Abstract
Weeds are one of the biotic factors that cause crop productivity losses worldwide. Due to the consequences to human health and the environment of the indiscriminate use of synthetic herbicides, alternative methods involving the use of the allelopathy phenomenon have been gaining prominence. Here, we explore the allelopathic effect of Jatropha gossypiifolia L. on the weed Bidens bipinnata L. and investigate its potential herbicidal allelochemicals. In vitro bioassays demonstrated that the use of J. gossypiifolia leaf powder was able to inhibit seed germination and early growth of B. bipinnata seedlings, obtaining significant reductions with increasing concentration. Bioguided fractionation of the aqueous extract indicated that the hexane and ethyl acetate fractions were bioactive in inhibiting weed growth. Metabolomics based on mass spectrometry and molecular networks was used to annotate the allelochemicals of the bioactive fractions, generating the dereplication of metabolites from the classes of alkaloids, phenolics, fatty acids, steroids, and terpenoids, which may be associated with herbicidal activity. These results point to the allelopathic effect of the J. gossypiifolia leaf powder and its putative herbicide allelochemicals, providing subsidies for future studies on the application of this species in alternative weed management strategies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Weeds are defined as any plant that grows in places unwanted by humans (Brighenti and Oliveira 2011), and their infestation constitutes a challenge in crop management worldwide. They often undermine agricultural productivity by competing for primary resources such as light, water, nutrients, and space. The estimated losses caused by weeds can reach approximately 34% of agricultural productivity, causing substantial financial losses (Singh et al. 2022).
Weed control is an important factor in ensuring productivity and quality in food production. Chemical control is the main method used (Pannacci et al. 2017); however, its indiscriminate use is associated with problems such as the development of herbicide-resistant species, destruction of natural enemies, destruction of native flora and fauna, and problems related to human health (Bhushan and Pathma 2021). The introduction of efficient and sustainable strategies has become a challenge in agriculture.
Plant species with allelopathic activity represent an alternative for the sustainable management of weeds, and their importance in agriculture is increasingly recognized (Pannacci et al. 2017; Macías et al. 2019). Allelopathy is the inhibitory or stimulating effect of one plant on another through the release of allelochemicals into the environment (Rice 1984). These compounds are present in all plant tissues and belong to the classes of phenolics, terpenoids, and alkaloids (Rice 1984; Latif et al. 2017). They can interfere with plant germination and growth, alter cell membranes and stomatal conductance, affect cell division, photosynthesis, respiration, protein synthesis, and enzymatic activities, and reduce mineral absorption (Jabran 2017; Silva and Mendes 2022).
Recent studies have demonstrated the allelopathic capacity of Jatropha gossypiifolia L. (Euphorbiaceae) (Lopes et al. 2022; Panda et al. 2020). This species is native to South and Central America with a pantropical distribution (Wu et al. 2019). Bioactive metabolites belonging to the classes of phenolic compounds, alkaloids, terpenoids, lignoids, and steroids have been reported in the stem, leaf, root, bark, seed, and latex (Wu et al. 2019; Silveira et al. 2020). Its wide distribution and diverse composition of bioactive metabolites suggest that this species may be useful for application in integrated weed management systems.
Aqueous extracts of J. gossypiifolia inhibit the seed germination and seedling growth of Bidens bipinnata L. (Asteraceae) under laboratory conditions and cause phytotoxicity in plants under greenhouse conditions (Lopes et al. 2022). B. bipinnata is a weed that adapts to both wet and dry situations (Xu and Deng 2017), causing productivity losses in cultivation areas by competing with different crops (Meissner and Beyers 1986), in addition to serving as a host for diseases (Miléo et al. 2007). Information on the allelopathic ability of J. gossypiifolia on B. bipinnata is still limited, such as the direct use of leaf powder and the identification of herbicidal allelochemicals, encouraging further investigation for weed control purposes.
The allelopathic potential of using powders from different parts of plants to inhibit the germination and early growth of other plant species has been widely demonstrated. Melissa officinalis L. shoot powder inhibited the germination and growth of roots and shoots of Amaranthus caudatus L., Digitaria sanguinalis L., and Lactuca sativa L. cv. Grand Rapids (Kato-Noguchi 2003). Brassicaceae plants’ seed powder was used to control Orobanche crenata Forsk. infestation of Pisum sativum L. (Ahmed et al. 2020). Pea seed powder acts as a natural herbicide for controlling weed-infested wheat plants (El-Rokiek et al. 2019). Artemisia argyi H.Lév. & Vaniot leaf powder exhibited a significant inhibitory effect on the germination and growth of weed seeds in pot and field experiments (Li et al. 2021). To the best of our knowledge, this is the first report on the assessment of the allelopathic effect of J. gossypiifolia leaf powder on a weed.
Thus, in this study, we explored the allelopathic effect of J. gossypiifolia leaf powder on B. bipinnata and investigated the potential allelopathic substances. First, in vitro bioassays with direct use of J. gossypiifolia leaf powder were carried out to evaluate its effect on the seed germination and early growth of B. bipinnata. Bioguided fractionation of the aqueous extract from J. gossypiifolia leaves was developed to determine biologically active fractions, which were characterized by metabolomics based on mass spectrometry and molecular networking.
Materials and methods
Plant material
Leaves of the aerial parts of J. gossypiifolia (in the adult stage with flowers and fruits) and B. bipinnata seeds were collected in the morning in a native area in the region of Bom Jesus, PI, Brazil (9°04′26.6″S 44°20′31.1″W and 9°04′56.8″ S 44°19′41.8″W, respectively). Exsiccates were deposited at the Graziela Barroso Herbarium (Federal University of Piauí, Teresina, PI, Brazil), under registration numbers TEPB 32,521 (B. bipinnata) and TEPB 32,525 (J. gossypiifolia), and the plants were registered by n° A0DB8D9 and AE123A5 in the National System for the Management of Genetic Heritage and Associated Traditional Knowledge (SisGen) (Lopes et al. 2022). The leaves were washed in running water and dried in an oven with forced air circulation at 45 °C for 72 h. After drying, the material was crushed in a knife mill and sieved through a 250 µm mesh sieve.
