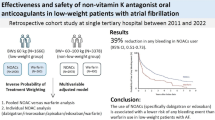
Abstract
Background
Direct oral anticoagulants (DOACs) are widely used for the treatment of venous thromboembolism (VTE) and for stroke prevention in atrial fibrillation (AF). However, evidence in obese and underweight patients is limited. We assessed the safety and effectiveness of DOACs and vitamin K antagonists (VKAs) in patients ≥ 120 kg or ≤ 50 kg enrolled in an observational prospective cohort study, the START-Register.
Methods
Adult patients started on anticoagulant therapy were followed up for a median of 1.5 years (IQR 0.6–2.8). Primary efficacy outcome was the occurrence of VTE recurrence, stroke and systemic embolism. Primary safety outcome was major bleeding (MB).
Results
10,080 AF and VTE patients were enrolled between March 2011 and June 2021, 295 patients weighted ≤ 50 kg and 82 patients ≥ 120 kg. Obese patients were significantly younger than underweight patients. Rates of thrombotic events were low and similar between DOACs and VKAs in underweight patients (1 event on DOACs therapy [0.9% 95% CI 0.11–5.39] and 2 on VKAs [1.1% 95% CI 0.01–47.68]) and in overweight patients (0 events on DOACs, 1 on VKAs [1.6%, 95% CI 0.11–5.79]. Two MB events occurred on DOACs (1.9%, 95% CI 0.38–6.00) and 3 on VKAs (1.6%, 95% CI 0.04–22.06) in the underweight group; 1 MB on DOACs (5.3% 95% CI 0.33–16.68) and 2 on VKAs (3.3%, 95% CI 0.02–130.77) in the overweight group.
Conclusions
DOACs seem to be effective and safe also for the treatment of patients with extreme body weights, both underweight and overweight. Further prospective studies are needed to support these findings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Direct oral anticoagulants (DOACs)–the thrombin inhibitor dabigatran, and the activated factor X (FXa) inhibitors apixaban, edoxaban, and rivaroxaban–are widely used for the treatment and secondary prevention of venous thromboembolism (VTE) and for ischemic stroke prevention in patients with non-valvular atrial fibrillation (AF) [1, 2]. DOACs are administered at fixed doses and do not require laboratory monitoring, resulting in a more convenient patient management. This strategy was found to be as effective and safe, if not safer, than treatment with vitamin K antagonists (VKAs) [3–12].
There is currently limited evidence in the literature to support the use of DOACs in obese and underweight patients. Studies that have specifically assessed the safety and effectiveness of DOACs in patients with extreme body weights had observational retrospective designs [13, 14]. A meta-analysis of the pivotal RCTs comparing DOACs with VKAs for the treatment of VTE addressed this issue and found no difference in VTE recurrence in patients with obesity treated with DOACs compared to VKAs, while DOACs significantly reduced the risk of major bleeding compared to VKAs [15]. In another meta-analysis, underweighted AF and VTE patients had a paradoxical increase of the risk of thromboembolism compared with non-low body weight patients, while AF overweight patients had fewer thromboembolic outcomes compared with AF patients with a non-high body weight [16].
In 2016, a guidance document from the International Society of Thrombosis and Haemostasis (ISTH), updated in 2021, suggested not to use DOACs in patients with a BMI > 40 kg/m2 or body weight > 120 kg because of limited clinical data and because available pharmacokinetic (PK)/pharmacodynamic (PD) evidence indicated decreased drug exposure, peak concentration, and shorter elimination half-lives with increasing body weight. No guidance was provided for patients with body weight < 50 kg [1, 2].
Aim of this prospective, cohort study is to assess the safety and effectiveness of DOACs and VKAs in patients weighting ≥ 120 kg and in patients ≤ 50 kg.
