ < 0.18, II—at 0.18 < Φ < 0.70, III—at Φ > 0.70. These areas may correspond to the microemulsions with different structure. The same conductivity profile was demonstrated for the microemulsions in the system Tween 80–ethanol–oleic acid–water; a decrease of conductivity at high water content was interpreted as structural transition from bicontinuous to direct microemulsion [26]. We can assume the existence of the reverse (W/O) microemulsion with isolated droplets at low concentrations of water (Φ < 0.18) in the system. The region of the conductivity decrease at high water content (Φ > 0.70) is characterized as a region of direct (O/W) microemulsion.
Thus, we can observe the gradual transition from reverse microemulsion to a direct one in the system NaDEHP–DEHPA–kerosene–water at T = 80 °C. The structure of the microemulsion changes with water concentration as follows: reverse microemulsion with isolated droplets in the region I, reverse percolate microemulsion (dynamic cluster of water droplets) and bicontinuous microemulsion (continuous surfactant film separating the water and oil domains) in the region II, and direct microemulsion (region III). For example, the gradual transitions from O/W to bicontinuous and W/O microemulsion with the growth of oil volume fraction from 0.1 to 0.9 were observed directly by freeze fracture electron microscopy [27].
The same change of the microemulsion structure is proposed at temperatures 60, 40 and 20 °C: reverse microemulsion with isolated droplets and reverse percolate microemulsion, which gradually transforms into bicontinuous microemulsion. The system does not reach a region of direct microemulsion at these temperatures.
Droplet Size
The hydrodynamic diameter of droplets of NaDEHP microemulsion with different water content was studied at 20 °C by dynamic light scattering method. Water concentration in the samples was higher than the threshold of volume-induced percolation. The dependence of hydrodynamic diameter of droplets from W for NaDEHP microemulsions with different DEHPA concentration is shown in Table1. Linear dependence is observed for the microemulsions with DEHPA and without co-surfactant.
The linear dependence of the hydrodynamic diameter of droplets on W is known for the systems with AOT, which is a close analogue of NaDEHP. For example, the expression for hydrodynamic diameter of AOT reverse micelles is shown [1]:
The expression for water core diameter of AOT reverse micelles is known [5]:
For AOT reverse micelles in toluene, the dependence d(W) obtained by dynamic light scattering method [28] can be described as:
The coefficient at W in Eq. 1 is lower than in the Eqs. 5–7. So, the effect of water content on droplet size for NaDEHP microemulsion is more weakly expressed than for the systems with AOT.
The growth of DEHPA concentration in the microemulsion leads to the increase of hydrodynamic diameter of the droplets and changes the slope of the lines d = kW + b (Table 1). The increase of DEHPA concentration in the organic phase of the microemulsion from 0.1 to 0.3 mol/L causes the increase of the coefficient at W from 0.038 to 0.249. The DEHPA addition into the microemulsion at a concentration less than 0.1 mol/L (in organic phase) results in the decrease of the coefficient at W from 0.099 to 0.038.
Thus, we observe the narrowing of the microemulsion region at DEHPA concentration growth from 0.1 to 0.5 mol/L (Fig. 2), a decrease in conductivity (Fig. 3), growth of the droplet size, and an increase of the coefficient at W (Table 1) in the DEHPA concentration range 0.1–0.3 mol/L. Analogous results are obtained for AOT microemulsions. The effect of substitution of Na+ by H+ as the counterion is studied in AOT-based W/O microemulsions. It has been shown that Na+ counterions screen electrostatic repulsions between polar headgroups of the surfactant molecules in droplets of AOT W/O microemulsion. Partial replacement of Na-AOT by H-AOT leads to the growth of the droplet size from 8 to 110 nm in the region of H-AOT mole fraction in the surfactant mixture 0 ≤ XH-AOT ≤ 0.52. Phase separation takes place with the further increase in the proportion of H-AOT in the surfactant mixture. When H-AOT mole fraction grows, screening of the electrostatic repulsion between sulfonate groups by the counterion weakens, the distance between the polar headgroups increases, and the droplet size rises. Further increase in the proportion of H-AOT leads to destabilization of the interface of the microemulsion droplets [29]. The increase of electrostatic repulsion between the polar phosphate groups with DEHPA introduction can also take place in the instigated NaDEHP microemulsion. But taking only the decrease of screening of the electrostatic repulsion of phosphate groups into account does not explain the decrease in the conductivity with DEHPA concentration growth at constant C NaDEHP, both before and after the percolation threshold.
