Abstract
Age-adjusted D-dimer (AADD) appears to increase the proportion of patients in whom pulmonary embolism (PE) can safely be excluded compared with conventional D-dimer (CDD), according to a limited number of studies. The aim if this study was to assess whether the use of an AADD might safely increase the clinical usefulness of CDD for the diagnosis of PE in our setting. Three hundred and sixty two consecutive outpatients with clinically suspected PE in whom plasma samples were obtained to measure D-dimer were included in this post hoc analysis of a previous study. CDD cutoff value was 500 ng/mL and AADD was calculated as (patient’s age × 10) ng/mL in patients aged >50. Sensitivity, specificity, clinical usefulness (i.e., proportion of true-negative tests among all patients with suspected PE), and the proportion of false negatives were calculated for both AADD and CDD among patients with low-to-moderate clinical probability of PE according to Well’s criteria. PE was confirmed in 98 patients (27 %). Among 331 patients with low-to-moderate clinical probability of PE, sensitivity and clinical usefulness were 100 and 27.8 % for CDD, respectively, and 100 and 36.5 % for AADD, respectively. In 29 patients aged >50 with CDD >500 ng/mL, AADD showed values under its normal cutoff point, without false negatives for the diagnosis of PE (0 %, 95 % CI 0–11 %). AADD increases clinical usefulness notably with respect to that of CDD in patients with clinical suspected PE without losing sensitivity in our cohort. The use of AADD apparently does not reduce the safety of CDD for the exclusion of PE.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pulmonary embolism (PE) is considered in the differential diagnosis of many clinical presentations, and it remains a diagnostic challenge in clinical practice [1]. Currently, the diagnosis of acute PE should follow a sequential diagnostic workup consisting of assessment of pretest probability (PTP) based on clinical evaluation followed by D-dimer testing in low/moderate clinical probability [2, 3]. Patients with a positive D-dimer testing, or those with high clinical PTP of PE, go on to multidetector computed tomography (MDCT) or ventilation–perfusion (V/Q) lung scanning [2, 3].
D-dimer testing has a high sensitivity, and has been proven a safe test in combination with non-high clinical PTP to rule out PE in outcome studies [4–6]. However, D-dimer testing has only a moderate specificity (40–60 %) for the PE diagnosis, leading to a high rate of false-positive results in multiple conditions [5, 7] [8]. As a result, the clinical usefulness of the test (i.e., the proportion of negative D-dimer tests in patients with suspected of PE and in whom this diagnosis may be safely ruled out) is low. Several studies have shown that the clinical usefulness of D-dimer with a conventional cutoff value of 500 ng/mL (CDD) is about 30 % [4, 6, 9–11]. Accordingly, 70 % of patients with suspected PE will require further evaluation with imaging techniques for PE detection (MDCT or V/Q lung scanning).
The CDD physiologically increases with aging making this test less useful in elderly patients compared with younger subjects in whom PE is suspected [12, 13]. Recent studies suggest an increased clinical usefulness of D-dimer testing for the diagnosis of PE when an age-adjusted D-dimer (AADD) cutoff is used in patients aged >50 [14–16]. However, with the exception of a prospective study [15], most of relevant reports using AADD are retrospective and limited to three research groups in Central Europe and the United States of America [17]. For this reason, it remains unknown whether the use of AADD in hospital settings across different geographic areas shows similar results in terms of both efficiency and safety. In this regard, the aim of our study is to evaluate in our local context whether the proposed AADD cutoff can safely improve the clinical usefulness of CDD cutoff value in a cohort of consecutive outpatients with suspected PE.
Patients and methods
Patients
Consecutive outpatients who presented to the emergency department (ED) with clinically suspected PE at Príncipe de Asturias University Hospital (Alcalá de Henares, Madrid, Spain) between September 2008 and October 2010 were included in a study of PE diagnosis with a three-month follow-up. Exclusion criteria were age younger than 18 years, pregnancy, patients already on therapeutic anticoagulation and logistic reasons (e.g., unavailability of MDCT, V/Q lung scanning or contrast pulmonary angiography). The study was approved by the local Ethics Committee and written informed consent forms were obtained from all patients.
