Abstract
Cancer-associated ischemic stroke (CAS) refers to a hypercoagulation disorder related to malignant tumors, especially adenocarcinoma. Carbohydrate antigen (CA) 125 is a mucinous serum marker that might reflect hypercoagulation status, but the association between CA 125 and CAS is unclear across various types of cancer. The aim of this study was to investigate the associations among tumor markers, coagulation markers, and clinical factors in acute ischemic stroke (AIS) patients with active cancer. Consecutive AIS patients with active cancer (a diagnosis or ongoing active therapy for cancer within 6 months) were prospectively enrolled at four hospitals. D-dimer, C-reactive protein (CRP), carcinoembryonic antigen (CEA), CA19-9, and CA 125 levels were measured. Of 120 AIS patients with active cancer, 47 were diagnosed with CAS. CA 125 had the strongest correlations with D-dimer and CRP (ρ = 0.543, p < 0.001 and ρ = 0.452, p < 0.001, respectively). The areas under the receiver-operating characteristic curves for the diagnosis of CAS were 0.812 (95% CI 0.718–0.878) for CA 125, 0.714 (95% CI 0.602–0.801) for CEA, and 0.663 (95% CI 0.552–0.759) for CA 19-9. Multivariable analysis revealed that CA 125 levels in the highest quartile (OR 2.91, 95% CI 1.68–5.53), multiple lesions in multiple vascular territories observed on diffusion-weighted imaging, the absence of dyslipidemia, and the absence of atrial fibrillation were independently associated with CAS. Increased CA 125 levels, which indicate hypercoagulability, were useful for diagnosing CAS in AIS patients with active cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer-associated ischemic stroke (CAS) is considered one etiology of cryptogenic stroke, and its pathophysiology is hypercoagulation disorder related to malignant tumors or the resulting general arteriovenous thrombosis [1, 2]. Although patients with CAS usually have active cancer, not all ischemic stroke patients with active cancer are diagnosed with CAS. Some ischemic stroke patients with active cancer are classified as having conventional stroke mechanisms with the coexistence of vascular risk factors [3]. The clinical status of CAS often affects oncological treatments and leads to the disability of performance status. Hence, it is essential to diagnose CAS at an early stage and consider the appropriate management of patients with CAS. Several studies have shown that increased D-dimer levels and diffusion-weighted imaging (DWI) lesions in multiple vascular territories were closely associated with CAS [4,5,6].
The association between cancer types and CAS has been investigated, and adenocarcinoma has been identified in many patients with CAS. Several tumor markers are useful for diagnosing adenocarcinoma and evaluating the progression status. Carcinoembryonic antigen (CEA) and carbohydrate antigen (CA) 19-9 are widely used as tumor markers for adenocarcinomas, such as gastric, biliary tract, colon, pancreatic, and lung cancers. CA 125, an adenocarcinoma marker, is generally used for the assessment of ovarian cancer or several gynecological diseases. It is also a mucinous serum marker that is expressed in patients with various diseases that are associated with hypercoagulation status [7, 8]. Indeed, increased CA 125 levels were associated with the incidence of stroke among patients with lung or gastric cancer [9, 10]. However, it is uncertain whether CA 125 levels are associated with hypercoagulability or the diagnosis of CAS compared with other tumor markers among patients with various types of active cancer. In the present study, we aimed to elucidate the utility of evaluating tumor markers, especially CA 125, in clinical settings among patients with active cancer.
