Abstract
To improve the hydrolysis resistant ability of trisiloxane surfactants, ethoxylated single-tail and double-tail trisiloxane surfactants of the general formulas Me3SiOSiMeR1OSiMe3 (R 1 = (CH2)3NHCH2CH(OH)CH2(OCH2CH2) x OCH3; x = 8.4, 12.9, 17.5, 22) and Me3SiOSiMeR2OSiMe3 (R 2 = (CH2)3NR3CH2CH(OH)CH2(OCH2CH2) x OCH3; R 3 = CH2(CH2) y CH3; x = 8.4, 12.9, 17.5, 22; y = 2, 6) were synthesized. Their structures were characterized by 1H NMR and 13C NMR. The surface activity and hydrolysis resistant properties of the trisiloxane surfactants prepared were also studied. The values of the critical micelle concentration of all trisiloxane surfactants prepared were at levels of 10−5 and 10−4 mol/L. They can reduce the surface tension of water to less than 24 mN/m. The hydrolysis resistant properties of double-tail trisiloxane surfactants are superior to those of single-tail trisiloxane surfactants. The double-tail trisiloxane surfactants 1B (x = 8.4; y = 2) and 2C (x = 12.9; y = 6) can be stable for 8 days in an acidic solution (pH 4.0) and 11 days in an alkaline environment (pH 10.0).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Trisiloxane surfactants are a relatively new agricultural adjuvant whose development began in the middle of the twentieth century. Due to their having many merits such as remarkably reducing the surface tension of water to 21 mN/m, having a good wetting power, stronger adhesion, an excellent spreading performance, a higher pore penetration coefficient, and a good anti-rain-washed performance, in recent decades trisiloxane surfactants have become of considerable interest to researchers in various countries [1–6]. General Electric Company’s Silwet® Super Spreaders (GESSP) of the general formula Me3SiOSi(Me)(R)OSiMe3 (R = (CH2)3O(CH2CH3O)8CH3), (CH2)3O(CH2CH3O)8H) are a trisiloxane agricultural adjuvant available on the market. They are all single-tail and branched-chain trisiloxane surfactants. Because of their branched tail, a specially flexible bond of Si–O–Si and an intensive methyl arrangement at the air/water interface, the GESSP have the unique ability to reduce the surface tension of water to approximate 21 mN/m and their aqueous solution can rapidly spread on low energy surfaces, and this is called superspreading or superwetting [4]. However, like other types of trisiloxane surfactants, they are very sensitive to environmental pH values. They hydrolyze rapidly when placed in an environment with pH values below 5 or above 9, and can only remain stable for 40 days even in an environment with a pH value of 7.0 [5, 6], this limits their application scope.
Recently hydrolysis-resistant disiloxane surfactants [5] of the general formula R 1SiMe2OSiQMe2 (R 1 = CH2CH(CH3)2, CH2CH2CH3, C(CH3)3, CH2CH2Si(CH3)3, etc.; Q = CH2CH2O(CH2CH2O) a (CH2CH(CH3)O) b R 2; R 2 = H or CH3) have been synthesized by Leatherman et al. They have also prepared hydrolysis resistant trisiloxane surfactants [6] with the general formula R 1Si(CH3)2OSi(CH3)QOSi(CH3)2 R 1 (R 1 = CH3, C(CH3)3, CH2(CH3)2; Q = CH2CH(R 2)CH2O(CH2CH2O) r (CH2CH2O) s R 3; R 2, R 3 = H, CH3). Compared with other siloxane surfactants, the hydrolysis-resistant ability of these two types of siloxane surfactant has been greatly improved; some of them can be stable for 3 months in an environment with pH values below 5 or above 10. This is due to a larger volume of the alkyl group (relative to methyl in the GESSP) bond to the Si atom in hydrophobic chain of the surfactant, or the larger volume of the methyl (relative to hydrogen atom in the GESSP) bond to the connection site between the hydrophilic group and the central Si atom in the Si–O chain, which increases the steric hindrance to attack on the Si atom for water molecules and inhibits the hydrolysis for these two types of trisiloxane surfactants. Regrettably, the structure of the siloxane needed to synthesize the above two types of hydrolysis-resistant siloxane surfactants is very special and is not readily available on the market, hence the industrialization of these two types of hydrolysis resistant siloxane surfactants is not easy.