Study design
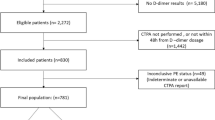
Consecutive outpatients who presented to the ED with clinically suspected PE were managed according to a strict local protocol for PE diagnosis, as detailed elsewhere (Fig. 1) [11]. Plasma samples to measure levels of D-dimer were obtained at enrollment. The D-dimer was measured at the end of study, and their results for the PE diagnosis were analyzed retrospectively.
Patients underwent clinical evaluation by the attending physician prior to undergoing any other test, and were categorized according to the 3-level Wells score in low, moderate, and high clinical probability groups [18]. In brief, since a validated high-sensitivity D-dimer assay was not available in our hospital at the time of enrollment, MDCT or V/Q lung scanning (in the presence of allergy to intravenous contrast agents or renal insufficiency) was done on all patients. A lower-limb venous compression ultrasonography (US) was done when MDCT or V/Q lung scanning showed no definite results for the diagnosis of PE, and a contrast pulmonary angiography was performed only in patients with inconclusive noninvasive workup.
PE was ruled out if: a negative result on MDCT along with a low or moderate clinical PTP according to Wells score; or normal V/Q lung scanning was found; or normal contrast pulmonary angiography; or low clinical PTP according to Wells score and V/Q lug scanning inconclusive with lower-limb US negative for DVT. Patients with PE ruled out did not receive anticoagulation, and were followed up over a three-month period. PE was confirmed if: a MDCT showing thrombi; or a high probability V/Q lung scanning and high clinical PTP; or inconclusive (low or moderate) V/Q lung scanning and moderate/high clinical PTP with DVT thrombosis shown by venous compression US of lower limbs; or a contrast pulmonary angiography showing thrombi; or presence of pulmonary emboli at necropsy.
Follow-up
All patients without PE and their respective general practitioners were contacted by phone by attending physicians to assess signs or symptoms suggestive of recurrent thromboembolic disease during the follow-up period or initiation of anticoagulant therapy for any reason.
Clinical charts were reviewed in case of hospital readmissions to assess the proportion of patients in whom thromboembolic events or death related to this condition occurred within 3 months after hospital discharge. Diagnostic failure was considered if fatal or nonfatal events related to venous thromboembolic disease were found during the follow-up period. Deaths were classified as caused by PE in case of an objective imaging test positive for PE prior to death, confirmation by autopsy, or if PE could not be confidently excluded as the cause of death.
Diagnostic tests
The techniques for performing MDCT, V/Q lung scanning and contrast pulmonary angiography have been described elsewhere [11]. A MDCT was positive for pulmonary embolism if contrast material outlined a central intraluminal defect or if a vessel was totally occluded in at least two different projections. A V/Q lung scanning was classified as normal, low, intermediate or high probability according to the Prospective Investigation of Pulmonary Embolism Diagnosis (PIOPED) study [19]. Lower-limb B-mode venous compression US was performed by trained staff. The examination consisted of a real-time B-mode examination of the common femoral and popliteal veins. The criterion for diagnosing deep-vein thrombosis (DVT) was incomplete compressibility of the vein [20].
Contrast pulmonary angiography was interpreted by a specialist in vascular radiology and the following criteria were considered diagnostic of PE: presence of repletion defects or sharp termination of one or more arteries greater than 2.5 mm in diameter. In case of doubt, a second experienced radiologist was asked for his or her opinion and the diagnosis was made by means of consensus.
Plasma samples
A blood sample to measure D-dimer levels was obtained by clean venipuncture immediately at enrollment. Blood samples were collected in plastic tubes containing 3.8 % trisodium citrate (9:1, vol:vol). Specimens were centrifuged at 3000×g during 15 min to obtain platelet poor plasma, which was aliquoted and stored at −70º C. The technician performing the analysis was unaware of the final diagnosis for each patient. At the end of study, the D-dimer ELISA assay (VIDAS, bioMérieux, Marcy l’Etoile, France) was performed.