Materials and methods
As detailed elsewhere [17] the START-Register (NCT02219984) is an inception, prospective, observational, multicenter, dynamic, independent study that enrolls adult patients who start anticoagulant therapy, whatever the indication of treatment and drug/dosage used. Authorisation to set up the registry was obtained from the Ethical Committee of the University Hospital ‘S. Orsola-Malpighi’, Bologna, Italy, in October 2011 (n = 142/2010/0/0ss). The same institution is charged with deploying and upkeeping the registry central database. The aim of the START-Register is to collect data on the effectiveness and safety of anticoagulant treatments, on the determinants of adverse events in patients who are anticoagulated, and on their quality of life and compliance to treatment. Patients are included only after providing signed informed consent and the study was conducted according the ethical principles for medical research as set out in the Declaration of Helsinki. All participating centers have professional personnel qualified by education, training and experience to perform the required tasks. All collected clinical material is property of the Arianna Anticoagulazione Foundation. All data were gathered using an electronic clinical report form (e-CRF) developed for the START-Register. Each participating center had access to the e-CRF by a specific account and password. All centers were invited to include patients consecutively in order to avoid as much as possible a selection bias. The e-CRF included all demographic patient data in anonymous form; only the enrolling center was able to connect the anonymous information with the name of each patient. The accuracy and completeness of data entry in the central database was monitored by dedicated study personnel, at the Arianna Foundation, who also solicits participating centers to contact, for the purpose of the study, patients lost to follow-up through a telephone call or their general practitioner. Information on the type, dosage, and duration of anticoagulant treatment was collected at study entry and during follow-up. Information on study outcomes occurring during follow-up was collected. At each participating center, patients were regularly followed with at least 1 in person visit at 6 and 12 months. A final visit, in person or by telephone contact was requested at the end of follow-up.
All therapeutic decisions were entirely left to the discretion of the treating physicians.
Here, we present the results of the cohort of the extreme weight patients (≤ 50 kg or ≥ 120 kg) who started anticoagulation for the treatment and secondary prevention of VTE and for ischemic stroke prevention in non-valvular AF. Cut-offs of 50 kg and 120 kg were established to define under and overweight patients respectively according to median weight of the Italian population. For the purpose of the present analysis, data were collected from March 2011 to June 2021; patients follow-up was registered until October 2021.
Primary endpoints of this study were the composite of VTE recurrence, stroke, systemic embolism (thrombotic events) for effectiveness and major bleeding events for safety. Information on and all-cause mortality and clinically relevant non-major bleeding was also collected. VTE recurrence was defined as objective diagnosis of symptomatic VTE by means of compression and/or color-doppler ultrasound, CT scan and laboratory tests. Stroke was defined as the occurrence of focal neurological symptoms lasting at least 24 h and supported by congruent ischemic lesions at CT or MRI scan. Systemic embolism was defined as symptomatic acute loss of blood flow to a peripheral artery, supported by objective evidence of embolism. Major bleeding (MB) and clinically relevant non-major bleeding (CRNMB) were defined according to the ISTH definitions [18, 19]. Thrombotic and bleeding events were adjudicated by the local investigators.
Statistical analysis
All variables were summarized with the usual descriptive statistics: categorical variables as absolute and relative frequency; age was described with mean and standard deviation, BMI as median and interquartile range (IQR). Chi-square test p value was applied for categorical variable; F test from ANOVA model for continuous variable. Kruskall–Wallis test was applied for median follow-up. Unadjusted incidence rate (IR) was calculated for efficacy and safety outcomes; 95% confidence interval (95% CI) estimates were based on the Poisson distribution.
A univariate and multivariable (adjusted for low dose) competing risk analysis was applied to explore the effect of treatment on primary efficacy outcome and on bleedings, Fine and Grey model was applied and sub-distribution hazard ratios (sHR) together with 95% CIs were calculated.
All analysis was performed using SAS v9.4.
Results
Among the 12,819 patients on DOAC or AVK therapy enrolled in the START-Register during the interval time March 2011–June 2021 after the occurrence of VTE or for stroke prevention in AF, 56 subjects were excluded from the analysis due to lack of weight data and, among the remainder, 2782 were excluded due to lack of follow-up until October 2021. Therefore, the analysis was performed on 10,080 subjects.
Patients were stratified by weight into three groups: 295 patients ≤ 50 kg, 9703 patients 50–119 kg and 82 patients ≥ 120 kg, hereinafter referred to as under, normal and overweight respectively. The decision to prescribe VKAs or DOACs was entirely left to attending clinicians.
The baseline characteristics of the population are summarized in Table 1. Briefly, obese patients were significantly younger than normal weight and underweight patients and there were significantly more women in the underweight group than in the other two groups.