Sodium bis(2-ethylhexyl)sulfosuccinate having a double-tail structure is now commonly used as a wetting agent, penetrant and emulsifier. Recently some traditional double-tail surfactants have been studied. The experimental results show that the area per molecule of double-tail surfactants is larger than that of corresponding single-tail surfactants [7, 8], and that their abilities to reduce the surface tension of water, to wet [9] and to spread [10] on low energy surfaces are superior to those of the corresponding single-tail surfactants.
If a large volume hydrophobic group is attached to the connection site between the hydrophilic group and the central Si atom in the Si–O chain in a traditional trisiloxane surfactant molecule, how does it alter the properties? Can these species maintain the high surface activity of traditional trisiloxane surfactants, and overcome their poor hydrolysis resistance? In this paper we report on the synthesis methods of double-tail trisiloxane surfactants using raw materials readily available on the market such as 3-aminopropylmethyldimethoxysilane and polyethylene glycol monomethyl ether, and so on. The interfacial properties and the hydrolysis-resistant performance of all trisiloxane surfactants prepared are also reported.
Experimental Procedures
Materials
3-Aminopropylmethyldimethoxysilane was obtained from the Nanjing Yudeheng Coupling Agent Plant, China. Hexamethyl disiloxane was from the Bengbu Hengyi Silicon Products Factory, Anhui, China. Polyethylene glycol monomethyl ether 400 (average degree of polymerization \( \left( {\overline{\text{DP}} } \right) \) = 8.4) was purchased from the Shanghai Haojiong Assistant Co., Ltd., China. Polyethylene glycol monomethyl ether 600 \( \left( {\overline{\text{DP}} = 12.9} \right) \) and Polyethylene glycol monomethyl ether 800 \( \left( {\overline{\text{DP}} = 17.5} \right) \) came from the Zhejiang Huangma Chemical Industry Group Co., Ltd., China. Polyethylene glycol monomethyl ether 1000 \( \left( {\overline{\text{DP}} = 22} \right) \) was obtained from the Shanghai Jinshan Chemical Co., Ltd., China. All the above chemicals were used as received. Paraffin wax was from the Shanghai Specimen and Model Factory, China. All other chemicals were of analytical grade. The water used was doubly distilled.
Synthesis
The synthesis route to double-tail trisiloxane surfactants is shown in Scheme 1. The specific method is as follows.
Procedure (a), (b) and (c) were carried out according to references [11], [12] and [13]. What was different was that the used solvent was toluene, rather than methanol in procedure (c). Products from the above procedure were characterized by FTIR, 1H NMR, 13C NMR and ESI-MS. Procedure (d) was implemented according to the reference [14] using toluene as the solvent. Its products were confirmed by FTIR.
Procedure (e): with a molar ratio of 1:1:2 and in the presence of toluene as solvent, the product of procedure (c) (ethoxylated trisiloxane), bromo-alkane (1-bromo-n-butane or 1-bromo-n-octane) and anhydrous sodium carbonate were placed under nitrogen in a three-necked flask equipped with a refluxing condenser and a magnetic stirrer. The mixture was heated to 80–110 °C for about 4–10 h. A yellowish-brown viscous liquid product was obtained after removing the inorganic salt and toluene.
Structural Characterization
Infrared spectroscopy was performed using a Bruker Tensor 27 FTIR spectrometer. The surfactants were directly smeared on a KBr plate. Mass spectroscopy was carried out on a Shimadzu LCMS-2010A liquid chromatography mass spectrometer (LC–MS) using electrospray ionization (ESI) in the positive-ion mode. The mobile phase was methanol. Samples were directly introduced into the ESI–MS after having been dissolved in methanol. Proton nuclear magnetic resonance (1H NMR) spectroscopy, carbon nuclear magnetic resonance (13C NMR) spectroscopy were carried out with a Varian Mercury-plus 300 spectrometer in CDCl3.
