Abstract
The association of bean yellow mosaic potyvirus (BYMV) was investigated earlier with the severe mosaic and stunting disease of V. faba. In the present study, we mechanically transmitted BYMV on V. faba to assess the impact on physiological, biochemical, and nutritional attributes. BYMV-inoculated plants exhibited severe symptoms, and their height, length of the pod, and seed yield (size and number) were reduced to half of the mock-inoculated V. faba. In BYMV-inoculated V. faba, chlorophyll a, b, and total (Chl a + Chl b) were lowered to 66.70%, 64.94%, and 66.19% respectively, and corroborated with the decrease in photosynthetic efficiency (Fv/Fm) from 0.36 to 0.26. An increase in membrane ion leakage and malondialdehyde was observed in inoculated V. faba indicating virus-induced physiological stress. The non-structural carbohydrates, total protein, and free proline contents were also significantly altered. More, the high accumulation of salicylic acid and other defense-related antioxidant enzymes (ascorbate peroxidase, guaiacol peroxidase, superoxide dismutase, and catalase) was observed to ameliorate virus-induced stress. An increase in polyphenols and flavonoids: gallic acid, protocatechuic acid, chlorogenic acid, caffeic acid, rutin, ferulic acid, quercetin, and kaempferol suggests the proactive action by BYMV-infected V. faba. A reduced accumulation of nutrition-related parameters while induced accumulation of anti-nutritional factors like tannin (as tannic acid) and phytate observed is suggestive of poor nutritional quality of the plants. The present study comprehensively elucidates the BYMV-induced perturbations in physio-biochemical and nutritional attributes of V. faba, diminishing the quality of plants and seeds, and raises serious concerns for the management measures against the BYMV.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Faba bean (Vicia faba L.), also known as broad bean or horse bean, is a species of bean in the Fabaceae family. V. faba is the fourth most important and oldest cultivated pulse crop worldwide (Mínguez and Rubiales 2021). Seeds of V. faba are a staple dietary protein source in North Africa, East Africa, Middle East, West, and East Asia (Karkanis et al. 2018; Makkouk et al. 2003). Processed food products are also a cheap source of high-quality protein (about 25–30% in seeds) in the human diet whereas green haulm, dry seeds, and straw are used as animal feed supplements (Karkanis et al. 2018). Other than the protein source, seeds of V. faba are rich in carbohydrates, mineral nutrients, vitamins, microelements (Iron and Zinc), and bioactive compounds (Maalouf et al. 2021). They are also rich in the amino acid, l-3,4-dihydroxyphenylalanine (L-DOPA) which is a precursor of dopamine having some potential for the treatment of Parkinson's and hypertension (Dhull et al. 2021).
Both the biotic and abiotic stress factors limit the yield potential of V. faba. Among the biotic stresses, about 50 viruses have been reported to infect V. faba worldwide (Kumari and Makkouk 2007; Schwinghamer et al. 2009) of which, bean yellow mosaic virus (BYMV, a species in the genus Potyvirus, family Potyviridae), is reported as a virus of economic importance (Cheng and Jones 2000). The severe mosaic disease of V. faba caused by BYMV was reported in Australia, Egypt, Iran, and India (Katoch et al. 2003; Radwana et al. 2008; Rohani et al. 2008; Younes et al. 2021). We have also identified BYMV infection in V. faba plants (Kaur et al. 2013) where infected plants were exhibiting the symptoms of dark green mosaic on leaves, stunting of plants, reduction in pod sizes, and low seed yield as compared to the healthy V. faba.
Compatible infection wherein the virus is able to infect the host plant is a controlled process whereby the virus maneuvers the host’s cellular functions to establish systemic infections. Conversely, the plant raises a multi-layered defense including physiological, biochemical, and metabolic processes to restrict viral infection and also to counteract the adverse effects induced by the virus (Whitham et al. 2006; Radwana et al. 2008). Such biotic stresses negatively affect the survival and fitness of the plant, induce aging, and synthesis of stress-induced biomolecules (Huseynova et al. 2018). The measurement of those induced biomolecules represents markers for stress determination (Kumar et al. 2016). A study by Radwan et al. (2010) revealed a higher accumulation of H2O2 and malondialdehyde (MDA) along with the induced activity of antioxidants while a reduction in the protein content in leaves of BYMV-infected V. faba. They suggested that the accumulation of representative antioxidants and metabolites is the response of plants against virus invasion. However, a comprehensive study exhibiting the virus-induced changes in physiological, biochemical, and nutritional attributes of V. faba is lacking. In the present study, we broadly demonstrate change in these attributes in BYMV-inoculated and mock-inoculated (healthy control) V. faba. More, the assessment of polyphenols, flavonoids, protein content, and other nutritive values of leaf, immature and mature seed tissues of BYMV-inoculated V. faba elucidates the virus-induced consequences. The strain of BYMV considered for this study is genetically diverse from the reported BYMV strains, though clad together with the Asian strains of BYMV (Kaur et al. 2013).