In vitro bioassays of seed germination and seedling growth
For the seed germination bioassay, pulverized leaves of J. gossypiifolia were mixed with sterilized quartz sand (25 g) in Petri dishes (diameter = 9 cm) and subsequently moistened with 10 mL of distilled water (Kato-Noguchi 2003). The powder concentrations used were 0, 10, 30, 100, and 300 mg per Petri dish. Then, thirty seeds of B. bipinnata, previously sterilized in sodium hypochlorite solution (2% w/v) for 15 min and washed four times with distilled water, were distributed on quartz sand in each Petri dish. The plates were placed in a germination chamber at 25 °C with a 12 h photoperiod for 7 days. The number of germinated seeds was counted daily for 7 days, with germinated seeds being those with a primary root protrusion ≥ 2.00 mm in length. The length of the primary root and hypocotyl was measured from the base of the stem to the tip of the root and from the stem to the plumular hook, respectively, using a digital calliper.
The evaluated variables were germination percentage (PG), germination speed index (GSI), and allelopathic effect response index (RI), according to their respective equations. PG = (N/A) × 100, where N = number of germinated seeds and A = total number of seeds. GSI = (G1/N1 + G2/N2…Gn/Nn), where G1, G2, and Gn corresponded to the number of seeds germinated in the first, second, and last counts, respectively, and N1, N2, and Nn were the number of days elapsed until the last count (Maguire 1962). RI = 1 − C/T (when T ≥ C) and RI = T/C − 1 (when T < C), where C is the control germination speed and T is the treatment germination speed; RI > 0 represents a stimulatory effect, RI < 0 represents an inhibitory effect, and the absolute value is consistent with the allelopathy intensity (Williamson and Richardson 1988).
For the seedling growth bioassay, B. bipinnata seeds were germinated on filter paper in a germination chamber at 25 °C and a 12 h photoperiod for 4 days. Then, 10 germinated seeds were separated and distributed over sterilized quartz sand (25 g) mixed with pulverized leaves of J. gossypiifolia in Petri dishes (diameter = 9 cm) moistened with 10 mL of distilled water. The plates were incubated in a germination chamber at 25 °C and a 12 h photoperiod for another 3 days, totaling 7 days of the experiment. Afterwards, the hypocotyl and radicle lengths were measured with the aid of a digital calliper.
The bioassays were conducted in a completely randomized design (CRD) with 4 replications. The Shapiro‒Wilk test was performed to test the normality of the data, which were subsequently submitted to analysis of variance (ANOVA) using SISVAR 5.6 software (Ferreira 2019), followed by regression analysis in SigmaPlot v12.0 (Systat Software Inc. Chicago, USA). The statistical significance criterion was p < 0.05.
Bioguided fractionation of aqueous plant extract
Powdered J. gossypiifolia leaves (192.56 g) were macerated with water three times for 6 days at room temperature (no growth of microorganisms in the aqueous extract was detected by visual and olfactory observations). The filtrates were combined and partitioned with hexane and ethyl acetate solvents in increasing order of polarity, obtaining hexane (HF: 0.1623 g), ethyl acetate (AF: 0.2719 g), and residual aqueous fractions (RF: 0.9558 g). The biological activity of the fractions was determined using the B. bipinnata growth bioassay.
For this purpose, HF, AF, and RF samples were resuspended in a 1% dimethylsulfoxide (DMSO) solution, and 1.5 mL was pipetted into Petri dishes (diameter = 5 cm) containing a filter paper disk, in which 10 B. bipinnata seeds previously germinated for 4 days at 25 °C and a 12 h photoperiod were deposited. The concentrations used for each treatment with the different fractions were 0.00, 0.63, 1.25, 2.50, and 5.00 mg mL−1. Hypocotyl and radicle lengths were measured after 7 days of incubation using a digital calliper. The bioassay was conducted in CRD with 4 replications in a 3 × 4 + 1 factorial. The first factor was the fraction type, and the second factor was the concentration. The data were submitted to ANOVA and regression analysis in SISVAR 5.6 software (Ferreira 2019).
Characterization of bioactive fractions by untargeted metabolomic analysis
Sample preparation
The bioactive samples, HF and AF, were resuspended in 1 mL of acetonitrile, vortexed (30 s), sonicated (10 min), and centrifuged for 10 min at 4 °C and 12,000 rpm. Then, 800 μL of the supernatant was removed, dried in N2, and resuspended in the mobile phase of the chromatographic system proportionally to the initial weight.
Liquid chromatography coupled to high resolution mass spectrometry (LC–HRMS) analysis
All samples were analysed in an Acquity H-Class liquid chromatography system (Waters®, Manchester, United Kingdom) coupled to a Xevo QTOF G2-XS mass spectrometer (Waters®, Manchester, United Kingdom) using an ACQUITY UPLC® CSH™ C18 column (2.1 × 100 mm, 1.7 µm). The analyses were adapted from Anhesine et al. (2019). The column temperature was set at 30 °C, and the mobile phase flow rate was maintained at 0.4 mL min−1. Mobile phase A was 0.1% formic acid aqueous solution, and phase B was 0.1% acetonitrile/formic acid. Gradient conditions were 0–18 min, 35% of B; 18–26 min, 100% of B; 26–30 min, 100% of B; 30.0–30.1 min, returned to initial condition 5% of B and remained until the end of the analysis for 6 min, totalling 36 min of running.
Positive (+) and negative (−) ion modes were recorded separately. The injection volume was 1 μL for both modes, and the instrument was operated in MSE mode in the m/z range of 50–1200, with an acquisition time of 0.5 s per scan. Other parameters were as follows: source temperature = 140 °C, desolvation temperature = 550 °C, desolvation gas flow = 900 L h−1, capillary voltage = 3.5 kV (+)/2.5 kV (−), cone voltage = 40 V. MSE analysis was operated at 6 V for low collision energy and a ramp of 20–50 V for high collision energy. Leucine enkephalin (molecular weight = 555.62; 200 pg μL−1) was used as a lock mass for accurate mass measurement. Analyses were performed in triplicate.