Methods
Study population
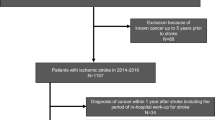
This was a four-center, hospital-based prospective study involving patients with acute ischemic stroke within 7 days after stroke onset who were hospitalized in the Hiroshima University Hospital, National Hospital Organization Kure Medical Center, Kawasaki Medical School Hospital, and Chikamori Hospital between November 2016 and September 2020. Of 2590 consecutive acute ischemic stroke patients, 329 patients with a history of cancer (12.7%) were enrolled. The patients with cancer were divided into those with active cancer (n = 153) and those with nonactive cancer (n = 176). Active cancer was defined as a diagnosis of cancer within 6 months before stroke onset, any treatment for cancer within the previous 6 months, or recurrent or metastatic cancer [11]. Blood samples were collected at admission to assess D-dimer and C-reactive protein levels, and tumor markers such as CEA, CA19-9, and CA 125 were principally obtained within 2 days after admission. Of the 153 patients with active cancer, 33 patients were excluded due to incomplete data for CEA, CA 19-9, and CA 125 on the same day. Finally, 120 ischemic stroke patients with active cancer were analyzed. The flowchart of patient selection is shown in Fig. 1.
Assessment of clinical characteristics
Ischemic stroke was defined as the sudden onset of acute neurological deficits duration greater than 24 h, with evidence of acute infarction on brain computed tomography or magnetic resonance imaging (MRI). The following clinical characteristics were recorded at admission: age, sex, body mass index (BMI), and classic vascular risk factors, including hypertension, diabetes mellitus, dyslipidemia, chronic kidney disease (CKD), atrial fibrillation, daily alcohol intake (> 40 g), smoking habit (current or previous smokers vs. never smokers), and history of stroke and deep venous thrombosis. The criteria for hypertension, diabetes mellitus, dyslipidemia, CKD, and atrial fibrillation were previously defined [12]. The type of cancer, current cancer treatment, and presence of systemic metastasis were also recorded. The type of cancer treatment was divided into the following classifications: before cancer treatment (stroke onset before starting treatment for cancer), surgical treatment, chemotherapy, combined therapy (chemotherapy and radiation), and other (including best supportive care). Data on antiplatelet or anticoagulant use before stroke onset were collected. In addition, acute reperfusion therapy (intravenous thrombolysis or/and endovascular treatment) was evaluated. Neurological severity was assessed according to the National Institutes of Health Stroke Scale (NIHSS) scores. Stroke subtypes were classified according to the criteria set by the Trial of ORG 10172 in Acute Stroke Treatment (TOAST) classification [13]. In principle, routine management was performed by 24-h electrocardiographic monitoring, carotid ultrasonography, transthoracic echocardiography, and brain imaging. Patients with conventional stroke mechanisms (small vessel occlusion, large artery atherosclerosis, or cardioembolism) were principally not considered to have CAS. Patients with other specific causes of stroke, such as artery dissection, vasculitis, aortic arch atheroma, and vasospasm, were also not considered to have CAS. Ultimately, CAS was comprehensively diagnosed by the attending physicians with a consensus of some stroke neurologists at each hospital based on the clinical status of cancer and stroke. Of 120 patients, 119 underwent MRI for the detection of new ischemic infarcts via DWI. Based on the DWI findings, patients were divided into three subgroups: (1) single lesions in one vascular territory, (2) scattered lesions in one vascular territory, and (3) multiple lesions in multiple vascular territories [14]. This study complies with the Declaration of Helsinki guidelines for investigations involving humans, and the study protocol was approved by the Ethics Committees of each hospital. As this study used clinical records, it was performed under the opt-out method. Informed consent for participation was not obtained from the participants.
Statistical analysis
Categorical variables are presented as numbers and percentages, and continuous variables are presented as the means with standard deviations (SDs) or medians (interquartile ranges). The statistical significance of intergroup differences was assessed using the Chi-square test for categorical variables and Student’s t test or the Mann–Whitney U test for continuous variables. Spearman’s correlation analysis for the associations between each tumor marker and the D-dimer and CRP levels was performed, because these values were not normally distributed. To predict CAS, receiver-operating characteristic (ROC) curves were constructed for CEA, CA 19-9, and CA 125 levels. The areas under the ROC curves (AUCs) were compared using a nonparametric method [15]. Multivariable logistic analysis was performed to identify indicators for CAS (model 1: age, sex, BMI, daily alcohol intake, current smoking, hypertension, diabetes mellitus, dyslipidemia, CKD, atrial fibrillation, history of stroke, history of deep venous thrombosis, systemic metastasis, D-dimer, CRP, and DWI lesions in multiple vascular territories; model 2: quartiles of each tumor marker level added to model 1) with a backward selection procedure using p > 0.10 of the likelihood ratio test as the exclusion criterion. Those analyses were performed using JMP 14.0 (SAS Institute, Inc., Cary, NC, USA).