The mean Body Mass Index (BMI) was 42.2 kg/m2 (IQR 39.4–45.2) in obese patients, defining these patients as morbidly obese.
DOACs were prescribed to 110 (37.3%) underweight patients (56.4% of them received a reduced dose), and to 20 (24.4%) morbidly obese patients, 2 of them received a reduced dose (10.0%). VKAs were prescribed to 185 (62.7%) underweight patients and to 62 (75.6%) overweight patients. Patients on VKAs showed a median time in therapeutic range (TTR) of 64% (IQR 50–77) in the low weight group, 66% (IQR 54–76) in normal weight and 70% (IQR 52–76) in the high weight group, respectively.
Median duration of follow-up was 1.5 years (interquantile range 0.6–2.8).
Study outcomes
Efficacy outcomes
Overall, a thrombotic event occurred in 3 underweight patients (1.0%), in 141 normal weight patients (1.4%), and in 1 overweight patient (1.2%).
In the underweight group, the primary efficacy outcome occurred in 1 patient on DOACs therapy (0.9%, IR 0.76, 95% CI 0.11–5.39) and in 2 patients on VKAs (1.1%, IR 0.61, 95% CI 0.01–47.68), while in the overweight group, the only event occurred on VKAs therapy (1.6%, IR 0.82, 95% CI 0.11–5.79). [Table 2]. In the normal weight group, the thrombotic outcome occurred in 89 patients on DOACs (2.2%, IR 1.46, 95% CI 1.18–1.79) and in 52 patients on AVKs (0.9%, IR 0.44, 95% CI 0.26–0.77).
The cumulative occurrence of thrombotic events did not show significant difference between DOACs and VKAs in the underweight group (sHR 1.42, 95% CI 0.14–14.07, p = 0.77), these data were confirmed also after adjusting for DOACs low-dose (sHR 4.90, 95% CI 0.50–48.32, p = 0.17).
Safety outcomes
During follow-up, MB or CRNMB occurred in a total of 569 patients.
There were 5 MB in the underweight group (1.7%), 234 in the normal weight group (2.4%), and 3 in the overweight group (3.7%).
In the underweight group, 2 events occurred in the DOAC group (1.9%, IR 1.50, 95% CI 0.38–6.00) and 3 in the VKA group (1.6%, IR 0.92, 95% CI 0.04–22.06). In overweight group, there was 1 MB in patients receiving DOACs therapy (5.3%, IR 2.35, 95% CI 0.33–16.68) and 2 in patients on VKAs (3.3%, IR 1.67, 95% CI 0.02–130.77). In the normal weight group, there were 96 MB in patients on DOACs (2.4%, IR 1.59, 95% CI 1.30–1.94) and 138 in those on VKAs (2.4%, IR 1.21, 95% CI 0.77–1.92).
CRNMB occurred in 9 underweight patients (3.1%), in 313 normal weighted (3.2%) and in 1 obese patient (1.2%).
CRNMB in underweight patients occurred in 1 patient on DOACs (0.9%, IR 0.75, 95% CI 0.11–5.33) and in 8 patients on VKAs (4.3%, IR 2.46, 95% CI 0.04–139.53). In overweight patients, the only event occurred in the DOAC group (5.3%, IR 2.33, 95% CI 0.33–16.68). In normal weight patients, 87 CRNMB occurred in the DOAC group (2.1%, IR 1.44, 95% CI 1.17–1.77) and 230 in the VKA group (4.1%, IR 2.04, 95% CI 1.29–3.22).
No significant difference in overall bleeding rates was detected between DOACs and VKAs in the underweight group (sHR 0.90, 95% CI 0.24–3.37, p = 0.88) or in the overweight group (sHR 3.17, 95% CI 0.46–21.92, p = 0.24).
Mortality outcomes
During follow-up, 35 underweight patients (11.9%), 702 normal weighted (7.2%) and 4 obese patients (4.9%) died.
In the underweight group, death occurred in 7 patients on DOACs (6.7%, IR 5.24, 95% CI 2.50–10.99) and in 28 patients on VKAs (15.1%, IR 8.33, 95% CI 1.73–39.98). Four overweight patients on VKAs died (6.5%, IR 3.26, 95% CI 1.22–8.69), none in the DOACs group. In the normal weight group, 201 patients on DOACs (4.6%, IR 3.21, 95% CI 2.79–3.69) and 501 patients on VKAs (8.9%, IR 4.25, 95% CI 3.14–5.75) died (p = 0.0094).