Feature-based molecular networking (FBMN)
Raw LC–HRMS data were imported into Progenesis QI® v2.0 software (Nonlinear Dynamics, Newcastle, UK) for peak alignment, pick picking, and deconvolution. The feature quantification table (CSV file) and the MS/MS spectral summary (MSP file) were exported and uploaded to the GNPS platform (Global Natural Products Social Molecular Network, https://gnps.ucsd.edu, Wang et al. 2016) using WinSCP (https://winscp.net) to generate an FBMN (Nothias et al. 2020) to investigate the metabolic profile of the dataset.
The data were filtered by removing all MS/MS fragment ions within ± 17 Da of the precursor m/z. MS/MS spectra were window filtered by choosing only the top 6 fragment ions in the ± 50 Da window throughout the spectrum. The precursor ion mass tolerance was set to 0.02 Da and the MS/MS fragment ion tolerance to 0.02 Da. A molecular network was then created where edges were filtered to have a cosine score above 0.7 and more than 4 matched peaks. Furthermore, edges between two nodes were kept in the network if and only if each of the nodes appeared in each other’s respective top 10 most similar nodes. Finally, the maximum size of a molecular family was set to 100, and the lowest scoring edges were removed from molecular families until the molecular family size was below this threshold.
The spectra in the network were then searched against GNPS spectral libraries (Wang et al. 2016; Horai et al. 2010). The library spectra were filtered in the same manner as the input data. All matches kept between network spectra and library spectra were required to have a score above 0.7 and at least 4 matched peaks. The molecular networks were visualized using Cytoscape software (Shannon et al. 2003). The FBMN results on the GNPS platform for the positive mode can be accessed at https://gnps.ucsd.edu/ProteoSAFe/status.jsp?task=a93b12ab83dc4188b878adb0cfc2d13e and for the negative mode at https://gnps.ucsd.edu/ProteoSAFe/status.jsp?task=d46fac0722e54e1aaa2b206ac0d17b52.
Molecular networks were visualized using Cytoscape software version 3.9.1 (Cytoscape Consortium, San Diego, CA, United States; Shannon et al. 2003). Nodes correspond to ion features, while edges between nodes represent calculated MS/MS cosine scores. The nodes were represented as pie charts, whose proportion corresponds to the feature abundance (peak area) in the samples (Nothias et al. 2020). The node size was dimensioned in relation to the sum of the peak areas obtained in the samples in which the feature was detected. Compounds with the same MS/MS spectrum but with different retention times were represented as separate nodes, indicating isomers (Mannochio-Russo et al. 2022).
Results and discussion
Effect of J. gossypiifolia leaf powder on the germination and initial growth of B. bipinnata
Seed germination and seedling growth of B. bipinnata were investigated after treatment with J. gossypiifolia leaf powder (Table S1) to deepen studies on the allelopathic effects of this plant. There was a decline in the germination percentage of B. bipinnata seeds with increasing doses of J. gossypiifolia leaf powder, which differed significantly from each other (p = 0.0001) (Table S2). Significant differences between concentrations (p = 0.0005) (Table S2) were also verified for the GSI data. The dose‒response curves for both parameters were fitted to a cumulative Gaussian distribution function (Fig. 1A and B), reaching 100% inhibition at the highest concentration of 300 mg per Petri dish.
Regarding the allelopathic effect response index (RI), there was a significant difference between the doses (p < 0.0000) (Table S2). The RI indicates a stimulatory effect on germination when its values are positive in relation to the control treatment, while negative values indicate inhibition (Williamson and Richardson 1988). The RI dose‒response curve was fitted to a modified Gaussian function (Fig. 1C) and showed an inhibitory effect for all evaluated concentrations of J. gossypiifolia leaf powder on the germination of B. bipinnata, with the maximum efficiency achieved with 300 mg per Petri dish. B. bipinnata seedling growth was measured by hypocotyl and radicle lengths (Table S1), for which there were significant differences between concentrations (p = 0.0154 and 0.0000, respectively) (Table S2). The dose‒response curves of these parameters were fitted to a logistic regression model (Fig. 1D and E), showing greater inhibition at 100 and 300 mg per Petri dish.
Panda et al. (2020) demonstrated that aqueous extracts of J. gossypiifolia leaves affected the germination and growth of Cicer arietinum L., as well as the water and chlorophyll contents, under laboratory conditions. Lopes et al. (2022) carried out in vitro bioassays and pot experiments to evaluate the allelopathic and phytotoxic effects of the aqueous extract from J. gossypiifolia leaves on B. bipinnata and showed an inhibitory effect on germination and initial growth. Flavonoids, phenolic acids, and terpenoids were detected in J. gossypiifolia aqueous extracts using attenuated total reflectance Fourier transform mid-infrared (ATR FT-MIR) spectroscopy and were suggested by the authors as possible herbicidal allelochemicals involved in the allelopathic mechanism.
Aqueous extracts of the leaves and stems of J. gossypiifolia and J. curcas completely inhibited the germination of Striga hermonthicca (Del.) Benth (Sawadogo/Ilboud et al. 2022). The aqueous methanol extract of J. curcas leaves showed an inhibitory effect on the germination of cress and the seedling growth of lettuce and alfalfa (Islam and Kato-Noguchi 2013). J. curcas leaf extract also inhibited the germination of wheat, mustard, sesame, black gram and other crops (Abugre and Sam 2010; Ma et al. 2011; Rejila and Vijayakumar 2011; Venkatesh et al. 2011; Tomar and Agarwal 2013). Mahmoud et al. (2016) demonstrated that an allelopathic effect of J. curcas on wheat was limited to intact green leaves, while dry plant parts amended in soils improved wheat grain yield in pot experiments.
Bioactive fractions of J. gossypiifolia aqueous extract in inhibiting the growth of B. bipinnata seedlings
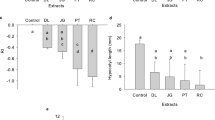
Seedling growth bioassays were performed using the hexane, ethyl acetate, and aqueous residual fractions obtained from the fractionation of the J. gossypiifolia aqueous extract to identify the fraction with an allelopathic inhibitory effect on the growth of B. bipinnata seedlings. For the hypocotyl length variable, there were significant differences between the treatments with the different types of fractions (p = 0.0000) (Table S3) and between the different concentrations evaluated (p = 0.0079), with interaction between the two factors (p = 0.0000) and differences from control treatments (p = 0.0000).