Statistical analysis
Baseline characteristics among the 2 groups of patients (with PE and without PE) were compared using the Student’s t test for variables with a parametric distribution, the Mann–Whitney test for variables with a nonparametric distribution and Chi-square test or Fisher exact test for binary variables. For all analyses, a two-tailed P value of less than 0.05 was considered to indicate statistical significance.
For D-dimer, we used two cutoff values: a conventional cutoff value of 500 ng/mL (CDD) and an age-adjusted D-dimer cutoff (AADD = patient’s age × 10 ng/mL), if age >50 years [14]. Diagnostic performances were assessed by diagnostic indexes from 2 × 2 contingency table analyses. Sensitivity, specificity, negative predictive value (NPV), positive predictive value (PPV) and clinical usefulness (defined as the proportion of true-negative tests among all patients with suspected PE) were calculated along with their corresponding 95 % confidence interval, for CDD alone, and for both CDD and AADD when patient’s age >50 years and CDD >500 ng/mL. These values were also calculated in the subgroup of patients with “low/moderate” clinical PTP of having PE according to the Wells score, in which VIDAS D-dimer test has been approved in clinical practice to rule out PE [2, 7, 21]. Analyses were done using SPSS software 17 (SPSS, Inc., Chicago, Illinois).
Results
Three hundred and eighty five consecutive outpatients with suspected PE who were evaluated at the ED of our hospital were included in this study. Twenty one patients (5 %) were excluded from the study due to lack of consent to be included in the study (9 patients), or unavailability to perform D-dimer test (12 patients). Therefore, 362 patients were included in the analysis with an age of 60 ± 19 years (mean ± SD; range 21–82 years). PE was diagnosed in 98 patients (27 %), and ruled out in the remaining 264 patients (73 %). None of these patients had thromboembolic events during the three-month period of follow-up (0 %, 95 % CI 0–1.4 %). No patient was lost to follow-up. Figure 1 shows the diagnostic strategy used in this study.
Most of demographic and clinical baseline characteristics were similar among patients with and without PE (Table 1). Patients with PE have a higher frequency of surgery in the preceding 4 weeks or recent immobilization, when compared with subjects without this condition (22.2 vs. 12.6 %). From a total sample of 362 patients, 291 were >50 years old (PE was confirmed in 81 patients, being ruled out in the remaining 210 subjects) (Table 1).
Table 2 shows 2 × 2 contingency table (Table 2A) and sensitivity, specificity, NPV, PPV and clinical usefulness (Table 2B) for both CDD and AADD in patients with clinically suspected PE. Two patients with high PTP according to Wells criteria showed false-negative values for CDD and AADD (Table 2A). Clinical usefulness for CDD is 25.7 % (93/362; 95 % CI 21–30 %), and 33.4 % for AADD (121/362; 95 % CI 28–38 %). Therefore, clinical usefulness of AADD results in a 7.7 % absolute increase when compared with that of CDD.
Table 3 shows 2 × 2 contingency table (Table 3A), sensitivity, specificity, NPV, PPV and clinical usefulness (Table 3B) for both CDD and AADD in 331 patients with low or moderate PTP according to Wells criteria. No false-negative cases were observed in this subset of patients for both CDD and AADD. Clinical usefulness is 27.8 % (78/331; 95 % CI 23.2–32.8 %) for CDD, and 36.5 % (121/331; 95 % CI 31.5–41.8 %) for AADD. Therefore, the use of AADD shows an 8.7 % absolute increment in clinical usefulness when compared with CDD. In 29 patients aged >50 years with CDD >500 ng/mL, AADD shows values under its calculated cutoff point, without false negatives for the diagnosis of EP (0 %, 95 % CI 0–11 %).