DEHPA is a weak acid (pKa = 1.4); it is well soluble in hydrocarbons and slightly soluble in water. In apolar solvents, DEHPA forms dimers (DEHPA)2 due to the hydrogen bonds of the phosphate groups. DEHPA introduction enhances the solubility of bis-(2-ethylhexyl)phosphates in apolar solvents due to the formation of complexes M(DEHP·DEHPA)n [30, 31]. DEHPA addition may cause an increase of NaDEHP solubility in the organic phase of the microemulsion. This results in a decrease in the number of NaDEHP molecules at the interface.
At the presence of relatively high concentrations of DEHPA, the solubility of NaDEHP in the organic solvent increases, and DEHPA acts as a co-solvent. It leads to the distribution of NaDEHP molecules from the oil–water interface into the bulk organic phase. The number of charge carriers in water phase reduces, and it decreases the conductivity. Water core diameter of the droplets increases with the reduction of the number of surfactant molecules at the interface. The narrowing of the microemulsion region at DEHPA concentration growth can also be explained by the transition of NaDEHP molecules from the interface into the bulk organic phase. Thus, DEHPA effect on the properties of NaDEHP microemulsion may be caused not only by the reduction of the screening of electrostatic repulsion of the phosphate groups, but also by the distribution of NaDEHP molecules from the oil–water interface into the bulk organic phase.
On the other hand, the expansion of the microemulsion region and the decrease of the coefficient at W at DEHPA concentration 0.1 mol/L are due to the formation of a mixed monolayer of DEHPA and NaDEHP molecules at the interface. In this case, at relatively low concentrations, DEHPA acts as a co-surfactant.
So, the effect of DEHPA on NaDEHP microemulsion has a dual nature and depends on the concentration of the extractant. At low values of DEHPA concentration, the effect of the extractant as a co-surfactant predominates. DEHPA molecules are localized at the interface and take part in the stabilization of the microemulsion droplets. With the growth of DEHPA concentration, the destabilizing effect of DEHPA on the microemulsion becomes predominant. DEHPA molecules can decrease the screening effect of Na+ counterions on the electrostatic repulsions of polar headgroups of the surfactant, and DEHPA can reduce the number of NaDEHP molecules at the interface due to the increase of NaDEHP solubility in the bulk organic phase of the microemulsion.
Copper Recovery
Extractant concentration in the microemulsion is an important parameter that affects metal recovery. The effect of “additional” DEHPA (i.e., introduced over the stoichiometric ratio DEHPA:NaOH = 1:1) on copper recovery from CuO particles was investigated. CuO is used as a model system of copper oxide ores and secondary raw materials. Microemulsion with the following composition was used for the leaching process: C NaDEHP = 1.6 mol/L (in the organic phase), W = 25. DEHPA concentration in the organic phase of the microemulsion was varied from 0 to 0.3 mol/L. These microemulsions are identical in structure (percolate microemulsion), but differ in the size of droplets.