Materials and methods
Plant material, BYMV culture, and mechanical sap transmission of virus
To raise plant material for experimentations, seeds of the Swarna Gaurav variety of V. faba were directly grown in 12-inch earthen pot filled with soil, vermicompost, and sand (3:1:1; v:v:v), and germinated seedlings were thinned to one plant per pot. A total of 60 pots with one plant in each were maintained in a glasshouse under natural daylight illumination and 25 ± 3 °C temperature. BYMV strain (Kaur et al. 2013), to be used for inoculations, was maintained on the Swarna Gaurav variety in the glasshouse. The virus was sap-inoculated on leaves of 4th node stage plants (40 days after seed sowing) to assess the BYMV-induced changes. Briefly, 1 g of infected leaf tissue was macerated in 10 mL ice-chilled 50 mM potassium phosphate buffer supplemented with 10 mM sodium sulfite (pH 7.0). The suspension was squeezed through double-layered muslin cloth and the clear sap obtained was mechanically rubbed on the carborundum pre-dusted leaf using a cotton swab onto the 30 seedlings (10 biological and 3 technical replicates). Mock inoculation was also performed on 30 seedlings (10 biological and 3 technical replicates) using inoculation buffer only and treated as a control (healthy) during all experiments. The severity of the disease symptom (green mosaic on leaf and plant stunting) was observed regularly, and growth and all parameters were recorded at 28 days post inoculations (dpi).
RNA isolation and reverse transcription-polymerase chain reaction (RT-PCR)
To ascertain the presence of BYMV in inoculated plants, total genomic RNA was isolated from 100 mg of youngest leaf tissue along with mock-inoculated and a positive control plant (Kaur et al. 2013) using TRIzol reagent (Invitrogen Co., Carlsbad, CA, USA) following the manufacturer’s instructions. CI-F and CI-R degenerate primers (Ha et al. 2008) capable of amplifying 700 bp DNA fragment from the conserved sequence within the cylindrical inclusion (CI) protein-coding region of the Potyviridae family were used for BYMV detection. All RNA preparations were treated with RNase-free DNase (50 µg/mL, Promega Co., WI, USA) to eliminate DNA contamination and assessed for quality check. For cDNA preparation, the reaction was set up in a final volume of 20 μL using 5 μg of genomic RNA, 5 U of RNase-H (Invitrogen Co. Carlsbad, CA, USA), 100 ng CI-R primer, 0.2 mM dNTPs, and 200 U M-MuLV reverse transcriptase (Invitrogen Co., Carlsbad, CA, USA), and synthesized following the manufacturer’s instructions. Next, the PCR was performed in a final volume of 25 μL using 1.5 μL of cDNA as template, 1X PCR buffer, 1.5 mM MgCl2, 0.2 mM dNTP, 100 ng of each CI-F and CI-R primers, and 1 U of Taq DNA polymerase (Promega Co., Madison, WI, USA). The PCR conditions were: initial denaturation at 94 °C for 5 min followed by 40 cycles of denaturation at 94 °C for 1 min, annealing at 56 °C for 45 s, extension at 72 °C for 1 min, and a final extension at 72 °C for 10 min. The size of the amplicon was assessed on 1% agarose gel with a standard DNA marker (Genei Pvt. Ltd., India).
Evaluation of BYMV-inoculated and mock-inoculated V. faba plant for different attributes
Plant growth parameters
The morphological attributes like plant height, pod length, pods per plant, seeds per pod, and total seed yield per plant were recorded from 120-day-old BYMV-inoculated and mock-inoculated (healthy) plants after germination. The data was recorded for 10 biological replicates and 3 technical replicates and shown in their respective measurement units.
Total moisture content
Total moisture content was estimated in BYMV and mock-inoculated V. faba using 2.0 g of fresh leaves from each plant immediately and after air drying under the shade. To calculate the percentage of total moisture, the estimated weight of leaves was put into the following formula:
Membrane ion leakage
Membrane ion leakage was measured following the method of Fan et al. (1997). Briefly, the youngest fully expanded leaves obtained from the BYMV and mock-inoculated plants were immersed in 10 mL of 0.4 M mannitol solution at room temperature (25 ± 3 °C) with 200 rpm shaking for 3 h, and the conductivity of the bathing solution was measured using a conductivity meter (Milwaukee Economical Pocket Tester, Noida, India). Total conductivity was determined by boiling leaves for 10 min in 10 mL of 0.4 M mannitol solution and expressed as the percentage of the initial conductivity versus the total conductivity.
Photosynthetic activity
Photosynthetic activity, a measurement of the maximum quantum yield of photosystem II (PS-II) performed on dark-adapted samples, was calculated in the 6th node leaves of BYMV and mock-inoculated V. faba following the method of Fan et al. (1997). The ratio of variable chlorophyll fluorescence (Fv) to maximum yield of chlorophyll fluorescence (Fm) was expressed as Fv/Fm. The 6th node leaves were first dark-adapted in leaf clips of the fluorometer for 10 min at room temperature prior to the measurement of Fv/Fm ratio for 3 technical replicates, where n = 10, with a Pocket PEA Chlorophyll Fluorimeter (PP Systems International, Inc., MA, USA).