Results
Of the 120 patients, 47 (39.2%) were diagnosed with CAS. Clinical data are shown in Table 1. Among the patients with CAS, a higher proportion were female, and there was a lower frequency of smoking, dyslipidemia, and atrial fibrillation. Although two patients had atrial fibrillation, they were comprehensively diagnosed with CAS based on clinical data of advanced-stage cancer. The patients with CAS had a higher frequency of systemic metastasis than those without CAS (70.2 vs. 35.6%, p < 0.001). Patients with CAS had a lower frequency of prior antiplatelet use than those without CAS. The details of antithrombotic medication are shown in Supplemental Table 1. Some patients with CAS had taken direct oral anticoagulants for deep venous thrombosis, pulmonary embolism, or atrial fibrillation prior to stroke onset. Regarding cancer type, patients with CAS had a higher frequency of lung adenocarcinoma, biliary tract/pancreatic cancer, and ovarian cancer than those without CAS (27.7 vs. 8.2%, 17.0 vs. 4.1%, and 8.5 vs. 0.0%, respectively). Of 47 patients with CAS, 36 patients (78.3%) had multiple DWI lesions in multiple vascular territories. The patients with CAS had higher levels of D-dimer, CRP, and each tumor marker than those without.
The correlation analysis of associations between D-dimer and CRP and each tumor marker is shown in Table 2. The correlation coefficients between CA 125 and D-dimer and CRP levels were higher than those between CEA or CA19-9 and D-dimer and CRP levels (ρ = 0.543, p < 0.001 and ρ = 0.452, p < 0.001, respectively). The AUCs of the ROC curve for each tumor marker in the diagnosis of CAS are shown in Fig. 2. The AUCs were 0.812 (95% confidence interval [CI] 0.718–0.878) for CA 125, 0.714 (95% CI 0.602–0.801) for CEA, and 0.663 (95% CI 0.552–0.759) for CA 19-9. The AUC of CA 125 was higher than that of CEA or CA 19-9 (p = 0.087 and p = 0.015, respectively). Multivariable logistic analysis showed that the absence of dyslipidemia, the absence of atrial fibrillation, increased D-dimer levels, and multiple lesions in multiple vascular territories were independently associated with CAS among patients with active cancer (Table 3, model 1). When adding the quartiles of CEA, CA 19-9, and CA 125 levels to the indicators, a CA 125 level, but not a CEA or CA 19-9 level, in the highest quartile was independently associated with CAS (odds ratio 2.91, 95% CI 1.68–5.53, p < 0.001) (Table 3, model 2).
The areas under the receiver-operating characteristic (ROC) curve (AUC) for each tumor marker for the diagnosis of cancer-associated stroke. The AUCs were 0.812 (95% confidence interval [CI] 0.718–0.878) for carbohydrate antigen (CA) 125, 0.714 (95% CI 0.602–0.801) for carcinoembryonic antigen (CEA), and 0.663 (95% CI 0.552–0.759) for CA 19-9
Discussion
In the present study, we found that increased CA 125 levels were closely associated with increased D-dimer and CRP levels among ischemic stroke patients with active cancer. Compared with CEA or CA 19-9 levels, CA 125 levels were suitable for predicting a diagnosis of CAS based on the ROC analysis. In addition, increased CA 125 levels were independently associated with CAS after adjusting for clinical factors, including D-dimer, CRP, and multiple DWI lesions.