Surface Tension (γ) and the Critical Micelle Concentration Determination
Properties were investigated under constant atmospheric conditions [32 ± 2 °C room temperature, 60 ± 3% relative humidity (RH)]. Aqueous solution surface tension values were measured using the Wilhelmy plate method using a BZY-1 completely automatic surface tensiometer (Shanghai Equity Instruments Factory, China). The critical micelle concentration (CMC) value was assessed at the intersection of the linear portions of the plot of the surface tension against the logarithm of the surfactant concentration. The surface tension at this intersection point is called the surface tension at CMC (γ cmc). All the surfactant solutions used to determinate the CMC value were tested within 1 h after having been prepared. Surfactant solutions were prepared with doubly distilled water. Prior to the measurements on surfactant solutions, the surface tension of the doubly distilled water was found to be 70.0 ± 0.3 mN/m.
Spreading Ability Determination
The spreading ability (SA) of surfactant solutions was evaluated by the following procedure. Using a syringe, exactly 17 μL of 0.1 wt.% surfactant solution prepared with a pH 7.0 buffer solution was deposited on paraffin wax. After 3 min, the average diameter of the drop was measured by means of a vernier caliper. Tests were run in triplicate. SA was calculated by the equation SA = (D/D 0)2, where D is diameter of drop of test solution drop (or emulsion) after 3 min, and D 0 = diameter of drop of distilled water applied to the surface in the same manner.
Hydrolysis Resistant Ability Determination
The hydrolysis resistant ability (HRA) of surfactants was measured by preparing 0.1 wt.% (pH 4.0, 7.0 and 10.0) surfactants solutions with buffer solutions and measuring their surface tension values within 10 min after their preparation. The first measurement time was used as starting time (time = 0) and then the surface tension values of surfactants solutions were measured till their values reach to 27 mN/m. The faster the surface tension value of the surfactant solution rises to reach this value, the poorer the HRA of the surfactant.
Results and Discussion
Synthesis of Surfactants
Four single-tail trisiloxane surfactants (1A, 2A, 3A and 4A) and eight double-tail trisiloxane surfactants (1B, 2B, 3B, 4B, 1C, 2C, 3C, and 4C) were prepared. Their molecular structures are shown in Scheme 1. In the synthesis procedure (c), toluene was used as the solvent in place of the methanol mentioned in the literature [13] to avoid the appearance of turbidity of the reaction liquid, which may result from the hydrolysis and condensation of trisiloxane in methanol hydrophilic. Anhydrous sodium carbonate was used in the procedure (e) to eliminate the production of hydrogen bromide, which reacts with the surfactants A to secondary amine salts and hinders the reaction between the hydrocarbon bromide and surfactants A. According to the law that the different chemical environments of H and C led to different chemical shifts, and by comparing the literatures [4, 15] and the chemical shifts of relative compounds, their 1H- and 13C-NMR spectra were analyzed. Assignments of the chemical shifts in 1H- and 13C-NMR spectra of 1A, 1B and 1C are listed in Tables 1 and 2.
Interfacial Properties
The equilibrium surface tensions of dilute aqueous solutions of all of the surfactants prepared were measured. The CMCs of the surfactants were estimated at the breaking point of the plot. The surface excess concentration (Γmax) and the surface area per molecule (a sm ) were calculated by applying Gibbs’ equations (Eqs. 1 and 2) in the steeply downward section just below CMC. The standard free energy of micellization (Δmin G 0) of trisiloxane surfactants was calculated by Eq. 3.
where R = 8.3144 J/mol K, N A is Avogadro’s number, Γmax and a sm are in mol/cm2 and Å/molecule, respectively. All of the data of CMC, γ cmc, SA, a sm and ΔG 0mic are listed in Table 3.