It was previously shown that the rate of copper leaching from a sample of cobalt–copper oxide ore into DEHPA containing microemulsion increases in the temperature range 50–80 °C; the effective activation energy of the leaching process was 38 kJ/mol. The obtained value of the activation energy corresponds to the process in which the total rate is influenced by the chemical reaction and the diffusion processes [32]. A temperature of 80 °C was chosen to provide the highest possible speed of the process and stability of the microemulsion. The reaction proceeds according to the equation:
The results of the DEHPA effect on copper recovery into NaDEHP microemulsion are presented in Fig. 6. In the absence of the extractant, the leaching rate is very low (curve 1 in Fig. 6). The rate of the microemulsion leaching (in the initial sections of the curves) increases considerably with the rise of DEHPA concentration (curves 2–4 in Fig. 6). We observe only the positive effect of DEHPA on the total rate of copper recovery into the microemulsion. The quantity of the metal recovered in the microemulsion also increases with the growth of the extractant concentration. So, the higher the concentration of DEHPA in NaDEHP microemulsion, the better the microemulsion leaching will be. The limitation is the region of existence of the microemulsion, which narrows with increasing of DEHPA concentration in the organic phase at C DEHPA > 0.1 mol/L.
Microemulsion for the leaching should have a broad region of existence and a high solubilization capacity of water for the processing of wet raw materials, for example, galvanic sludge. DEHPA concentration in the microemulsion is required to be as high as possible to provide the desired rate of the process and recovery of metal. The maximum value of W cr is observed at NaDEHP concentrations of 1.6 mol/L and at a DEHPA concentration of 0.1 mol/L. At a DEHPA concentration of 0.3 mol/L, W cr values are slightly lower, but the leaching rate and the metal recovery are significantly higher than at C DEHPA = 0.1 mol/L. At C DEHPA > 0.3 mol/L, essential narrowing of the microemulsion region is observed. Based on these results, we can recommend microemulsion in the system NaDEHP–DEHPA–kerosene–water with C NaDEHP = 1.6 mol/L, C DEHPA = 0.3 mol/L (in the organic phase) for the leaching of non-ferrous metals. The rate of diffusion in the reverse microemulsion with percolated structure is higher than in the reverse microemulsion with isolated droplets, so water content in the microemulsion is recommended to be in the range of W values from 8 (the percolation threshold) to 32 (the boundary of microemulsion existence at 20 °C).
The results obtained in this study are considered as a basis for the elaboration of the process of non-ferrous metal leaching from complex ores and secondary raw materials by means of DEHPA-containing NaDEHP microemulsion.
Conclusion
The effect of DEHPA on region of existence, electrical conductivity and droplet size of NaDEHP microemulsions is depends on the DEHPA concentration.
The narrowing of the microemulsion region is observed with the growth of DEHPA concentration in the organic phase from 0.1 to 0.5 mol/L. Based on the change of conductivity and viscosity of the microemulsions, we suppose the effect of volume-induced percolation of conductivity at volume fraction of water Φ = 0.18 (W = 8); the transition from reverse microemulsion with isolated droplets to reverse percolate microemulsion takes place. The increase of DEHPA concentration in the organic phase from 0.1 to 0.4 mol/L leads to the reduction of electrical conductivity of the microemulsions, and does not affect the percolation threshold. Hydrodynamic diameter of droplets of microemulsion in the system DEHPNa–DEHPA–kerosene–water increases linearly with \(W = C_{{H_{2} O}} /C_{\text{NaDEHP}}\) growth. The rise of DEHPA concentration in the organic phase from 0.1 to 0.3 mol/l leads to the increase of the hydrodynamic diameter of the droplets, and causes the growth of the coefficient at W from 0.038 to 0.249, i.e., it increases the slope of the lines.
The opposite effect takes place in a DEHPA concentration range of 0.0 to 0.1 mol/L (in the organic phase). In this case, the presence of DEHPA leads to the expansion the region of existence of the microemulsion, does not affect the conductivity and decreases the slope of the lines d(W).