Estimation of chlorophyll pigment
To assess the chlorophyll damage in V. faba due to BYMV inoculations as compared to the mock-inoculated control plants, estimation of chlorophyll a (Chl a), chlorophyll b (Chl b), and total chlorophyll (Chl a + Chl b) pigments was carried out. 100 mg of leaf tissue, collected from each treatment, was macerated in 10 mL of 80% acetone and the lysate was centrifuged at 5000 rpm for 10 min. The supernatant was transferred to fresh test tubes and absorbance was recorded at 664 nm and 647 nm in a UV spectrophotometer (Shimadzu, UV-160, Japan). Chl a, Chl b and Chl a + Chl b pigments were calculated using the formula of Lichtenthaler and Wellbum (1983) as follows:
where results were expressed as milligram per gram of fresh weight (mg g−1 FW). D, optical density at respective nm; V, final volume of chlorophyll extract in 80% acetone; and W, fresh weight of the tissue from which chlorophyll was extracted.
Sugar (non-structural carbohydrate)
Sugar estimation was carried out following an improved method of Gerchacov and Hatcher (1972). Briefly, 200 mg of fresh leaf tissue each from the BYMV and mock-inoculated V. faba were crushed in 80% methanol. The extract was centrifuged at 10,000 rpm for 10 min and obtained supernatant was incubated in a water bath at 70 °C. To this supernatant, equal volume of 5% phenol and 5 mL of sulfuric acid were added and absorbance was measured at 640 nm. A standard curve was plotted using the different concentrations of standard glucose solutions and sugar estimation was done on the background of this standard curve and results were expressed as mg g−1 of fresh weight (mg g−1 FW).
Total protein content
Total protein from leaf and mature seed tissues of BYMV and mock-inoculated V. faba was estimated using the method of Bradford (1976). Briefly, 50 mg of dry tissue was extracted independently in 10 mL of alkaline medium (0.1 N NaOH) for 2 h at 90 °C. The debris was pelleted at 10,000 rpm and 1 mL of the collected supernatant was added to 5 mL of alkaline reagent (2% Na2CO3 prepared in 0.1 N NaOH and 0.5% hydrated CuSO4 in 1% sodium potassium tartarate) and allowed to stand for 10 min after thorough mixing. To this solution, 0.5 mL of Folin reagent, diluted in 1:1 (v/v), was added and mixed immediately. The data was recorded at 595 nm absorbance and results were expressed as mg g−1 of dry weight (mg g−1 DW).
Free proline content
Free proline was determined according to the method of Bates et al. (1973). Briefly, 500 mg of leaf samples was homogenized in 3% (w/v) sulfosalicylic acid and filtered through 0.2 µ Whatman filter paper. After the addition of ninhydrin and glacial acetic acid, the resulting mixture was heated at 100 °C for 1 h in a water bath and the reaction was stopped by placing on ice-bath for 30 min. The mixture was extracted with toluene and the absorbance of the fraction with toluene aspired from the liquid phase was read at 520 nm absorbance. Proline concentration was determined using the calibration curve and expressed as micromole proline per gram of fresh weight (µM g−1 FW).
Total phenolic content
Phenolic content in the leaf, stem, and immature and mature seeds was estimated by the Folin–Ciocalteu assay (Folin and Ciocalteu 1927). Total phenolics were extracted from 100 mg dried powder of leaf, stem, immature, and mature seed tissues independently in 50% methanol following centrifugation at 10,000 rpm for 10 min. The supernatants were collected in fresh test tubes and mixed with 0.5 mL Folin–Ciocalteu reagent and 2 mL of 20% Na2CO3 before incubation at room temperature for 2 h in dark and optical density measured at 720 nm absorbance. The phenol content was measured using gallic acid as standard and results were expressed as mg g−1 of gallic acid equivalents (GAE).
Specific phenolic and flavonoid compounds
The specific phenolic and flavonoid compounds: gallic acid, protocatechuic acid, chlorogenic acid, caffeic acid, rutin, ferulic acid, quercetin, kaempferol, and salicylic acid were estimated in the leaf, stem, immature, and mature seed tissues of BYMV-inoculated and mock-inoculated V. faba by HPLC (Prominence HPLC, Shimadzu) using their respective standards and results were expressed as µg g−1 of dry weight (µg g−1 DW).
Malondialdehyde (MDA) content
Lipid peroxidation expressed as MDA and was determined as 2-thiobarbituric acid (TBA) reactive metabolites (Qiu et al. 2007). Briefly, 300 mg of fresh leaf samples collected from each treatment was homogenized independently in 5 mL of 5% trichloroacetic acid. The homogenate was clarified by centrifuged at 5000 rpm for 15 min. 1 mL of supernatant was then mixed with 2.5 mL of TBA and heated at 100 °C in a water bath for 20 min and quickly chilled on ice. The mixture was centrifuged at 10,000 rpm for 5 min and the absorbance of the resulting supernatant was measured at 532 nm and 600 nm. By subtracting the non-specific absorbance at 600 nm, the MDA in leaves was determined by its molar extinction coefficient (155 mM−1 cm−1). Results were presented as nM g−1 MDA FW.