Discussion
In this observational, prospective cohort study we have compared the incidence rates of thrombotic and bleeding events in patients with extreme body weights treated with DOACs and VKAs. Overall, rates of thrombotic events were similar across body weight groups and between DOACs and VKAs in the subgroups of underweight and overweight patients. There was a trend toward more major bleeding events in overweight than in underweight patients, with no statistically significant difference between treatment groups.
Overall, the low number of events documented in our study seems to support the possibility to use the DOACs in patient populations with extreme body weights. In particular, the effectiveness and safety of the DOACs appears at least comparable to that of VKAs in patients with a body weight of equal to or lower than 50 kg. In the overweight population enrolled in our study, with a mean BMI of 42.2 kg/m2, the number of events was reassuringly very low, but the small number of patients in this subgroup does not allow any firm conclusion.
Our results are in keeping with the results of Aloi and colleagues, who compared VTE recurrence rates between patients receiving DOAC therapy who weighed > 120 kg and those who weighed < 120 kg in the Veterans Integrated Service Network (VISN) 8 database [20]. Also in this retrospective study the number of events was low and not statistically different between the two groups.
In another retrospective cohort study on VTE patients, Cardinal et al. showed that the risk of recurrent VTE is not associated with BMI, whereas MB occurred more frequently in underweight than in normal or overweight patients treated with DOACs [21]. These results also support our findings for the obese population, but show an inverse trend in terms of safety. The lower incidence of bleeding events in the underweight population enrolled in our study may be, at least in part, explained by the high proportion of patients receiving low-dose DOACs in our study.
Cohen and colleagues compared the risk of recurrent VTE, MB, and CRNMB among VTE patients with obesity and morbid obesity treated with apixaban or warfarin and found a lower risk of CRNMB with the DOAC compared to warfarin across non-obese, obese/non-morbid, and morbidly obese patients. [22] The incidence of the other outcome events was comparable between the treatment groups, again supporting the use of DOACs in obese patients.
Finally, in a real-world Korean retrospective study, patients with non-valvular AF and low weight included in the Korean National Health Insurance Service (NHIS) database, So-Ryoung Lee and colleagues included 21,679 patients with a body weight < 60 kg and showed a better effectiveness and safety of DOACs versus warfarin [23]. This result remained consistent in patients with extremely low body weight (< 50 kg). The main limitation of this study was the poor TTR control in Asian patients treated with warfarin [23]. In our study, the underweight population showed a lower median TTR than other groups, but it was still 64% (IQR 50–77), demonstrating a good level of anticoagulation also in this population.
The participating centers, expert in managing anticoagulation, prescribed VKAs to majority of extreme body weights patients, following 2016 and 2021 ISTH recommendation [1, 2], showing a good TTR control both in under and overweighted patients.
Finally, a lower mortality rate was found among DOACs treated patients of any weight category. This difference may be related, at least in part, to the selection criteria adopted by physicians in choosing anticoagulant treatment. For example, the higher prevalence of severe renal failure in VKAs patients may be one factor to explain the higher mortality rate recorded in this population in spite of the weight category. Data are not shown as they are beyond the scope of this study.
Differences in outcome rates of thrombotic events and bleeding events were observed between DOACs and VKAs in normal weight patients. This finding, which is out of the scope of the present study, has been already reported and discussed in a previous paper by this group [24].
This study presents a number of limitations. First, the observational design requires extreme caution in interpreting direct comparisons between drugs due to the high risks of bias of these studies. Second, the small sample size of under and overweighted patients does not allow to reach firm conclusions on the effectiveness and safety of anticoagulant drugs in these populations.
Finally, in this study there was no central adjudication of outcome events. Moreover, we decided to focus our analysis on patients body weight, therefore no clinical outcomes data on patients BMI are available. Strength of the study is the multicentric design with a prospective collection of the patient data, together with accuracy and completeness of follow-up for patients enrolled.
In conclusion, DOACs seem to be effective and safe also for the treatment of patients with extreme body weights, both underweight and overweight. Further prospective studies are needed to support these findings.
Data availability
Data cannot be shared openly, to protect study participant privacy.