Discussion
In the present study, AADD shows a higher clinical usefulness for ruling out PE compared with CDD (33.7 vs. 25.7 %) when applied to patients with any grade of clinical probability of PE. Therefore, our results show that the use of AADD results in an 8 % absolute increase compared to the clinical usefulness of CDD in patients in whom PE is clinically suspected. However, it should be noted that D-dimer testing is not suitable for ruling out PE in patients with high PTP, only being approved for the exclusion of this condition in patients with low or moderate PTP [2, 7, 21]. In our local setting, CDD and AADD show a clinical usefulness of 27.8 and 36.5 %, respectively, in the subset of patients with low or moderate PTP for the exclusion of PE. Again, the use of AADD results in an 8.7 % increase in the clinical usefulness within this subset of patients. The most relevant studies in Europe dealing with AADD in patients of any age such as that of Douma et al. [14], comprising three cohorts, as well as the validation prospective study reported by Righini et al. [15], show a clinical usefulness with the use of AADD of 42, 51, 40 and 39.8 %, respectively, among patients with non-high PTP of PE.
These reports apparently show a slightly higher clinical usefulness compared with that observed in our study in patients with low or moderate PTP of PE. These differences are probably related to the model used to categorize groups of patients with non-high PTP of PE, which differs from ours. In our case, we use the 3-category Wells score (low, moderate, and high PE probability), while both Douma and Righini use the two-level Wells score in a high proportion of patients (PE likely or PE unlikely), which shows a lower prevalence of PE and positive D-dimer results [22]. If clinical usefulness of AADD were calculated in the studies by Douma and Righini in all patients with suspected PE, the following result would have been found: 32.5 % (560/1721 patients) in the derivation cohort by Douma [14], 33 % (1093/3306 patients) in the validation cohort 1 by Douma [14], 36.5 % (663/1812 patients) in the validation cohort 2 by Douma [14], and 34.7 % (1154/3324 patients) in the study by Righini [15]. These results are similar to the findings in our study showing a clinical utility for AADD of 33.4 %. As above mentioned, the absolute increase in the clinical usefulness of AADD with respect to CDD is about 8–9 %, similar to that reported in the studies by Douma and Righini (range 5.15–11.1 %) [14, 15].
An interesting finding is the safety analysis when using AADD. The use of AADD implies increasing the conventional D-dimer cutoff point (500 ng/mL) in patients aged >50, which might result in false negatives and a lower sensitivity of the test compromising its safety to exclude PE. The most relevant studies with a large sample size such as the aforementioned by Douma et al. [14], and Righini et al. [15], as well as a recent report by Woller et al. [16], find lower false-negative rates when AADD is used (0.6, 0.3, and 1.5 %, respectively); these authors point out the fact that AADD appears to be safe when it is used in patients with non-high PTP of PE. In our study, AADD ruled out PE in 29 patients aged >50 in whom CDD was >500 ng/ml, with no false-negative results (0 %; 95 % CI 0–11.7 %). This result implies that AADD probably represents a safe test, but the range of the confidence interval does not allow one to draw firm conclusions in this regard.
In our study, the prevalence of PE is 27 %, higher than that reported in a cohort from the United States of America by Penaloza (5.1 %) [22] and Woller (10.6 %) [16], but in line with those observed in European cohorts by Douma (24 %) [14], Righini (19 %) [15], Penaloza (28 %) [23], and Jaffrelot (31 %) [24].
A major strength in our study is the strict diagnostic protocol used in suspected cases of PE, as every patient underwent imaging tests to confirm or exclude this condition, and was closely followed up for more than 3 months. The main limitations of our study are: (1) although the cohort included consecutive patients with suspected PE and samples were collected at the moment when clinical suspicion was considered, D-dimer test results were obtained retrospectively at the end of the study; (2) the study is limited to only one Spanish center; (3) in regard to the safety of AADD, although no false-negative result was observed, we could not draw firm conclusions as previously mentioned due to a relatively wide 95 % CI.