Antioxidative enzyme activity
For antioxidant enzyme activity assays, 1 g of fresh leaf tissue from BYMV and mock-inoculated V. faba were homogenized in 3 mL of 100 mM Tris–HCl (pH = 7.8) containing 1 mM dithiothreitol, 1 mM EDTA, and 5 mM MgCl2. After proper mixing, 20 mg of polyvinyl polypyrrolidone was added and the homogenate was centrifuged at 14,000 rpm for 15 min at 4 °C. The supernatant was immediately used for enzyme activity measurements. Catalase (CAT) was detected at 240 nm by the rate of decomposition of H2O2 as described by Aebi (1984). Glutathione peroxidase (GPx) activity was assayed by the oxidation increase of NADPH at 340 nm according to Goldberg and Spooner (1984). Total Ascorbate peroxidase (APx) activity was determined as the decrease in absorbance of ascorbate at 298 nm by the method of Gerbling et al. (1984). The activity of superoxide dismutase (SOD) was assayed by its ability to inhibit the photochemical reduction of nitroblue tetrazolium (NBT) following the method of Beauchamp and Fridovich (1971).
Assessment of nutritional quality
The nutritional parameters of BYMV-inoculated and mock-inoculated V. faba were measured in 1 g leaf samples independently following the procedures described elsewhere (Singh et al. 2014) and data represented in their respective values.
Statistical analyses
All experiments for the assessment of different parameters or treatments were performed in triplicates, where n = 10. All data were subjected to an independent t test and shown as the mean ± SE (n = 10). Significant values of the test between pairs of mean values were determined at p > 0.05. All statistical tests were carried out using SPSS v.27.0 for Windows software (SPSS Inc., Chicago, IL).
Results and discussion
Morphological impact of BYMV on V. faba
The association of BYMV with the severe leaf mosaic and plant stunting disease of V. faba was identified earlier by us (Fig. 1a, Kaur et al. 2013). Mechanical (sap) inoculations of BYMV on V. faba led to the development of mosaic symptom at 15 dpi and the size of the entire plant and pod was reduced significantly at 60 dpi (Fig. 1b,1c), as was observed in naturally infected field-grown plants (Kaur et al. 2013). In BYMV-inoculated plants, the height was compromised by more than 46% as the average height decreased from 76.7 ± 6.05 cm to 41.24 ± 6.84 cm (Fig. 1d). Other plant vigor-related attributes like pod number per plant, number of seed per pod, and total seed yield per plant were also reduced by 43.6% (from 7.1 ± 1.34 to 4.0 ± 1.06 g), 47.5% (from 3.4 ± 0.5 to 1.8 ± 0.6 g) and 48% (from 19.3 ± 1.59 g to 10.06 ± 2.33 g) respectively (Fig. 1e–g). The RT-PCR with CI-F and CI-R degenerate primers confirmed the presence of BYMV in all the inoculated plants by showing corresponding 700 bp DNA fragments but not in mock-inoculated (control) plants (data not shown).
BYMV-infected V. faba exhibiting severe mosaic symptom on leaves in filed condition (a). Mock (left) and BYMV-inoculated (right) plants of V. faba exhibiting no symptom and, mosaic and stunting symptom respectively at 28 dpi (b). The pod of mock and BYMV-inoculated plants at 60 dpi showing size variability (c). Plant growth parameters including plant height (d), pod number per plant (e), seed number per pod (f) and total seed yield per plant (g) were recorded at 90 dpi. Values are the means of three replicates ± standard deviation (SD)
Alteration in physiological and biochemical attributes of V. faba due to BYMV inoculation
Studies revealed that the virus infection negates the survival and fitness of the plant, perturbs nutritional values, and manifests the synthesis of stress-induced biomolecules as was observed in previous studies (Radwana et al. 2008, 2010; Kumar et al. 2016). The plant exhibits adaptive physiological and biochemical features to cope with and ameliorate the deleterious effects of the virus and help the plant sustain under stress (Huseynova et al. 2018). And hence, BYMV-induced changes in both physiological (total moisture, membrane ion leakage, photosynthetic efficiency) and biochemical (sugar proline, chlorophyll pigments, MDA, antioxidant enzymes, polyphenols, and specific phenolics) attributes were assessed in BYMV-inoculated and mock-inoculated V. faba.