Hypercoagulability due to cancer leads to arterial and venous thrombotic events, including CAS. The mechanisms observed in ischemic stroke patients with cancer are very heterogeneous because of intravascular coagulopathy, nonbacterial thrombotic endocarditis, paradoxical embolism, tumor occlusion, and conventional stroke mechanisms due to shared vascular risk factors [1, 3]. Increased D-dimer levels and multiple infarct lesions in multiple vascular territories might be associated with the presence of active cancer among ischemic stroke patients based on several observational studies [4, 16,17,18]. These indicators were also associated with occult cancer among cryptogenic stroke patients without a cancer diagnosis at the time of stroke onset [19]. Therefore, accumulating evidence has shown that increased D-dimer levels and multiple infarct lesions in multiple vascular territories are associated with cancer-associated stroke or hypercoagulability. In the present study, the absence of a conventional stroke mechanism (dyslipidemia or atrial fibrillation), increased D-dimer levels, and multiple DWI lesions were associated with CAS without considering the influence of tumor markers. Although the definition of CAS is still not fully unified worldwide, the diagnosis of CAS based on attending physicians at each hospital in the present study was supported by the findings of previous reports.
Our novel and interesting findings were that CA 125 levels were closely associated with D-dimer or CRP levels among patients with active cancer. In addition, CA 125 levels were superior to CEA or CA 19-9 in the diagnosis of CAS. CA 125, which was initially used as an ovarian cancer biological marker, has been characterized to be a transmembrane mucin, MUC 16 [20]. Therefore, CA 125 is also a characterized biological marker of mucinous cancers, including ovarian cancer, lung adenocarcinoma, pancreatic cancer, and breast cancer. As several cases with gynecological disease could have systemic embolisms through increased CA 125 levels [7, 21], increased CA 125 levels might reflect hypercoagulability. Four patients with metastatic cancer (one patient had pancreatic cancer, and three patients had lung cancer) had brain infarcts with other recurrent thromboembolic diseases through markedly increased CA 125 levels [22]. Interestingly, CA 125 is also proposed to be a biological marker of heart failure, because it is released by mesothelial cells as a response to serosal effusions and inflammation [23]. In the present study, the correlational relationships between CA 125 levels and D-dimer or CRP levels were the highest compared with those between CEA or CA 19-9 levels and D-dimer and CRP levels. Long et al. showed that ischemic stroke patients with gastric cancer had significantly higher CA 125 levels than nonstroke patients with gastric cancer [9]. Xie et al. found that ischemic stroke patients with lung cancer had significantly higher CA 125 levels than nonstroke patients with lung cancer [10]. However, a few studies have investigated whether increased CA 125 levels were associated with cancer-associated coagulability and CAS compared with other tumor markers in ischemic stroke patients across various types of cancer. We found that the predictive ability of CA 125 for the diagnosis of CAS was higher than that of CEA or CA 19-9. In addition, increased CA 125 levels were independently associated with CAS after adjusting for baseline characteristics, including D-dimer or CRP levels. We speculate that CA 125 might be a suitable biological marker of CAS via cancer-associated hypercoagulability and inflammation. In the present study, the incidences of lung cancer (adenocarcinoma) and biliary tract/pancreatic cancer were higher in CAS patients, consistent with the previous studies [4, 6]. Although CA 125 might not be used for monitoring the cancer activity in patients with lung cancer or biliary tract/pancreatic cancer in the daily clinical setting, it might be a useful indicator of a high risk of CAS in patients with those cancers. Further studies on whether CA 125 is useful for predicting the future incidence of CAS are required in patients with various cancers.