The unfolding of the interaction effect, in which the levels of one factor are compared within each level of the other factor, showed significant differences between the concentrations only for the HF treatment (p = 0.0000, Table S3), whose curve dose‒response was fitted to a cumulative Gaussian distribution function (Table 1). Therefore, treatments with AF (p = 0.0556) and RF (p = 0.1542) showed a nonvariable effect between the evaluated doses (0.63; 1.25; 2.50, and 5.00 mg mL−1). However, all treatments with different concentrations of AF differed significantly in relation to the control treatment, while RF was significantly similar to the control, and HF differed only at 1.25 and 2.50 mg mL−1 (Table 1).
At a concentration of 1.25 mg mL−1, treatments with AF and HF did not differ, showing reductions in hypocotyl length of 10.31 and 12.66 mm, respectively. At 2.5 mg mL−1, HF had a stronger effect in suppressing hypocotyl development, reaching a mean value of 7.84 mm, approximately half the hypocotyl length value for the control treatment with 1% DMSO (15.00 mm), differing from AF, which had an average value of 12.60 mm. AF was, therefore, the bioactive fraction in reducing hypocotyl development at all concentrations evaluated, while HF bioactivity was dose dependent, and RF did not show bioactivity.
Regarding the radicle length, the treatments with the different types of fractions also differed statistically (p = 0.0000) (Table S4), as well as for the fraction concentration factor (p = 0.0002). There was an interaction between the two factors (p = 0.0001), which differed from the control treatments (p = 0.0000). The unfolding of the interaction effect showed significant differences between the concentrations for the HF (p = 0.0000) and RF (p = 0.0014) samples (Table S4), which presented dose‒response curves fitted to the cumulative Gaussian distribution function (Table 2), while for AF, there was no significant difference between the evaluated concentrations.
When evaluating the effect of the different types of fractions within each of the doses, it was observed that all fractions differed significantly from each other at concentrations of 0.63, 1.25, and 2.50 mg mL−1 (Table 2), with AF reaching the lowest mean values of radicle length, followed by FH with an intermediate effect and RF with a lower inhibitory effect. The strongest inhibitory effect of AF was 7.40 mm at 2.50 mg mL−1, with a reduction of more than 50% compared to the control treatments, which were approximately 22 mm. At a concentration of 5.00 mg mL−1, there was no significant difference in FA and FH, which differed from FR, which presented lower performance.
In general, the mechanism of action of allelochemicals in the target plant is related to interference in its vital activities. They affect respiration, photosynthesis, stomatal opening, chlorophyll content, nutrient absorption, inhibition of oxygen uptake by mitochondria, protein synthesis, plasma membrane permeability, cell division and elongation, organic synthesis, hormone balance, and enzymatic activities (Pires and Oliveira 2011). Silva et al. (2022) demonstrated the inhibition of the primary root growth of Euphorbia heterophilla L. by ethyl acetate and hexane fractions obtained from the methanolic extract of the aerial parts of Lonchocarpus cultratus (Vell.) A.M.G. Azevedo & H.C. Lima and observed a possible correlation between flavonoids and allelopathy.
The application of an ethyl acetate extract obtained from Agerantum conyzoides L. leaves completely controlled Amaranthus spinosus L. at a concentration of 20%, similar to 2,4-D (Erida et al. 2021). Ethyl acetate and hexane fractions of the aqueous extract from Cryptomeria japonica (Thunb. Ex L.f.) leaves inhibited the hypocotyl and radicle lengths of Robinia pseudoacacia L., and cryptomeridiol was shown to be the active component in the allelopathic effect (Tanaka et al. 2020). Our results pointed to AF and HF as bioactive fractions of the aqueous extract of J. gossypiifolia leaves in inhibiting the initial growth of B. bipinnata, with stronger bioactivity demonstrated by the FA fraction, suggesting the presence of metabolites with allelopathic herbicidal activity in these fractions.
Metabolite annotation of the bioactive fractions of J. gossypiifolia aqueous extract
The AF and HF fractions of the J. gossypiifolia aqueous extract, which showed inhibitory activity on the growth of B. bipinnata seedlings, were analysed by LC‒MSE in both positive and negative modes (Fig. S1). Preprocessing the dataset resulted in a total of 16,544 and 18,199 mass peaks for the positive and negative modes, respectively. Spectral data were used to generate molecular networks in FBMN mode.
The computed FBMNs revealed putative annotations via an automated library spectral match with the GNPS public spectral libraries, which found a match for 69 out of 1711 spectral nodes in the positive mode and 31 out of 1942 spectral nodes in the negative mode (Fig. S2). However, 53 hits were initially rejected due to excessive mass errors (> 5 ppm). These matches were manually evaluated and compared to the literature, resulting in level 2 annotations according to the Metabolomic Standards Initiative (MSI) (Sumner et al. 2007).
The molecular network uses spectral similarity to group metabolites since similar molecular structures will generate similar fragmentation patterns obtained by tandem mass spectrometry analysis. Molecular families and molecules similar only to themselves are represented by clusters and singlets, respectively. In the cluster, similar molecules (nodes) are connected by edges, whose thickness represents the level of their similarity (Aron et al. 2020).
A total of 30 compounds were annotated by library matching of nodes within and outside families (i.e., clusters or singlets) (Table 3 and Fig. S3), including alkaloids, fatty acids, phenolic compounds, terpenoids and steroids. Flavonoids were the most abundant class of metabolites among the annotated compounds, with representatives of the subclasses of flavone, flavanone, methoxylated flavonol and flavone, and C- and O-glycosylated flavonoid. Representative molecular networks of some flavonoids are shown in Fig. 2.
Molecular networks of some flavonoid subclasses generated from the feature-based molecular networking (FBMN) workflow and annotated based on spectral correspondences in the GNPS platform. Each node represents a tandem mass spectrometry (MS/MS) spectrum, while the edges connecting them represent MS/MS fragmentation similarity (cosine > 0.7). Pie charts indicate the relative abundance of ions in the HF (green) and AF (yellow) fractions. Node sizes are relative to the summed peak areas of the precursor ion
Apigenin 7-O-rhamnoglucoside (15) was found exclusively in AF, while the other flavonoids were present in both extracts; however, they showed a higher concentration in AF, as well as caffeic acid (18) (Table 3). Sinapic acid (23), 2-hydroxy-4-methoxybenzophenone (24), and 2-methoxycinnamaldehyde (25) showed the highest concentrations in HF. The phenolic compounds present exclusively, or mostly, in AF may be associated with its stronger allelopathic effect on B. bipinnata radicle development compared to HF, which showed an intermediate effect. The effect of HF on hypocotyl length inhibition was statistically similar to that of AF depending on the dose.