Alteration in physiological attributes
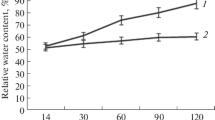
Change in membrane permeability is one of the first detectable attributes of disease and is expressed as ion leakage (Epple et al. 2003). In the present study, the leaves of BYMV-inoculated plants showed 27.73% conductivity than 11.83% as compared to the mock-inoculated control (healthy) samples (Fig. 2a). An increase of 55.6% in ion leakage was observed and has a clear correlation with the plant stress due to the infection of BYMV (Radwana et al. 2010). Though, the total moisture content was not much affected and relatively lowered only to 4.8% in the BYMV-inoculated plants and was 84.85% as compared to the mock-inoculated control plants (89.65%) (Fig. 2b). A reduction in moisture is suggestive of morphological damage in the xylem cells as the disfigured xylem and poor water transportation were observed in N. tabacum cv. White Burley due to the infection of Cucumber mosaic virus (CMV, Kumar et al. 2016). It has been shown that the decrease in photosynthetic efficiency, i.e. Fv/Fm ratio, is due to a decrease in efficiency of photosystem-II (PS-II) in the BYMV-infected plants (Huseynova et al. 2018). In the present study, Fv/Fm ratio was found to decrease to 0.26 in BYMV-inoculated leaf samples while it was observed 0.36 in the healthy samples (Fig. 2c) and suggests a decrease in the photosynthetic efficiency in V. faba due to the BYMV infection.
Image showing physiological changes in terms of moisture content, photosynthetic efficiency and membrane ion leakage in mock (Healthy) and BYMV-inoculated (Inoculated) V. faba. The data was measured in the leaf samples collected at 28 dpi. The x-axis represents the measuring unit. Values are means of three replicates ± standard deviation (SD)
Alteration in biochemical attributes
As a response to biotic as well as abiotic stresses plants accumulate a higher amount of different types of compatible solutes like proline, sucrose, and polyols (Serraj and Sinclair 2002). These biochemicals protect the plant by further maneuvering defense processes like cellular osmotic adjustment, protection of membrane integrity, reactive oxygen species (ROS) detoxification, and protein (enzyme) stabilization (Islam et al. 2003). And therefore, alteration in sugar, protein, proline, chlorophyll, lipid peroxidase (MDA), antioxidative enzymes, phenols, and specific phenolic contents was assessed. The total protein in the leaf and mature seed tissues of mock-inoculated V. faba was found 31.8 and 33.9 μg of per g tissue respectively, whereas it increased to 33.1 and 35.9 μg of per g tissue respectively in BYMV-inoculated V. faba (Fig. 3a, Table S1). These results indicated an increment of 3.9% and 5.67% protein in the BYMV-infected samples (Table S1). Accumulation of proteins in TMV-infected tobacco plants was also reported previously due to induced pathogenesis-related protein (Fraser 1982). MDA (a byproduct of lipid peroxidation) is a suitable marker for assessing the peroxidation and integrity of the membrane system both under biotic and abiotic stresses (Shaik and Ramakrishna 2014). During this study, the MDA in the leaves of BYMV-inoculated V. faba increased to a concentration of 0.6 nM/g of FW from 0.44 nM/g of FW in mock-inoculated leaves (Fig. 3b). Also, the membrane ion leakage (measured as % conductivity) is directly proportional to the level of MDA and therefore an increase of 55.6% conductivity was observed in BYMV-inoculated V. faba (Fig. 2a). Studies revealed the increase of MDA production and membrane ion leakage during drought and heat (Savicka and Škute 2010) as well as against the infection of CMV in tobacco (Kumar et al. 2016) and BYMV in faba beans (Radwana et al. 2010). Our results also corroborated well with the earlier findings.
The virus-infected leaves are characterized by reduced photosynthetic rate leading to a decrease in the concentration of soluble sugars (non-structural carbohydrate) and often starch accumulation (structural carbohydrate) (Shalitin and Wolf 2000). Studies revealed that sugar metabolism is influenced by the infection of CMV in melon (Tecsi et al. 1996) and tobacco plants (Kumar et al. 2016). During this study, the total soluble sugar was found to decrease to 38.96 mg g−1 FW in leaves of BYMV-inoculated V. faba from 61.46 mg g−1 FW in mock-inoculated plants (Fig. 3c). The increase in total protein was found associated with the reduction in sugar in tomato fruits infected with tomato yellow leaf curl virus (Singh et al. 2014). Our findings are consistent with these results as an increase in total protein together with a decrease in soluble sugar was observed in BYMV-infected V. faba.
Chlorophyll pigment is critical in the photosynthesis system for the production of food in the form of carbohydrates and was observed seriously affected by the viral infection (Kumar et al. 2016; Ananthu and Umamaheswaran 2019). In this study, a reduction of 66.70%, 64.94%, and 66.19% in chl a, chl b, and total (chl a + chl b) pigments respectively was observed in leaves of BYMV-inoculated V. faba as compared to the mock-inoculated control plants (Fig. 3d–f, Table S2). The reduction in chlorophyll pigments indicated a poor photosynthetic system in the virus-infected leaves and therefore poor assimilation of non-structural sugars was observed. In an earlier study, the morphophysiological changes in the leaves of V. faba due to BYMV infection were observed and found that virus-infected cells had lower chloroplast pigmentation compared to the cells of uninoculated plants at 21 dpi (Elbeshehy et al. 2014). A highly gradual decline in photosynthetic pigments was also observed by Hemidia (2005) in BYMV-infected samples along with an increase in carbohydrates and soluble proteins. Our results are consistent with these findings as reduced photosynthetic efficiency (Fv/Fm), lesser chlorophyll pigments and soluble sugar was found in the BYMV-inoculated V. faba. It is extrapolated that reduced photosynthetic efficiency may be due to improper chloroplast structures including dilated grana and thylakoids. This is evident in a study that showed ultrastructural changes in chloroplasts with an increased stromal area which became spherical in shape and some lost their envelopes, either partially or totally, with the cylindrical inclusion body of varied shapes (Radwana et al. 2008).