The CMCs of all trisiloxane surfactants prepared are in the 10−5–10−4 mol/L range, and their γ cmc values in the 18.6–23.6 mN/m range as seen in Table 3, i.e. thus exhibiting a high efficiency. In general, the CMC and γ cmc values of polyoxyethylene surfactants increase with an increase of the number of oxyethylene units, because of the enhancement of hydrophilicity of the surfactant with the enlargement of its hydrophilic group [16]. Basically, the variation trend of CMC and γ cmc values of the same type of surfactants, such as 1A, 2A, 3A and 4A, is in agreement with the above-mentioned trend as seen in Table 3. The variation in the CMC values of the different types of trisiloxane surfactants having the same number of oxyethylene units and different hydrophobic groups, can also be elucidated by the variation of molecular hydrophilicity. Comparison with the corresponding types of A and B trisiloxane surfactants, the decreases of CMC values of the type of C double-tail trisiloxane surfactants (1C, 2C, 3C and 4C) results from a larger volume of the n-octyl group in the type C surfactant molecules and the weakening of hydrophilicity of C surfactants. A smaller volume of the n-butyl group in B double-tail trisiloxane surfactants molecules causes little change to the totally molecular hydrophilicity. Hence the corresponding CMC values of A and B surfactants do not differ in any significant way. The values of γ cmc of double-tail trisiloxane surfactants are generally higher than those of the corresponding single-tail trisiloxane surfactants as seen in Table 3. This is attributed to the preponderance of highly surface active methyl substituents in single-tail trisiloxane surfactants, and these are closely packed on the surface of the water, whereas this closely packed frames are destroyed by the increase in higher surface energy methylene groups in double-tail trisiloxane surfactants, thus resulting in an increase in the γ cmc values.
In general, the value of the surface area per molecule (a sm ) appears to be determined by the area occupied by the hydrated hydrophilic groups, rather than by the hydrophobic groups [17]. As can be seen from Table 3, except for the a sm values of 1C and 4C, all other a sm values of the double-tail trisiloxane surfactants are larger than those of single-tail trisiloxane surfactants. It indicates that the a sm values of double-tail trisiloxane surfactants are not only determined by the area occupied by the hydrated hydrophilic groups, but also depend on the volume of the hydrophilic groups and their configuration at the water surface.
The spreading ability (SA) of double-tail trisiloxane surfactants solutions on low energy surfaces is poorer than that of single-tail trisiloxane surfactant solutions. It may be related to the higher γ cmc values and to the poor flexibility of hydrophobic groups in double-tail trisiloxane surfactants.
Hydrolysis Resistant Ability
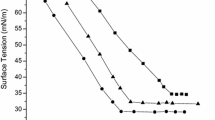
The hydrolysis-resistant performances of all trisiloxane surfactants prepared are shown in Figs. 1, 2, 3. The different variations in surface tension values of different surfactants solutions with the same pH value as time elapses, suggest some differences in hydrolysis resistance. The less the surface tension varies, the stronger is the hydrolysis resistant ability of the surfactant. No matter if the environment is acid, alkaline or neutral, the hydrolysis resistant ability of type C double-tail trisiloxane surfactants is better, and type A single-tail trisiloxane surfactant worse. On the one hand, the attachment of another hydrophobic group to a single-tail trisiloxane surfactant results in a double-tail trisiloxane surfactant. Obviously the hydrophilicity of the latter is less than the former’s. The larger the attached hydrophobic group is, the weaker the hydrophilicity of the double-tail trisiloxane surfactant is, and consequently the more difficult it is for the water molecule to attack the Si atom. On the other hand, in the double-tail trisiloxane surfactant molecule another hydrophobic group surrounding the Si–O chain increases the steric hindrance for the water molecule to attack the Si atom. Figures 1, 2, 3 also show that the hydrolysis resistant ability of the same type of trisiloxane surfactants decreases with the increase in the number of oxyethylene units. This is due to the increase in hydrophilicity of the surfactant with an increase in the number of oxyethylene units.
By inspecting Figs. 1, 2, 3, the conclusion can be reached that in acidic conditions (pH 4.0), the hydrolysis resistant abilities of surfactants 1B, 1C and 2C, are better than that of other surfactants, for the surface tensions of their aqueous solutions had no obvious change within 8 days, while the values of the surface tension of aqueous solutions of 1A and 2A change significantly within 3 and 1 days, respectively. In an alkaline environment (pH 10.0), 1B and 2C are the better hydrolysis-resistant surfactants, for they can be stable for up to 11 days. Wheras, in neutral conditions (pH 7.0), the hydrolysis-resistant performances of 1C, 2C and 3C are superior to those of other trisiloxane surfactants, i.e. they can withstand hydrolysis for 60 days.