Phenolic compounds constitute the most important and common class of plant allelochemicals in the ecosystem, with not only physiological functional capacity but also allelopathic potential, interfering with several important plant enzymes and physiological processes (Latif et al. 2017). Phenolic acids are the main allelochemicals, which have a primary effect in sensitive species to reduce hydraulic conductivity and net nutrient uptake by roots and thus eventually growth (Blum 1996). Furthermore, they can inhibit photosynthesis in plants, decrease energy metabolism, and inhibit cell division and root branching (Li et al. 2010). In the field, mixtures of phenolic acids and other organic compounds can inhibit plant growth even at individual concentrations below their inhibitory levels (Blum 1996). Caffeic and synapic acids, found in HF and AF fractions, have previously been reported in numerous plant species with an allelopathic effect (Blum 1996; Bertin et al. 2003; Mahdavikia and Saharkhiz 2015).
Many flavonoids act as strong inhibitors of the germination and growth of weeds and crops, playing important physiological roles in plants and protecting them against biotic stress. Aglycones and flavonoid glycosides are released by root exudation and tissue degradation or leaching and are found in soil solutions and root exudates (Weston and Mathesius 2013). Flavonoids produced by roots play roles in signaling to microbes and other plants, as well as protection against soil pathogens, and their accumulation in roots is highly dependent on biotic and abiotic environmental conditions (Rao 1990). Flavonoids act primarily as regulators of auxin transport and degradation, in addition to showing affinity for many enzymes necessary for mitochondrial respiration in plants and animals (Weston and Mathesius 2013).
Apigenin (14) delayed the germination speed of L. sativa seeds and promoted shoot and root growth inhibition at 3 mmol L−1, which was potentiated with increasing concentration (Liu et al. 2011). Zhang et al. (2017) demonstrated that luteolin (19) and eriodictyol (17), bioactive flavonoids identified in Canadian Conyza L. leachates, significantly decreased seed germination and seedling growth of Agrostis stolonifera L. and L. sativa, respectively. Apigenin-6-C-glycoside (13), isolated from the hydroalcoholic extract of Machaerium eriocarpum Benth. leaves, demonstrated allelopathic inhibitory activity on the germination and growth of sorghum, as well as inhibition of the number of lateral roots in cucumber (Bento et al. 2018). 7-Hydroxy-6,8-dimethoxycoumarin (11) was found in the bark of Ailanthus altissima (Mill.) Swingle (Caramelo et al. 2021) and leaves of aerial parts of the genus Artemisia (Tan et al. 1998; Ivănescu et al. 2021) have been reported to have allelopathic effects. The allelopathic activity of quercetins is also well described in the literature (Golisz et al. 2007; Fernández-Aparicio et al. 2021).
Quinoline (1, 2), indole (3, 4), and pyrrole (6) alkaloids were detected in both bioactive fractions but showed higher concentrations in AF, and pyrrolidine alkaloid (5) was the major alkaloid in HF (Table 3). Representative molecular networks of some alkaloids are shown in Fig. 3. The major presence of most alkaloids in AF may also be associated with the greater inhibitory activity of this fraction on the radicle. Many alkaloids have strong allelopathic effects on weeds and crops as growth inhibitors. Inhibition of seed germination by quinoline alkaloids synthesized by plants has been reported (Aerts et al. 1991). Lovett and Hoult (1995) studied the defense mechanisms of barley (Hordeum spp.) through the release of gramine and hordenine alkaloids from plant roots; these alkaloids have been shown to have allelopathic effects in seedling bioassays. The indole alkaloid gramine in barley behaves as a photophosphorylation uncoupler (Andreo et al. 1984). Alkaloids can inhibit plant growth by several mechanisms, including interference with DNA, enzyme activity, protein biosynthesis, and membrane integrity in developing plants (Latif et al. 2017).
Isomers of the monoterpenes carveol (26 and 27, Table 3) and citronellal (28, 29) were detected in both fractions, but mostly in HF (Fig. 4), as well as steroids (30). Terpenoids perform multiple biological activities in plants, such as acting as hormones, photosynthetic pigments, electron transporters, structural components of membranes, and mediators of polysaccharide assembly, in addition to acting in communication and defense (Kim and Shin 2003). Due to their allelopathic activity, they are considered possible leaders in the development of new agrochemicals based on natural products (Macías et al. 2008, 2019).
Monoterpenes are volatile metabolites that have been described as responsible for allelopathic interactions in several plants. Inhibition of germination has been reported for several monoterpenes in pure form or as mixtures of essential oils. Monoterpenes such as citronellal, citronellol, linalol, and cineol inhibit germination and initial seedling growth of weeds such as Cassia occidentalis L., Amaranthus viridis L., Echinochloa crus-galli L. (P.). Beauv, and B. pilosa under in vitro conditions (Singh et al. 2002, 2004). Singh et al. (2006) demonstrated that citronellal causes severe phytotoxicity in weeds. Registration in the patent bank was found involving the use of citronella oil as an herbicide (Ryan and Morris 1999).
Citronellal isomers detected in HF are, therefore, one of the likely allelochemicals responsible for the inhibitory effect on hypocotyl and radicle development observed for this fraction. Chaimovitsh et al. (2017) demonstrated that (+)-citronellal can promote enantioselective disruption of plant microtubule assembly at high and low dosages, while (−)-citronellal had dose-dependent activity, indicating that these compounds have specific isomeric activity.
Terpenoids, lignoids, and steroids were previously detected in J. gossypiifolia extracts (Wu et al. 2019). Silveira et al. (2020) detected several phenolic acids, triterpenoids, and flavonoids, including apigenin and luteolin, in the hydroalcoholic extract of J. gossypiifolia. Many metabolites still need to be identified in this species, considering the number of molecular networks generated in the FBMN mode. However, among the annotated compounds, many have already been registered in the literature as allelopathic and with herbicidal potential, which explains the effect on the reduction of hypocotyl and radicle lengths of B. bipinnata observed in our studies.