References
Martin K, Beyer-Westendorf J, Davidson BL, Huisman MV, Sandset PM, Moll S (2016) Use of the direct oral anticoagulants in obese patients: guidance from the SSC of the ISTH. J Thromb Haemost Blackwell Publish Ltd 14:1308–1313
Martin KA, Beyer-Westendorf J, Davidson BL, Huisman MV, Sandset PM, Moll S (2021) Use of direct oral anticoagulants in patients with obesity for treatment and prevention of venous thromboembolism Updated communication from the ISTH SSC Subcommittee on Control of Anticoagulation. J Thrombos Haemost 19:1874–1882
Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, Breithardt G, Halperin JL, Hankey GJ, Piccini JP, Becker RC, Nessel CC, Paolini JF, Berkowitz SD, Fox KAA, Califf RM (2011) Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 365:883–891
Buller HRDHGMMMMSPMRGSSSLSASMVPWP (2013) Edoxaban versus Warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med 369:1406–1415
Buller HR, Prins MH, Lensin AW, Decousus H, Jacobson BFMECJVPWP, Agnelli G, Cohen A, Berkowitz SDBHDB, Misselwitz FGARGSSSA (2010) Oral rivaroxaban for symptomatic venous thromboembolism. New Eng J Med Massachusetts Med Soc 363:2499–2510
Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, Waldo AL, Ezekowitz MD, Weitz JI, Špinar J, Ruzyllo W, Ruda M, Koretsune Y, Betcher J, Shi M, Grip LT, Patel SP, Patel I, Hanyok JJ, Mercuri M et al (2013) Edoxaban versus warfarin in patients with atrial fibrillation. New Eng J Med Massachusetts Med Soc 369:2093–2104
Schulman S, Kearon C, Kakkar AK, Mismetti P, Schellong S, Eriksson H, Baanstra D, Schnee J, Goldhaber SZ (2009) Dabigatran versus warfarin in the treatment of acute venous thromboembolism. New Eng J Med Massachusetts Med Soc 361:2342–2352
Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, Wang S, Alings M, Xavier D, Zhu J, Diaz R, Lewis BS, Darius H, Diener H-C, Joyner CD, Wallentin L (2009) Dabigatran versus warfarin in patients with atrial fibrillation. New Eng J Med Massachusetts Med Soc 361:1139–1151
Bailey AL. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. Cardiology Review. 2012
Schulman S, Kakkar AK, Goldhaber SZ, Schellong S, Eriksson H, Mismetti P, Christiansen AV, Friedman J, le Maulf F, Peter N, Kearon C (2014) Treatment of acute venous thromboembolism with dabigatran or warfarin and pooled analysis. Circulation 129:764–772
Granger CB, Alexander JH, McMurray JJV, Lopes RD, Hylek EM, Hanna M, Al-Khalidi HR, Ansell J, Atar D, Avezum A, Bahit MC, Diaz R, Easton JD, Ezekowitz JA, Flaker G, Garcia D, Geraldes M, Gersh BJ, Golitsyn S, Goto S et al (2011) Apixaban versus warfarin in patients with atrial fibrillation. New Eng J Med Massachusetts Med Soc 365:981–992
Agnelli G, Buller HR, Cohen A, Curto M, Gallus AS, Johnson M, Masiukiewicz U, Pak R, Thompson J, Raskob GE, Weitz JI (2013) Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med. https://doi.org/10.1056/NEJMoa1302507
Lorenz MA, Linneman TW (2022) Comparing safety and efficacy of direct oral anticoagulants versus warfarin in extreme obesity. J Pharm Pract. https://doi.org/10.1177/08971900221116809
Perino AC, Fan J, Schmitt S, Guo JD, Hlavacek P, Din N, Kothari M, Pundi K, Russ C, Emir B, Turakhia MP (2021) Anticoagulation treatment and outcomes of venous thromboembolism by weight and body mass index: insights from the veterans health administration. Circ Cardiovasc Qual Outcomes 14:e008005