The strict regulation of proline in plants is essential for maintaining the osmotic potential of tissues (Wang et al. 2012), whereas the accumulation of free proline is an indicator of stress (Mazid et al. 2011). Estimation of free proline content revealed a significant increase of 29.31% (increased to 34.32 μM g−1 FW in BYMV-inoculated from 24.26 μM g−1 FW in mock-inoculated plants) (Fig. 3g, Table S2) and indicated that the inoculated plants have undergone stress due to the BYMV infection. The results are consistent as an increase of proline was observed in CMV-infected tobacco plants to counterbalance the disturbed electrolyte system and to maintain the osmotic potential of infected tissues (Kumar et al. 2016). The phenolic compounds in plant cells are secondary metabolites that have strong free radical scavenging capacity (Huang et al. 2006) and are reported to accumulate in response to pathogen attacks (Gachon et al. 2004). In the present study, increase in 9.41%, 14.94%, 1.61%, and 17.46% in the phenolic contents of leaf, stem, immature, and mature seed samples respectively was observed in the BYMV-inoculated V. faba as compared to mock-inoculated plants (Fig. 3h, Table S3).
Specific phenolic compounds could open a way for revealing plant strategies against pathogen attacks in plant–virus interactions (Chaerle et al. 2007). In the present study, status of V. faba specific phenolic compounds: gallic acid, protocatechuic acid, chlorogenic acid, caffeic acid, rutin, ferulic acid, quercetin, and kaempferol were observed. The amount of gallic acid was increased by 5.37% and 24.04% in BYMV-inoculated leaf and stem tissues respectively compared to the mock-inoculated plants, whereas in immature and mature seeds, it was reduced by 32.87% and 10.09% respectively (Fig. 4a, Table S4). Similarly, protocatechuic acid was increased by 20.74%, 34.53%, and 6.89% in infected leaf, stem and mature seed samples. However, it was reduced to 17.5% in immature seeds (Fig. 4b). An increase of 12.56% and 32.32% was observed in infected leaf and stem sampled for chlorogenic acid, whereas it was reduced by 11.36% in immature seeds and 7.73% in mature seeds (Fig. 4c). Caffeic acid also followed the same trend and was increased by 7.13% and 43.02% in infected leaf and stem sampled whereby reduced by 3.0 9% and 14.05% in infected immature seeds and mature seeds respectively (Fig. 4d). Rutin and ferulic acid were the only polyphenols that showed an increasing trend in all tissue types. Rutin was increased by 7.33%, 61.14%, 56.00%, and 14.13% in all infected leaf, stem, immature seeds, and mature seeds respectively (Fig. 4e). The level of ferulic acid increased by 30.69%, 33.10%, 71.43%, and 1.62% in all infected leaf, stem, immature seeds, and mature seeds respectively (Fig. 4f). The level of quercetin was increased by 68.67% and 83.33% in infected leaf and stem samples respectively, whereas decreased by 88.89% and 33.33% in immature and mature seeds (Fig. 4g). Kaempferol remained unchanged in the leaf and immature seed samples of BYMV-inoculated plants, whereas increased up to 10% in infected stem and decreased by 9.09% in infected mature seeds (Fig. 4h). Interestingly, the level of salicylic acid was increased by 71.69%, 48.5%, 27.38%, and 38.71% in leaf, stem, and immature and mature seed samples respectively of BYMV-inoculated plant samples (Fig. 4i). The estimation of jasmonic acid (JA) was also attempted in both BYMV and mock-inoculated V. faba. However, the amount of JA was below the detection threshold limit in the healthy samples and therefore could not be detected in BYMV-inoculated samples.
Image showing changes in the specific polyphenols (gallic acid, protocatechuic acid, chlorogenic acid, rutin, ferulic acid, quercetin, caffeic acid, kaempferol and salicylic acid) in mock (Healthy) and BYMV-inoculated (Inoculated) V. faba. The data was measured in the leaf, stem and immature seed samples collected at 28 dpi and mature seeds at 30 days post harvesting. The x-axis represents the measuring unit. Values are means of three replicates ± standard deviation (SD)
During this study, phenolic compounds showed either their up- or down-regulation in various plant parts. Higher accumulation of gallic acid, protocatechuic acid, chlorogenic acid, caffeic acid, rutin, ferulic acid, quercetin, and salicylic acid in the leaf samples was found due to the BYMV infection. Also, a high accumulation of rutin, ferulic acid, and salicylic was observed in immature seeds of BYMV-infected V. faba, whereas the concentration of protocatechuic acid, rutin, ferulic acid, and salicylic acid was high in mature seeds as compared to the healthy controls. These specific polyphenols are the exemplary compounds present not only in V. faba but also in many other plants species and have recently been reported to inhibit the growth cycle of seasonal influenza and other human viruses (Kim and Chung 2018; Khan et al. 2020; Chojnacka et al. 2021). In a study, Parvez et al. have clearly shown that quercetin can inhibit genome replication in human cells, and coincubation with nucleoside analogs enhances its efficacy against the Hepatitis B virus (Parvez et al. 2019). Cumulatively, our study is a first study that has shown the regulation of these polyphenols against a plant virus infection for the first time; however, this need to be evidenced further by the molecular studies.