Conclusions
J. gossypiifolia leaf powder demonstrated an allelopathic inhibitory effect on the germination and initial growth of the weed B. bipinnata. Untargeted metabolomic analysis of hexane and ethyl acetate fractions from the J. gossypiifolia aqueous extract, which were bioactive in inhibiting the growth of B. bipinnata seedlings, revealed the presence of several secondary metabolites, including alkaloids, phenolics, and terpenoids, probably associated with allelopathic herbicidal activity.
Considering the search for eco-friendly weed control methods, the present investigation provides subsidies for future studies involving the use of J. gossypiifolia in integrated weed management systems as a strategy to reduce dependence on synthetic chemical herbicides. Assessing the effectiveness of using the powder under field conditions is a fundamental step in this process.
Data availability
All relevant material was included in the manuscript or the Supplementary Material.
References
Abugre S, Sam SJQ (2010) Evaluating the allelopathic effect of Jatropha curcas aqueous extract on germination, radicle and plumule length of crops. Int J Agric Biol 12:769–772
Aerts RJ, Snoeijer W, van der Meijden E, Verpoorte R (1991) Allelopathic inhibition of seed germination by Cinchona alkaloids? Phytochemistry 30:2947–2951. https://doi.org/10.1016/S0031-9422(00)98229-3
Ahmed SEDAEG, Messiha NK, El-Masry RR, El-Dabaa MAT (2020) The dual allelopathic capacity of two Brassicaceae plants’ seed powder in controlling Orobanche crenata infesting Pisum sativum as well as stimulating its growth and yield. Bull Natl Res Cent 44:1–8. https://doi.org/10.1186/s42269-020-0276-6
Andreo CS, Orellano EG, Niemeyer HM (1984) Uncoupling of spinach thylakoids by gramine. Zeitschrift Für Naturforschung C 39:746–748. https://doi.org/10.1515/znc-1984-7-812
Anhesine NB, Bueno PCP, Torres RB, Lopes NP, Cavalheiro AJ (2019) Non-polar and polar chemical profiling of six Casearia species (Salicaceae). Biochem Syst Ecol 87:103954. https://doi.org/10.1016/j.bse.2019.103954
Aron AT, Gentry EC, McPhail KL, Nothias LF, Nothias-Esposito M, Bouslimani A, Dorrestein PC (2020) Reproducible molecular networking of untargeted mass spectrometry data using GNPS. Nat Protoc 15:1954–1991. https://doi.org/10.1038/s41596-020-0317-5
Bento CC, Tangerina MMP, Zanatta AC, Sartori ALB, Franco DM, Hiruma-Lima CA, Vilegas W, Almeida LFR, Sannomiya M (2018) Chemical constituents and allelopathic activity of Machaerium eriocarpum Benth. Nat Prod Res 34:884–888. https://doi.org/10.1080/14786419.2018.1508136
Bertin C, Yang X, Weston LA (2003) The role of root exudates and allelochemicals in the rhizosphere. Plant Soil 256:67–83. https://doi.org/10.1023/A:1026290508166
Bhushan LS, Pathma J (2021) Impact of pesticides on the environment: a global perspective. Plant Cell Biotechnol Mol Biol 22:1–14
Blum U (1996) Allelopathic interactions involving phenolic acids. J Nematol 28:259
Brighenti AM, Oliveira MF (2011) Biologia de plantas daninhas. In: Oliveira RS Jr, Constantin J, Inoue HM (eds) Biologia e manejo de plantas daninhas. Curitiba, Omnipax, pp 1–36
Caramelo D, Pedro SI, Marques H, Simão AY, Rosado T, Barroca C, Gallardo E (2021) Insights into the bioactivities and chemical analysis of Ailanthus Altissima (Mill.) Swingle. Appl Sci 11:11331. https://doi.org/10.3390/app112311331
Chaimovitsh D, Shachter A, Abu-Abied M, Rubin B, Sadot E, Dudai N (2017) Herbicidal activity of monoterpenes is associated with disruption of microtubule functionality and membrane integrity. Weed Sci 65:19–30. https://doi.org/10.1614/WS-D-16-00044.1
El-Rokiek KG, El-Din SAS, El-Wakeel MA, El-Awadi MES, Dawood MG (2019) Allelopathic potential of the pea seed powder as natural herbicide for controlling weeds infested wheat plants. Bull Natl Res Cent 43:1–9. https://doi.org/10.1186/s42269-019-0248-x
Erida G, Saidi N, Hasanuddin H, Syafruddin S (2021) Herbicidal effects of ethyl acetate extracts of billygoat weed (Ageratum conyzoides L.) on spiny amaranth (Amaranthus spinosus L.) growth. Agronomy 54:211–220. https://doi.org/10.3390/agronomy11101991
Fernández-Aparicio M, Masi M, Cimmino A, Vilariño S, Evidente A (2021) Allelopathic effect of quercetin, a flavonoid from Fagopyrum esculentum roots in the radicle growth of Phelipanche ramosa: quercetin natural and semisynthetic analogues were used for a structure-activity relationship investigation. Plants 10:543. https://doi.org/10.3390/plants10030543
Ferreira DF (2019) SISVAR: a computer analysis system to fixed effects split plot type designs. Revista Brasileira De Biometria 37:529–535. https://doi.org/10.28951/rbb.v37i4.450
Golisz A, Lata B, Gawronski SW, Fujii Y (2007) Specific and total activities of the allelochemicals identified in buckwheat. Weed Biol Manage 7:164–171. https://doi.org/10.1111/j.1445-6664.2007.00252.x
Horai H, Arita M, Kanaya S, Nihei Y, Ikeda T, Suwa K, Nishioka T (2010) MassBank: a public repository for sharing mass spectral data for life sciences. J Mass Spec 45:703–714. https://doi.org/10.1002/jms.1777
Islam AKMM, Kato-Noguchi H (2013) Allelopathic prospective of Ricinus communis and Jatropha curcas for bio-control of weeds. Acta Agriculturae Scandinavica, Section B-Soil & Plant Science 63:731–739. https://doi.org/10.1080/09064710.2013.865073