Boonyawat K, Caron F, Li A, Chai-Adisaksopha C, Lim W, Iorio A, Lopes RD, Garcia D, Crowther MA (2017) Association of body weight with efficacy and safety outcomes in phase III randomized controlled trials of direct oral anticoagulants: a systematic review and meta-analysis. J Thrombosis Haemost 15:1322–1333
Mai V, Marceau-Ferron E, Bertoletti L, Lacasse Y, Bonnet S, Lega JC, Provencher S (2021) Direct oral anticoagulants in the treatment of acute venous thromboembolism in patients with obesity: a systematic review with meta-analysis. Pharmacol Res 163:105317
Antonucci E, Poli D, Tosetto A, Pengo V, Tripodi A, Magrini N, Marongiu F, Palareti G, Testa S, Paoletti O, Pengo V, Falanga A, Lerede T, Guazzaloca G, Poli D, Marcucci R, Piana A, Cibecchini F, Ruocco L, Lucarelli G et al (2015) The Italian START-Register on anticoagulation with focus on atrial fibrillation. PLoS ONE 10(5):e0124719
Schulman S, Kearon C (2005) Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thrombosis Haemostasis. https://doi.org/10.1111/j.1538-7836.2005.01204.x
Kaatz S, Ahmad D, Spyropoulos AC, Schulman S (2015) Definition of clinically relevant non-major bleeding in studies of anticoagulants in atrial fibrillation and venous thromboembolic disease in non-surgical patients: Communication from the SSC of the ISTH. J Thromb Haemostasis 13:2119–2126
Aloi KG, Fierro JJ, Stein BJ, Lynch SM, Shapiro RJ (2021) Investigation of direct-acting oral anticoagulants and the incidence of venous thromboembolism in patients weighing ≥120 kg Compared to Patients Weighing <120 kg. J Pharm Pract 34:64–69
Cardinal RM, D’Amico F, D’Addezio A, Dakers K, Castelli G (2021) Safety and efficacy of direct oral anticoagulants across body mass index groups in patients with venous thromboembolism: a retrospective cohort design. J Thromb Thrombolysis. https://doi.org/10.1007/s11239-020-02361-8
Cohen A, Sah J, Lee T, Rosenblatt L, Hlavacek P, Emir B, Keshishian A, Yuce H, Luo X (2021) Effectiveness and Safety of Apixaban vs. Warfarin in venous thromboembolism patients with obesity and morbid obesity. J Clin Med 10:200
Lee SR, Choi EK, Park CS, do Han K, Jung JH, Oh S, Lip GYH (2019) Direct oral anticoagulants in patients with nonvalvular atrial fibrillation and low body weight. J Am Coll Cardiol 73:919–931
Palareti G, Antonucci E, Legnani C, Mastroiacovo D, Poli D, Prandoni P, Tosetto A, Pengo V, Testa S, Ageno W (2020) Bleeding and thrombotic complications during treatment with direct oral anticoagulants or vitamin K antagonists in venous thromboembolic patients included in the prospective, observational START2-register. BMJ Open. https://doi.org/10.1136/bmjopen-2020-040449
Acknowledgements
The following Investigators and Centers participated to the START2-Register: Benilde Cosmi, UO Angiologia e Malattie della Coagulazione, Azienda Ospedaliero Universitaria. S. Orsola—Malpighi, Bologna. Walter Ageno, Giovanna Colombo, Dipartimento di Emergenza e Accettazione, Centro Trombosi. ed Emostasi, Ospedale di Circolo, Università dell’Insubria, Varese. Doris Barcellona, Struttura Dipartimentale di Emostasi e Trombosi, AOU di Cagliari, Dipartimento di Scienze Mediche e Sanità Pubblica, Università di Cagliari, Cagliari. Barbara Bresciani, Cardiologia, Ospedale di Bentivoglio ASL Bologna, Bologna. Eugenio Bucherini, Struttura Semplice Dipartimentale, Medicina Vascolare-Angiologia, Ospedale Civile Faenza, Faenza (RA). Paola Casasco, Centro diagnosi trombosi e sorveglianza terapia anticoagulanti, Immunoematologia Trasfusionale, Ospedale SS. Antonio e Margherita, Tortona (AL). Paolo Chiarugi, Ambulatorio Antitrombosi per la sorveglianza dei pazienti in terapia