There are several limitations in this study. First, our study had a small sample size, and not all ischemic stroke patients with active cancer could be evaluated for each tumor marker (CEA, CA 19-9, and CA 125), which might lead to potential selection bias. However, baseline characteristics, including neurological severity, did not generally differ from those in the previous studies of acute ischemic stroke patients with active cancer [18]. Second, the definition of CAS was not unified across hospitals in the study, because we adopted the attending physician’s diagnosis with consensus by several stroke neurologists at each hospital. Indeed, two patients with atrial fibrillation (one with paroxysmal atrial fibrillation) were diagnosed with CAS by attending physicians, because those patients with an advanced stage of cancer experienced multiple infarcts despite anticoagulation therapy. In addition, not all patients with an undetermined etiology were considered to have CAS. The diagnosis of CAS was based on the attending physician’s comprehensive judgements, and well-known indicators, such as D-dimer levels and multiple ischemic lesions in multiple vascular territories, were associated with CAS in the present study. Third, active cancer might cause atherosclerotic development or hypercoagulability even in the context of conventional stroke mechanisms. Potential mechanisms, such as paradoxical embolism, should also be considered in patients with active cancer who experience deep venous thrombosis. Whether the status of cancer-associated hypercoagulability, which influences conventional stoke mechanisms, was considered CAS should be discussed in future studies. In addition, cancer treatment-related stroke, for example, radiation-induced vasculopathy or chemotherapy-induced coagulopathy, may be other important issues in the management of stroke patients with active cancer.
In conclusion, increased CA 125 levels, which indicated cancer-associated hypercoagulability or inflammation, were useful for the diagnosis of CAS among ischemic stroke patients with active cancer. Further large and prospective studies are needed to determine whether CA 125 is useful for the early diagnosis of cancer-associated thrombosis or stroke among patients with various types of active cancer.
Availability of data and materials
The data that support the findings of this study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
References
Bang OY, Chung JW, Lee MJ, Seo WK, Kim GM, Ahn MJ et al (2020) Cancer-related stroke: an emerging subtype of ischemic stroke with unique pathomechanisms. J Stroke 22:1–10
Dardiotis E, Aloizou AM, Markoula S, Siokas V, Tsarouhas K, Tzanakakis G et al (2019) Cancer-associated stroke: pathophysiology, detection and management (Review). Int J Oncol 54:779–796
Kim SG, Hong JM, Kim HY, Lee J, Chung PW, Park KY et al (2010) Ischemic stroke in cancer patients with and without conventional mechanisms: a multicenter study in Korea. Stroke 41:798–801
Schwarzbach CJ, Schaefer A, Ebert A, Held V, Bolognese M, Kablau M et al (2012) Stroke and cancer: the importance of cancer-associated hypercoagulation as a possible stroke etiology. Stroke 43:3029–3034
Finelli PF, Nouh A (2016) Three-territory DWI Acute infarcts: diagnostic value in cancer-associated hypercoagulation stroke (Trousseau Syndrome). AJNR Am J Neuroradiol 37:2033–2036
Shen Y, Li Y, Chen C, Wang W, Li T (2020) D-dimer and diffusion-weighted imaging pattern as two diagnostic indicators for cancer-related stroke: a case-control study based on the STROBE guidelines. Medicine (Baltimore) 99:e18779
Akaishi T, Kuroda H, Tateyama M, Yoshida Y, Otsuki T, Watanabe M et al (2015) Recurrent cerebral infarction synchronous with menorrhagia caused by endometrial stromal sarcoma. J Neurol Sci 358:509–511
Shao B, Wahrenbrock MG, Yao L, David T, Coughlin SR, Xia L et al (2011) Carcinoma mucins trigger reciprocal activation of platelets and neutrophils in a murine model of Trousseau syndrome. Blood 118:4015–4023
Long H, Qin K, Chen J, Chen Y, Chen L, Zeng J et al (2018) Biomarkers of gastric cancer-related ischemic stroke and its underlying pathogenesis. Medicine (Baltimore) 97:e0493