Abbreviations
- CMC:
-
Critical micelle concentration
- GESSP:
-
General Electric Company’s Silwet® Super Spreaders
- \( \overline{DP} \) :
-
Average degree of polymerization
- FTIR:
-
Fourier transform infrared
- 1H NMR:
-
Proton nuclear magnetic resonance
- 13C NMR:
-
Carbon nuclear magnetic resonance
- ESI:
-
Electrospray ionization
- ESI–MS:
-
Electrospray ionization–mass spectrometry
- HPLC–MS:
-
High performance liquid chromatography–mass spectrometry
- RH:
-
Relative humidity
- SA:
-
Spreading ability
- HRA:
-
Hydrolysis resistant ability
- γ cmc :
-
The surface tension of surfactant solution at the CMC
References
Wagner R, Wu Y, Czichocki G, Berlepsch HV, Weiland B, Rexin F, Perepelittchenko L (1999) Silicon-modified surfactants and wetting: 1. Synthesis of the single components of Silwet L 77 and their spreading performance on a low-energy solid surface. Appl Organomet Chem 13:611–620
Svitova TF, Hill RM, Radke CJ (2001) Spreading of aqueous trisiloxane surfactant solutions over liquid hydrophobic substrates. Langmuir 17:335–348
Zhang Y, Zhang GY, Han F (2006) The spreading and superspreading behavior of new glucosamide-based trisiloxane surfactants on hydrophobic foliage. Colloids Surf A 276:100–106
Zhang GD, Han F, Zhang GY (2006) Synthesis and interfacial properties of a new family trisiloxanes. Acta Chimi Sin 64:1205–1208
Leatherman MD, Policello GA, Rajaraman SK (2007) Hydrolysis resistant organomodified disiloxane surfactants. US Patent 20,070,088,091
Policello GA, Leatherman MD, Peng WQ, Rajaraman SK, Xia ZJ (2007) Hydrolysis resistant organomodified trisiloxane surfactants. US Patent 20,070,184,005
Nave S, Eastoe F, Penfold J (2000) What is so special about Aerosol-OT? Part 1. Aqueous systems. Langmuir 16:8733–8740
Nave S, Paul A, Eastoe F, Pitt AR, Heenan RK (2005) What is so special about Aerosol-OT? Part 2. Phenyl-tipped surfactants. Langmuir 21:10021–10027
Zheng Y, Han D, Wang H (2007) Solution properties of double-tail surface surfactants. Petrochem Technol 36:285–288
Simončič B, Rozman V (2007) Wettability of cotton fabric by aqueous solutions of surfactants with different structures. Colloids Surf A 292:236–245
Zhang GD, Han F, Zhang GY (2005) Synthesis of (3-aminopropyl)trisiloxane. New Chem Mater 32:52–54
Guo LM, Wu SX (2005) Synthesis of oligo ethylene glycol diglycidyl ether by phase transfer catalysis. Fine Chem 22(supplement):108–111
Zhang GD, Han F, Zhang GY (2006) Synthesis and characterization of a series of trisiloxanes. China Surfactant Deterg Cosmet 36:73–80
Wang M, Song ZG, Xiang GW, Jiang H (2007) Green synthesis of 1-bromobutane. Chem Ind Times 21:30–31
Han F, Zhang GY (2004) Synthesis and interfacial properties of glucosamide-based trisiloxane surfactants. Acta Chimi Sin 62:733–737
Zhu YY (2003) The relationship between surfactant structure and properties. Petroleum Industry Press, Beijing, p 46
Han F, Zhang GY (2004) New family of Gemini surfactants with glucosamide-based trisiloxane. Colloid Surf A 237:79–85
Acknowledgments
The authors wish to thank the following people who offered their assistance in structural characterization. They include Tao Cai and Haiyang Gao. The financial support of the Natural Science Research Project of Department of Education of Guangdong Province (No. 04J016) and the Science and Technology Program of Guangdong Province (No. 2005B16001155) are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Peng, Z., Lu, C. & Lai, J. Synthesis and Properties of Novel Double-Tail Trisiloxane Surfactants. J Surfact Deterg 12, 331–336 (2009). https://doi.org/10.1007/s11743-009-1134-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11743-009-1134-6