anticoagulante. orale, U.O. Analisi Chimico-Cliniche, Azienda Ospedaliero Universitaria Pisana, Pisa. Antonio Chistolini, Alessandra Serrao, Sezione Ematologia, Dipartimento di Biotecnologie. Cellulari ed Ematologia, Azienda Ospedaliero Universitaria Policlinico Umberto I, Roma. Valeria De Micheli, Centro Emostasi e Trombosi, Ospedale di Lecco, Lecco. Marcello Di Nisio, Medicina Vascolare e Malattie Trombemboliche, Ospedale SS.ma Annunziata, Pescara. Anna Falanga, Teresa Lerede, Luca Barcella, USC SIMT, Centro Emostasi e Trombosi, Ospedale Papa Giovanni XXIII, Bergamo. Vittorio Fregoni UOC Medicina Generale ASST Val Lario, Sondalo (Sondrio). Elvira Grandone, Donatella Colaizzo, Centro Trombosi Casa del Sollievo e della Sofferenza, S. Giovanni Rotondo (Foggia). Antonio Insana, Laboratorio Analisi, Ospedale Mauriziano, Torino. Giuseppe Malcangi, Centro Emofilia e Trombosi, Policlinico di Bari, Bari. Catello Mangione, Trasfusionale, Ospedale di Galatina, Galatina (LE). Giuliana Martini, Centro Emostasi, Spedali Civili Di Brescia, Brescia. Lucilla Masciocco, Pasquale Saracino, Centro Controllo Coagulazione, Dipartimento di Medicina-Geriatria-Lungodegenza, Ospedali Riuniti Riuniti-Foggia, Presidio Ospedaliero Lastaria, Lucera (Foggia). Daniela Mastroiacovo, Dipartimento di Angiologia, Ospedale SS. Filippo e Nicola, Avezzano (L’Aquila). Marco Marzolo, UOC Medicina Interna, Ospedale di Rovigo, Rovigo. Vincenzo Oriana, Centro Emofilia, Servizio Emostasi e Trombosi, Azienda Bianchi Melacrino. Morelli, Reggio Calabria. Carmelo Paparo, Patologia Clinica, Ospedale Maggiore, Chieri (Torino). Simona Pedrini, Federica Bertola, Servizio di Laboratorio, Istituto Ospedaliero Fondazione. Poliambulanza Brescia, Brescia. Vittorio Pengo, Gentian Denas, Elisa Bison, Dipartimento di Scienze Cardio-Toraco-Vascolari, Centro Trombosi, AOU Padova, Padova. Antonietta Piana, Francesco Cibecchini, Centro Prevenzione e Cura Malattie Tromboemboliche. Azienda Ospedaliera Universitaria San Martino IST, Genova. Pasquale Pignatelli, Daniele Pastori, Centro Trombosi, Clinica Medica Policlinico Umberto I°, Roma. Gian Marco Podda, Simone Birocchi, Medicina Interna ASST Santi Paolo e Carlo, Milano. Daniela Poli, Rossella Marcucci, Niccolò Maggini, SOD Malattie Aterotrombotiche, Azienda. Ospedaliero Universitaria-Careggi, Firenze. Angelo Porfidia, UOC Medicina Generale, Policlinico Universitario Gemelli, Roma. Serena Rupoli, Clinica Ematologica, Ospedali Riuniti Ancona, Ancona. Domizio Serra, Centro Trombosi, Ospedale Evangelico Internazionale, Genova. Sophie Testa, Oriana Paoletti, Centro Emostasi e Trombosi, Laboratorio Analisi chimico-cliniche. e microbiologiche, Azienda Socio Sanitaria Territoriale di Cremona, Cremona. Andrea Toma, Pietro Barbera, UOC di Patologia Clinica, Ambulatorio Terapia Anticoagulante. Orale, O.C. “L.Cazzavillan”, Arzignano (Vicenza). Alberto Tosetto, Divisione di Ematologia, Ospedale San Bortolo, Vicenza. Claudio Vasselli, Laboratorio Patologia Clinica, Policlinico Casilino, Roma. Adriana Visonà, Beniamino Zalunardo UOC Angiologia, Ospedale San Giacomo Apostolo, Castelfranco Veneto (Treviso). Maddalena Loredana Zighetti, SIMT- AO San Paolo, Milano.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Guarascio, M., Bertù, L., Donadini, M.P. et al. DOACs use in extreme body-weighted patients: results from the prospective START-register. Intern Emerg Med 18, 1681–1687 (2023). https://doi.org/10.1007/s11739-023-03334-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11739-023-03334-4