In conclusion, AADD notably increases the clinical usefulness of CDD in patients with suspected PE in our local setting without losing sensitivity. The use of AADD does not appear to diminish the safety of CDD, though we could not draw firm conclusions in this regard.
References
Squizzato A, Luciani D, Rubboli A, Di Gennaro L, Landolfi R, De Luca C, Porro F, Moia M, Testa S, Imberti D, Bertolini G (2013) Differential diagnosis of pulmonary embolism in outpatients with non-specific cardiopulmonary symptoms. Intern Emerg Med 8(8):695–702
Stein PD, Woodard PK, Weg JG, Wakefield TW, Tapson VF, Sostman HD, Sos TA, Quinn DA, Leeper KV Jr, Hull RD, Hales CA, Gottschalk A, Goodman LR, Fowler SE, Buckley JD (2006) Diagnostic pathways in acute pulmonary embolism: recommendations of the PIOPED II investigators. Am J Med 119(12):1048–1055
Moores LK, King CS, Holley AB (2011) Current approach to the diagnosis of acute nonmassive pulmonary embolism. Chest 140(2):509–518
van Belle A, Büller HR, Huisman MV, Huisman PM, Kaasjager K, Kamphuisen PW, Kramer MH, Kruip MJ, Kwakkel-van Erp JM, Leebeek FW, Nijkeuter M, Prins MH, Sohne M, Tick LW (2006) Christopher Study Investigators. Effectiveness of managing suspected pulmonary embolism using an algorithm combining clinical probability, D-dimer testing, and computed tomography. JAMA 295(2):172–179
Stein PD, Hull RD, Patel KC, Olson RE, Ghali WA, Brant R, Biel RK, Bharadia V, Kalra NK (2004) D-dimer for the exclusion of acute venous thrombosis and pulmonary embolism: a systematic review. Ann Intern Med 140(8):589–602
Carrier M, Righini M, Djurabi RK, Huisman MV, Perrier A, Wells PS, Rodger M, Wuillemin WA, Le Gal G (2009) VIDAS D-dimer in combination with clinical pre-test probability to rule out pulmonary embolism. A systematic review of management outcome studies. Thromb Haemost 101(5):886–892
Di Nisio M, Squizzato A, Rutjes AW, Büller HR, Zwinderman AH, Bossuyt PM (2007) Diagnostic accuracy of D-dimer test for exclusion of venous thromboembolism: a systematic review. J Thromb Haemost 5(2):296–304
Kabrhel C, Mark Courtney D, Camargo CA Jr, Plewa MC, Nordenholz KE, Moore CL, Richman PB, Smithline HA, Beam DM, Kline JA (2010) Factors associated with positive D-dimer results in patients evaluated for pulmonary embolism. Acad Emerg Med 17(6):589–597
Heit JA, Minor TA, Andrews JC, Larson DR, Li H, Nichols WL (1999) Determinants of plasma fibrin D-dimer sensitivity for acute pulmonary embolism as defined by pulmonary angiography. Arch Pathol Lab Med 123(3):235–240
Perrier A, Roy PM, Aujesky D, Chagnon I, Howarth N, Gourdier AL, Leftheriotis G, Barghouth G, Cornuz J, Hayoz D, Bounameaux H (2004) Diagnosing pulmonary embolism in outpatients with clinical assessment D-dimer measurement, venous ultrasound, and helicoidal computed tomography: a multicenter study. Am J Med 116(5):291–299
Flores J, García-Avello A, Alonso E, Ruíz A, Navarrete O, Álvarez C, Lozano C, Arribas I (2014) Tissue plasminogen activator as a novel diagnostic aid in acute pulmonary embolism. Vasa 43(6):450–458. doi:10.1024/0301-1526/a000392
Righini M, Goehring C, Bounameaux H, Perrier A (2000) Effects of age on the performance of common diagnostic tests for pulmonary embolism. Am J Med 109(5):357–361
Righini M, Nendaz M, Le Gal G, Bounameaux H, Perrier A (2007) Influence of age on the costeffectiveness of diagnostic strategies for suspected pulmonary embolism. J Thromb Haemost 5(9):1869–1877