The high accumulation of total phenolics in different plant parts of BYMV-infected V. faba tempted us to further study the activity of non-enzymatic detoxification of reactive oxygen species (ROS). The level of ROS in plants is not only regulated by the antioxidant enzyme activities but also by the presence or absence of antioxidant metabolites, such as phenolic compounds and flavonoids. It is reported that the higher phenolic contents proportionally increased due to higher total antioxidant capacity (Cai et al. 2004). Also, when plants are exposed to pathogens, they produce ROS that induces programmed cell death in the plant cells to restrict the pathogen and limit the disease process (Apel and Hirt 2004). Therefore, the activity of antioxidant enzymes was assayed in leaf and mature seed samples of V. faba. The activity of APx was increased by 18.0% and 6.5% in leaf and seeds of BYMV-inoculated plants respectively (Fig. 5a, Table S5). CAT enzyme activity was induced by 59.5% and 60.0% in leaf and seeds of BYMV-infected samples respectively (Fig. 5b). SOD enzyme revealed 30.07% and 20.9% increase in leaf and seeds respectively (Fig. 5c). The enzymatic activity of GPx was also induced by 40% and 54.6% in the leaf and seed sample of infected V. faba plants (Fig. 5d). As a result, a modified antioxidant status was observed in BYMV-inoculated plants of V. faba. To support this, Radwana et al. also observed modifications in proline, phenolics and antioxidant enzyme activities in BYMV-inoculated V. faba (Radwan et al. 2010). It is reported that phenolic compounds are free radical scavenging molecules that are rich in their antioxidant activity (Dat et al. 2000). More, the need for proline in maintaining the elevated ROS level and shift in nitrogen metabolism has been reported earlier (Cai et al. 2004; Hare and Cress 1997). Some studies revealed that the proline may also act as a potent scavenger of elevated reactive oxygen species (ROS) (Hare and Cress 1997; Chen and Dickman 2005) and the 29.31% increase in proline in BYMV-inoculated V, faba observed during the present study is corroborating well with the findings.
Image showing comparative changes in antioxidant enzyme activities in leaf tissue of mock (Healthy) and BYMV-inoculated (Inoculated) V. faba. The data was measured in the leaf samples collected at 28 dpi and seed at 30 days post harvesting. The x-axis represents the measuring unit. Values are means of three replicates ± standard deviation (SD)
Nutritional quality after infection of BYMV
The nutritional composition of mature seeds obtained from the BYMV-inoculated and mock-inoculated V. faba was evaluated and the results are described in Table 1. A significant decrease in dietary fiber (24.58%), protein (40.72%), fat (21.00%), total carbohydrate (31.25%), phosphorus (38.46%), zinc (14.67%), copper (18.62%), manganese (16.91%), and iron (7.70%) was observed, whereas the concentrations of tannins (measured as tannic acid), phytate, and total soluble sugar were increased by 31.91%, 12.50%, and 4.50%, respectively in seeds of BYMV-inoculated plants than in mock-inoculated (control) samples. V. faba also is reported to have a number of anti-nutrients including phytates, vicine, convicine, saponins, lectins, raffinose, stachyose, tannins, trypsin inhibitors, and protease inhibitors (Labba et al. 2021), present in mature seeds. The presence of anti-nutritional factors, such as phytate and tannins, has significant negative effects on nutritional quality (Towo et al. 2006). They are reported to negatively influence the bioavailability of minerals and alter the absorption of proteins in the diet (Luo et al. 2008). During human consumption, both phytate and tannins can bind to minerals, such as iron (Fe), and reduce its digestibility and bioavailability to cause iron deficiency in humans (Towo et al. 2006). Our results revealed that BYMV infections not only altered the physiological and biochemical attributes but also diminished the nutritional value of plants and their seeds.
Data availability
The data are available in the laboratory.