Xie X, Chen L, Zeng J, Qin C, Cheng D, Wei X et al (2016) Clinical features and biological markers of lung cancer-associated stroke. J Int Med Res 44:1483–1491
Lee AY, Levine MN, Baker RI, Bowden C, Kakkar AK, Prins M et al (2003) Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med 349:146–153
Naito H, Nezu T, Hosomi N, Aoki S, Kinoshita N, Kuga J et al (2018) Controlling nutritional status score for predicting 3-mo functional outcome in acute ischemic stroke. Nutrition 55–56:1–6
Adams HP, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL et al (1993) Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 24:35–41
Kang DW, Chalela JA, Ezzeddine MA, Warach S (2003) Association of ischemic lesion patterns on early diffusion-weighted imaging with TOAST stroke subtypes. Arch Neurol 60:1730–1734
DeLong ER, DeLong DM, Clarke-Pearson DL (1988) Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 44:837–845
Kim K, Lee JH (2014) Risk factors and biomarkers of ischemic stroke in cancer patients. J Stroke 16:91–96
Lee EJ, Nah HW, Kwon JY, Kang DW, Kwon SU, Kim JS (2014) Ischemic stroke in patients with cancer: is it different from usual strokes? Int J Stroke 9:406–412
Kneihsl M, Enzinger C, Wünsch G, Khalil M, Culea V, Urbanic-Purkart T et al (2016) Poor short-term outcome in patients with ischaemic stroke and active cancer. J Neurol 263:150–156
Gon Y, Sakaguchi M, Takasugi J, Kawano T, Kanki H, Watanabe A et al (2016) Plasma D-dimer levels and ischaemic lesions in multiple vascular regions can predict occult cancer in patients with cryptogenic stroke. Eur J Neurol 24(3):503–508
Das S, Batra SK (2015) Understanding the unique attributes of MUC16 (CA125): potential implications in targeted therapy. Cancer Res 75:4669–4674
Aiura R, Nakayama S, Yamaga H, Kato Y, Fujishima H (2021) Systemic thromboembolism including multiple cerebral infarctions with middle cerebral artery occlusion caused by the progression of adenomyosis with benign gynecological tumor: a case report. BMC Neurol 21:14
Jovin TG, Boosupalli V, Zivkovic SA, Wechsler LR, Gebel JM (2005) High titers of CA-125 may be associated with recurrent ischemic strokes in patients with cancer. Neurology 64:1944–1945
Miñana G, Núñez J, Sanchis J, Bodí V, Núñez E, Llàcer A (2010) CA125 and immunoinflammatory activity in acute heart failure. Int J Cardiol 145:547–548
Acknowledgements
We express our gratitude to Mayumi Oda at Chikamori Hospital, Mari Okamoto at Kawasaki Medical School, and Makiko Miyamoto at National Hospital Organization Kure Medical Center and Chugoku Cancer Center for their secretarial assistance. This study was supported by a research grant from the Japan Society for the Promotion of Science KAKENHI (grant numbers 17K17907 and 20K16579).
Funding
This study was supported by a research grant from the Japan Society for the Promotion of Science KAKENHI (grant numbers 17K17907 and 20K16579).
Author information
Authors and Affiliations
Contributions
TN: drafted and revised the manuscript for intellectual content, and collected data. NH, HN, SA, TT, TK, TS, DK, YM, TY, YY, NO, YS, NK, TK, HU, TO, and HM: revised the manuscript for intellectual content, and collected data.
Corresponding author
Ethics declarations
Conflict of interest
Hirofumi Maruyama received grants from Daiichi Sankyo Co., Ltd.; these grants are unrelated to the submitted work. All other authors declare that they have no conflicts of interest.
Ethical approval
This study complies with the Declaration of Helsinki guidelines for investigations involving humans, and the study protocol was approved by the Ethics Committees of each hospital (Hiroshima University Hospital; E-608, National Hospital Organization Kure Medical Center; 29–01, Kawasaki Medical School Hospital; 2621, and Chikamori Hospital; 223). As this study used clinical records, it was performed under the opt-out method.
Informed consent
Informed consent for participation was not obtained from the participants.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nezu, T., Hosomi, N., Naito, H. et al. Clinical characteristics and tumor markers in ischemic stroke patients with active cancer. Intern Emerg Med 17, 735–741 (2022). https://doi.org/10.1007/s11739-021-02862-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11739-021-02862-1