The dual effect of DEHPA on NaDEHP microemulsion is explained by a combination of two actions. At low values of DEHPA concentration, the effect of co-surfactant predominates. DEHPA molecules are localized at the interface and take part in the stabilization of the microemulsion droplets. With increasing of DEHPA concentration, the destabilizing effect becomes predominant. DEHPA molecules can decrease the screening effect of Na+ counterions on the electrostatic repulsions of polar headgroups of the surfactant, and DEHPA can increase the solubility of NaDEHP in the organic phase of the microemulsion and reduce the number of NaDEHP molecules at the interface.
The rate of copper leaching into the microemulsion increases considerably with the rise of DEHPA concentration. The quantity of metal recovered in the microemulsion also increases with the extractant concentration growth. No dual effect of DEHPA on copper recovery is observed. We can recommend the following composition of the microemulsion for non-ferrous metals leaching: C NaDEHP = 1.6 mol/L, C DEHPA = 0.3 mol/L (in the organic phase); W = 8–32.
References
Kumar P, Mittal KL (eds) (1999) Handbook of microemulsion science and technology. Marcel Dekker, New York
McEvoy E, Donegan S, Power J et al (2007) Recent advances in the development and application of microemulsion EKC. Electrophoresis 28:193–207
López-Quintela MA, Tojo C, Blanco MC et al (2004) Microemulsion dynamics and reactions in microemulsions. Curr Opin Colloid Interface Sci 9:264–278
Xu X-J, Gan LM (2005) Recent advances in the synthesis of nanoparticles of polymer latexes with high polymer-to-surfactant ratios by microemulsion polymerization. Curr Opin Colloid Interface Sci 10:239–244
Pileni MP (1997) Nanosized particles made in colloidal assemblies. Langmuir 13:3266–3276
Capek I (2004) Preparation of metal nanoparticles in water-in-oil (w/o) microemulsions. Adv Colloid Interface Sci 110:49–74
Salager JL, Forgiarini AM, Bullon J (2013) How to attain an ultralow interfacial tension and three-phase behavior with a surfactant formulation for enhanced oil recovery: a review. Part 1. optimum formulation for simple surfactant-oil-water ternary systems. J Surfact Deterg 16:449–472
Watarai H (1997) Microemulsions in separation sciences. J Chromatogr A 780:93–102
Tondre C (1999) Surfactant-based colloidal particles as the extracting phase for the removal of metal ions from aqueous environments: kinetic and applied aspects. ACS Symp Ser 740:139–157
Osseo-Asare K (1988) Enhanced solvent extraction with water-in-oil microemulsions. Sep Sci Technol 23:1269–1284
Brejza EV, Perez de Ortiz SE (2000) Phenomena affecting the equilibrium of Al(III) and Zn(II) extraction with Winsor II microemulsions. J Colloid Interface Sci 227:244–246
Zhao YY, Tao Z, Chuan-Bo X, Xue-Mei X, Ling L, Zhan-Yu L (2008) Study on the extraction of cobalt and nickel from NH4SCN solution by Winsor II microemulsion system. Sep Purif Technol 60:174–179
He D, Yang C, Ma M, Zhuang L, Chen X, Chen S (2004) Studies of the chemical properties of tri-n-octylamine–secondary octanol–kerosene–HCl–H2O microemulsions and its extraction characteristics for cadmium (II). Colloids Surf A 232:39–47
Yurtov EV, Murashova NM (2011) Leaching the metals with extractant containing microemulsions. Theor Found Chem Eng 45:726–730
Faure A, Tistchenko AM, Zemb T, Chachaty C (1985) Aggregation and dynamical behavior in sodium diethyl phosphate/ware/benzene inverted micelles. J Phys Chem 89:3373–3378