Ivănescu B, Burlec AF, Crivoi F, Roșu C, Corciovă A (2021) Secondary metabolites from Artemisia genus as biopesticides and innovative nano-based application strategies. Molecules 26:3061. https://doi.org/10.3390/molecules26103061
Jabran K (2017) Manipulation of allelopathic crops for weed control. Springer, Cham, pp 65–75. https://doi.org/10.1007/978-3-319-53186-1
Kato-Noguchi H (2003) Assessment of allelopathic potential of shoot powder of lemon balm. Sci Hortic 97:419–423. https://doi.org/10.1016/S0304-4238(02)00159-0
Kim KU, Shin DH (2003) The importance of allelopathy in breeding new cultivars. FAO Plant Production and Protection Paper (FAO)
Latif S, Chiapusio G, Weston LA (2017) Allelopathy and the role of allelochemicals in plant defence. In: Becard G (ed) Advances in botanical research: how plants communicate with their biotic environment. Academic Press, pp 19–54
Li ZH, Wang Q, Ruan X, Pan CD, Jiang DA (2010) Phenolics and plant allelopathy. Molecules 15:8933–8952. https://doi.org/10.3390/molecules15128933
Li J, Chen L, Chen Q, Miao Y, Peng Z, Huang B, Guo L, Liu D, Du H (2021) Allelopathic effect of Artemisia argyi on the germination and growth of various weeds. Sci Rep 11:1–15. https://doi.org/10.1038/s41598-021-83752-6
Liu Y, Zhang CX, Wei SH, Cui HL, Huang HJ (2011) Compounds from the subterranean part of Johnsongrass and their allelopathic potential. Weed Biol Manage 11:160–166. https://doi.org/10.1111/j.1445-6664.2011.00416.x
Lopes RWN, Morais EM, Lacerda JJJ, Araújo FDS (2022) Bioherbicidal potential of plant species with allelopathic effects on the weed Bidens bipinnata L. Sci Rep 12:1–12. https://doi.org/10.1038/s41598-022-16203-5
Lovett J, Hoult A (1995) Allelopathy and self-defense in barley. In: Inderjit, Dakshini KMM, Einhellig FA (eds) Allelopathy. American Chemical Society, Washington, pp 170–183. https://doi.org/10.1021/bk-1995-0582.ch013
Ma Y, Chun J, Wang S, Chen F (2011) Allelopathic potential of Jatropha curcas. Afric J Biot 10:11932–11942. https://doi.org/10.5897/AJB11.733
Macías FA, Galindo JLG, García-Díaz MD, Galindo J (2008) Allelopathic agents from aquatic ecosystems: potential biopesticides models. Phytochem Rev 7:155–178. https://doi.org/10.1007/s11101-007-9065-1
Macías FA, Mejías FJR, Molinillo JMG (2019) Recent advances in allelopathy for weed control: from knowledge to applications. Pest Manage Sci 75:2413–2436. https://doi.org/10.1002/ps.5355
Maguire JD (1962) Speed of germination-aid in selection and evaluation for seedling emergence and vigor. Crop Sci 2:176–177. https://doi.org/10.2135/cropsci1962.0011183X000200020033x
Mahdavikia F, Saharkhiz MJ (2015) Phytotoxic activity of essential oil and water extract of peppermint (Mentha×piperita L. CV. Mitcham). J Appl Res Med Aromat Plants 2:146–153. https://doi.org/10.1016/j.jarmap.2015.09.003
Mahmoud A, Singh SD, Muralikrishna KS (2016) Allelopathy in jatropha plantation: effects on seed germination, growth and yield of wheat in north-west India. Agr Ecosyst Environ 231:240–245. https://doi.org/10.1016/j.agee.2016.06.042
Mannochio-Russo H, Almeida RF, Nunes WDG, Bueno PCP, Carabalho-Rodríguez AM, Bauermeister A, Dorrestein PC, Bolzani VS (2022) Untargeted metabolomics sheds light on the diversity of major classes of secondary metabolites in the Malpighiaceae botanical family. Front Plant Sci 13:1–19. https://doi.org/10.3389/fpls.2022.854842
Meissner RNPC, Beyers EA (1986) Allelopathic influence of Tagetes-and Bidens-infested soils on seedling growth of certain crop species. S Afr J Plant Soil 3:176–180. https://doi.org/10.1080/02571862.1986.10634217
Miléo LJ, Silva JF, Bentes JLS, Christofoleti PJ (2007) Plantas daninhas hospedeiras alternativas de Colletotrichum guaranicola em cultivos de guaraná no Estado do Amazonas. Planta Daninha 25:771–782. https://doi.org/10.1590/S0100-83582007000400014
Nothias LF, Petras D, Schmid R, Dührkop K, Rainer J, Sarvepalli A, Dorrestein PC (2020) Feature-based molecular networking in the GNPS analysis environment. Nat Methods 17:905–908. https://doi.org/10.1038/s41592-020-0933-6
Panda A, Jali P, Mahalik G (2020) Effects of allelochemicals released by Jatropha gossypiifolia L. on morphology of Cicer arietinum L. In: Rawat AK (ed) Advances in agronomy. AkiNik Publications, pp 125–136
Pannacci E, Lattanzi B, Tei F (2017) Non-chemical weed management strategies in minor crops: a review. Crop Prot 96:44–58. https://doi.org/10.1016/j.cropro.2017.01.012
Pires NM, Oliveira VR (2011) Alelopatia. In: Oliveira RS Jr, Constantin J, Inoue MH (eds) Plantas daninhas e seu manejo. Curitiba, Omnipax, pp 95–123
Rao AS (1990) Root flavonoids. Bot Rev 56:1–84. https://doi.org/10.1007/BF02858531
Rejila S, Vijayakumar N (2011) Allelopathic effect of Jatropha curcas on selected intercropping plants (Green chilli and sesame). J Phytol 3:1–3
Rice EL (1984) Allelopathy, 2nd edn. Academic Press, Orlando, Florida, p 400
Ryan RE, Morris S (1999) Citronella oil as an herbicide for control of ragwort, docks, nettles, or thistles. Canadense patent n° 233445C
Sawadogo/Ilboudo TCD, Yonli D, Sourabie S, Zerbo P, Traoré H, Boussim JI (2022) Aqueous extracts from indigenous plant in Burkina Faso with bio-herbicide properties to reduce Striga hermonthica (Del.) Benth propagation. CABI Agric Biosci 3:61. https://doi.org/10.1186/s43170-022-00129-z
Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Ideker T (2003) Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res 13:2498–2504. https://doi.org/10.1101/gr.1239303
Silva JRO, Mendes KF (2022) Alelopatia no controle de plantas daninhas: mecanismos fisiológicos e ecológicos. In: Mendes KF, Silva AA (eds) Plantas daninhas: biologia e manejo. Oficina de Textos, pp 40–55
Silva EMBM, Ximenez GR, Silva E, Peixoto MA, Pilau EJ, Pomini AM, Oliveira SM (2022) Phytotoxic activity and identification of chemicals constituents in Lonchocarpus cultratus (Vell.) AMG Azevedo & HC Lima aerial parts (Fabaceae). S Afr J Bot 144:305–315. https://doi.org/10.1016/j.sajb.2021.08.042
Silveira RS, Leal GC, Molin TRD, Faccin H, Gobo LA, Silveira GD, Souza MTS, Lameira OA, Carvalho LM, Viana C (2020) Determination of phenolic and triterpenic compounds in Jatropha gossypiifolia L. by Ultra-high performance liquid chromatography-tandem mass spectrometric (UHPLC-MS/MS). Braz J Pharm Sci 56:1–12. https://doi.org/10.1590/s2175-97902019000417262
Singh HP, Batish DR, Kaur S, Ramezani H, Kohli KR (2002) Comparative phytotoxicity of four monoterpenes against Cassia occidentalis. Ann Appl Biol 141:111–116. https://doi.org/10.1111/j.1744-7348.2002.tb00202.x
Singh HP, Batish DR, Kaur S, Vaid S, Kohli KR (2004) Weed suppressing ability of some volatile monoterpenes. J Plant Dis Prot 19:821–828
Singh HP, Batish DR, Kaur S, Kohli RK, Arora K (2006) Phytotoxicity of the volatile monoterpene citronellal against some weeds. Z Naturforsch C J Biosci 61:334–340. https://doi.org/10.1515/znc-2006-5-606
Singh M, Kukal MS, Irmak S, Jhala AJ (2022) Water use characteristics of weeds: a global review, best practices, and future directions. Front Plant Sci 12:794090. https://doi.org/10.3389/fpls.2021.794090
Sumner LW, Amberg A, Barrett D, Beale MH, Beger R, Daykin CA, Viant MR (2007) Proposed minimum reporting standards for chemical analysis: chemical analysis working group (CAWG) metabolomics standards initiative (MSI). Metabolomics 3:211–221. https://doi.org/10.1007/s11306-007-0082-2
Tan RX, Zheng WF, Tang HQ (1998) Biologically active substances from the genus Artemisia. Planta Med 64:295–302. https://doi.org/10.1055/s-2006-957438
Tanaka S, Tomita R, Saijo H, Takahashi K, Ashitani T (2020) Growth-inhibitory activity of components in Cryptomeria japonica leaves against Robinia pseudoacacia. J For Res 25:192–197. https://doi.org/10.1080/13416979.2020.1747150
Tomar NS, Agarwal RM (2013) Influence of treatment of Jatropha curcas L. leachates and potassium on growth and phytochemical constituents of wheat (Triticum aestivum L.). Am J Plant Sci 4:1134–1150. https://doi.org/10.4236/ajps.2013.45140
Venkatesh A, Tapasya S, Kumar RV, Gurunathan N (2011) Allelopathic effects of different accessions of Jatropha curcas on field crops. Range Manag Agrofor 32:40–44
Wang M, Carver JJ, Phelan VV, Sanchez LM, Garg N, Peng Y, Bandeira N (2016) Sharing and community curation of mass spectrometry data with Global Natural Products Social Molecular Networking. Nat Biotechnol 34:828–837. https://doi.org/10.1038/nbt.3597
Weston LA, Mathesius U (2013) Flavonoids: their structure, biosynthesis, and role in the rhizosphere, including allelopathy. J Chem Ecol 39:283–297. https://doi.org/10.1007/s10886-013-0248-5
Williamson GB, Richardson D (1988) Bioassays for allelopathy: measuring treatment responses with independent controls. J Chem Ecol 14:181–187. https://doi.org/10.1007/bf01022540
Wu Q, Patocka J, Nepovimova E, Kuca K (2019) Jatropha gossypiifolia L. and its biologically active metabolites: a mini review. J Ethnopharmacol 234:197–203. https://doi.org/10.1016/j.jep.2019.01.022
Xu Z, Deng M (2017) Identification and control of common weeds. Springer, Dordrecht
Zhang HY, Qi SS, Dai ZC, Zhang M, Sun JF, Du DL (2017) Allelopathic potential of flavonoids identified from invasive plant Conyza canadensis on Agrostis stolonifera and Lactuca sativa. Allelopath J 41:223–237. https://doi.org/10.26651/2017-41-2-1098
Funding
LA, YMSG, and AARS were supported by the Coordination for the Improvement of Higher Education Personnel (CAPES, Finance Code 001). AMP was supported by the São Paulo Research Foundation (FAPESP, grant number 2019/04314-6).
Author information
Authors and Affiliations
Contributions
LA and YMSG conducted all bioassays and sample preparation, data collection and analysis. AARS and AMP performed LC‒MS analyses and data processing in Progenesis software. JJJL performed the statistical analyses of the bioguided fractionation and regression analyses. FDSA designed and coordinated the research and performed the feature-based molecular networking. LA, YMSG, and FDSA wrote the manuscript (original draft). All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Communicated by F. Araniti.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
de Almeida, L., Gaspar, Y.M., Silva, A.A.R. et al. Allelopathic effect and putative herbicidal allelochemicals from Jatropha gossypiifolia on the weed Bidens bipinnata. Acta Physiol Plant 46, 61 (2024). https://doi.org/10.1007/s11738-024-03689-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-024-03689-x