Douma RA, le Gal G, Söhne M, Righini M, Kamphuisen PW, Perrier A, Kruip MJ, Bounameaux H, Büller HR, Roy PM (2010) Potential of an age adjusted D-dimer cut-off value to improve the exclusion of pulmonary embolism in older patients: a retrospective analysis of three large cohorts. BMJ 340:c1475
Righini M, Van Es J, Den Exter PL, Roy PM, Verschuren F, Ghuysen A et al (2014) Age-adjusted D-dimer cutoff levels to rule out pulmonary embolism: the ADJUST-PE study. JAMA 311(16):1117–1124
Woller SC, Stevens SM, Adams DM, Evans RS, Lloyd JF, Snow GL, Bledsoe JR, Gay DZ, Patten RM, Aston VT, Elliott CG (2014) Assessment of the safety and efficiency of using an age-adjusted D-dimer threshold to exclude suspected pulmonary embolism. Chest 146(6):1444–1451
Schouten HJ, Geersing GJ, Koek HL, Zuithoff NP, Janssen KJ, Douma RA, van Delden JJ, Moons KG, Reitsma JB (2013) Diagnostic accuracy of conventional or age adjusted D-dimer cut-off values in older patients with suspected venous thromboembolism: systematic review and meta-analysis. BMJ 346:f2492
Wells PS, Ginsberg JS, Anderson DR, Kearon C, Gent M, Turpie AG, Bormanis J, Weitz J, Chamberlain M, Bowie D, Barnes D, Hirsh J (1998) Use of a clinical model for safe management of patients with suspected pulmonary embolism. Ann Intern Med 129(12):997–1005
Investigators PIOPED (1990) Value of the ventilation/perfusión scan in acute pulmonary embolism: results of the prospective investigation of pulmonary embolism diagnosis (PIOPED). JAMA 263(20):2753–2759
Lensing AW, Prandoni P, Brandjes D, Huisman PM, Vigo M, Tomasella G, Krekt J, Wouter Ten Cate J, Huisman MV, Büller HR (1989) Detection of deep-vein thrombosis by real-time B-mode ultrasonography. N Engl J Med 320(6):342–345
Kruip MJ, Slob MJ, Schijen JH, van der Heul C, Buller HR (2002) Use of a clinical decision rule in combination with D-dimer concentration in diagnostic workup of patients with suspected pulmonary embolism: a prospective management study. Arch Intern Med 162(14):1631–1635
Ceriani E, Combescure C, Le Gal G, Nendaz M, Perneger T, Bounameaux H, Perrier A, Righini M (2010) Clinical prediction rules for pulmonary embolism: a systematic review and meta-analysis. J Thromb Haemost 8(5):957–970
Penaloza A, Roy PM, Kline J, Verschuren F, LE Gal G, Quentin-Georget S, Delvau N, Thys F (2012) Performance of age-adjusted D-dimer cut-off to rule out pulmonary embolism. J Thromb Haemost 10(7):1291–1296
Jaffrelot M, Le Ven F, Le Roux PY, Tissot V, Rame E, Salaun PY, Le Gal G (2012) External validation of a D-dimer age-adjusted cut-off for the exclusion of pulmonary embolism. Thromb Haemost 107(5):1005–1007
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study has been funded in part by the Research Foundation of Hospital Príncipe de Asturias FIB-PI/1010061.
Conflict of interest
The authors declare that they have no conflict of interest.
Statement of human and animal rights
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Written informed consent forms were obtained from all patients.
Rights and permissions
About this article
Cite this article
Flores, J., García de Tena, J., Galipienzo, J. et al. Clinical usefulness and safety of an age-adjusted D-dimer cutoff levels to exclude pulmonary embolism: a retrospective analysis. Intern Emerg Med 11, 69–75 (2016). https://doi.org/10.1007/s11739-015-1306-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11739-015-1306-5