Yu Z-J, Neuman RD (1995) Reversed micellar solution-to-bicontinuous microemulsion transition in sodium bis(2-ethylhexyl)phosphate/n-heptane/water system. Langmuir 11:1081–1086
Kurumada K, Nagamine Sh, Tanigaki M (1999) Structure and properties of bis(2-ethylhexyl)phosphoric acid microemulsions with a network structure. Effect of counter ions. Colloids Surf A 148:305–311
Yurtov EV, Murashova NM (2004) Phase equilibria and nonequilibrium structures in the sodium di-2-ethylhexyl phosphate-decane-water system. Colloid J 66:629–634
Gaonkar AG, Neuman RD (1987) Interfacial activity, extractant selectivity and reversed micellization in hydrometallurgical liquid/liquid extraction systems. J Colloid Interface Sci 119:251–261
Li Q, Li T, Wu J (2002) Water solubilization capacity and conductance behaviors of AOT and NaDEHP systems in the presence of additives. Colloids Surf A 197:101–109
Chakraborty I, Moulik SP (2005) Physicochemical studies on microemulsions 9. Conductance percolation of AOT-derived W/O microemulsion with aliphatic and aromatic hydrocarbon oils. J Colloid Interface Sci 289:530–541
Cid-Samamed A, Garcia-Rio L, Fernandez-Gandara D, Mejuto JC, Morales J, Pérez-Lorenzo M (2008) Influence of n-alkyl acids on the percolative phenomena in AOT-based microemulsions. J Colloid Interface Sci 318:525–529
Gradzielski M, Hoffmann H (1999) Rheological Properties of Microernulsions. In: Kumar P, Mittal KL (eds) Handbook of microemulsion science and technology. Marcel Dekker, New York, pp 357–386
Kljajic A, Bester-Rogac M, Trost S, Zupet R, Pejovnik S (2011) Characterization of water/sodium bis(2-ethylhexyl) sulfosuccinate/sodium bis(amyl) sulfosuccinate/n-heptane mixed reverse micelles and w/o microemulsion systems: the influence of water and sodium bis(amyl) sulfosuccinate content. Colloids Surf A 385:249–255
Borkovec M, Eicke H-F, Hammerich H, Das Gupta B (1988) Two percolation processes in microemulsions. J Phys Chem 92:206–211
Mehta SK, Kaur K, Kaur G, Bhasin KK (2009) Percolating phenomenon in microemulsions: effect of external entity. In: Fanun M (ed) Microemulsions: properties and applications. CRC Press, USA, pp 59–76
Burauer S, Belkoura L, Stubenrauch C, Strey R (2003) Bicontinuous microemulsions revisited: a new approach to freze fracture electron microscopy (FFEM). Colloids Surf A 228:159–170
Eicke H-F (1986) Aqueous nanophases in liquid hydrocarbons stabilized by ionic surfactants. In: Parfitt D, Eicke H-F (eds) Interfacial phenomena in apolar media. Marcel Dekker Inc, Basel, pp 41–89
Takashina S, Yoshida M, Gotoh K, Oshitani J (2008) Phase behavior and size variation of AOT-based W/O microemulsions by substituting H+ for Na+ as the counterion. Colloids Surf A 325:52–56
Hanson C (ed) (1971) Recent advances in liquid–liquid extraction. Pergamon Press, New York
Rydberg J, Cox M, Musikas C, Choppin GR (eds) (2004) Solvent extraction principles and practice. Marcel Dekker, New York
Yurtov EV, Murashova NM. (2008) Microemulsion leaching of metals. In: Moyer, B.A. (ed.) Solvent extraction: fundamentals to industrial applications, proceedings of ISEC 2008 international solvent extraction conference, the Canadian Institute of Mining, Metallurgy and Petroleum, Montreal, pp.1597–1602
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Murashova, N.M., Levchishin, S.Y. & Yurtov, E.V. Effect of Bis-(2-ethylhexyl)Phosphoric Acid on Sodium Bis-(2-ethylhexyl)Phosphate Microemulsion for Selective Extraction of Non-Ferrous Metals. J Surfact Deterg 17, 1249–1258 (2014). https://doi.org/10.1007/s11743-014-1598-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11743-014-1598-x