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126. https://doi.org/10.1016/S0076-6879(84)05016-3
Ananthu N, Umamaheswaran K (2019) Effect of viral infection on carbohydrate and chlorophyll contents in ginger (Zingiber officinale Rosc.). Int J Curr Microbiol App Sci 8:862–867
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55:373–399. https://doi.org/10.1146/annurev.arplant.55.031903.141701
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207. https://doi.org/10.1007/BF00018060
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assay and an assay applicable to acrylamide gels. Anal Biochem 44:276–287. https://doi.org/10.1016/0003-2697(71)90370-8
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Cai YZ, Luo Q, Sun M, Corke H (2004) Antioxidant activity and phenolic compounds of 112 traditional chinese medicinal plants associated with anticancer. Life Sci 74:2157–2184. https://doi.org/10.1016/j.lfs.2003.09.047
Chaerle L, Lenk S, Hagenbeek D, Buschmann C, van Der Straeten D (2007) Multicolor fluorescence imaging for early detection of the hypersensitive reaction to tobacco mosaic virus. J Plant Physiol 164:253–326. https://doi.org/10.1016/j.jplph.2006.01.011
Chen C, Dickman MB (2005) Proline suppresses apoptosis in the fungal pathogen Colletotrichum trifolii. Proc Natl Acad Sci USA 102:3459–3464. https://doi.org/10.1073/pnas.040796010
Cheng Y, Jones RAC (2000) Biological properties of necrotic and non-necrotic strains of bean yellow mosaic virus in cool season grain legumes. Ann Appl Biol 36:215–227. https://doi.org/10.1111/j.1744-7348.2000.tb00028.x
Chojnacka K, Skrzypczak D, Izydorczyk G, Mikula K, Szopa D, Witek-Krowiak A (2021) Antiviral properties of polyphenols from plants. Foods 10:2277. https://doi.org/10.3390/foods10102277
Dat J, Vandenabeele S, Vranová E, Van Montagu M, Inzé D, van Breusegem F (2000) Dual action of the active oxygen species during plant stress responses. Cell Mol Life Sci 57:779–795. https://doi.org/10.1007/s000180050041
Dhull SB, Kidwai MK, Noor R, Chawla P, Rose PK (2021) A review of nutritional profile and processing of faba bean (Vicia faba L.). Legume Sci. https://doi.org/10.1002/leg3.129
Elbeshehy EF, Almaghrabi OA, Mahmoud WMA, Elazzazy A (2014) Effect of biosynthesized silver nanoparticles on physiological parameters of Vicia faba infected by Bean yellow mosaic virus. J Pure App Microbiol 8:803–812
Epple P, Mack AA, Morris VR, Dangl JL (2003) Antagonistic control of oxidative stress-induced cell death in Arabidopsis by two related, plant-specific zinc finger proteins. Proc Natl Acad Sci USA 100:6831–6836. https://doi.org/10.1073/pnas.1130421100
Fan L, Zheng S, Wang X (1997) Antisense suppression of phospholipase D retards abscisic acid- and ethylene-promoted senescence of postharvest arabidopsis leaves. Plant Cell 9:2183–2196. https://doi.org/10.1105/tpc.9.12.2183
Folin O, Ciocalteu V (1927) On tyrosine and tryptophane determinations in proteins. J Biol Chem 73:627–650. https://doi.org/10.1016/S0021-9258(18)84277-6
Fraser RSS (1982) Are pathogenesis-related proteins involved in acquired systemic resistance of tobacco plants to tobacco mosaic virus? J Gen Virol 58:305–313. https://doi.org/10.1099/0022-1317-58-2-305
Gachon C, Baltz R, Saindrenan P (2004) Over-expression of a scopoletin glucosyltransferase in Nicotiana tabacum leads to precocious lesion formation during the hypersensitive response to tobacco mosaic virus but does not affect virus resistance. Plant Mol Biol 541:137–146. https://doi.org/10.1023/B:PLAN.0000028775.58537.fe
Gerbling KP, Grahame JK, Fisher KH, Latzko E (1984) Partial purification and properties of soluble ascorbate peroxidases from pea leaves. J Plant Physiol 115:59–67. https://doi.org/10.1016/S0176-1617(84)80051-6
Gerchacov SM, Hatcher PG (1972) Improved technique for analysis of carbohydrates in sediments. Limnol Oceanogr 17:938–143. https://doi.org/10.4319/lo.1972.17.6.0938
Goldberg DM, Spooner RJ (1984) Glutathione reductase. In: Bergmeyer HU (Ed). Methods in enzymatic analysis. Enzymes 1: Oxidoreductases, transferases. vol. III. Verlag Chemie, Weinheim. pp 43–49
Ha C, Coombs S, Revill PA, Harding RM, Vu M, Dale JL (2008) Design and application of two novel degenerate primer pairs for the detection and complete genomic characterization of potyviruses. Arch Virol 153:25–36. https://doi.org/10.1007/s00705-007-1053-7
Hare PD, Cress WA (1997) Metabolic implications of stress induced proline accumulation in plants. Plant Growth Regul 21:79–102
Hemidia SK (2005) Effect of Bean yellow mosaic virus on physiological parameters of Vicia faba and Phaseolus vulgaris. Int J Agric Biol 7:154–157
Huang YC, Chang YH, Shao YY (2006) Effects of genotype and treatment on the antioxidant activity of sweet potato in Taiwan. Food Chem 98:529–538. https://doi.org/10.1016/j.foodchem.2005.05.083
Huseynova IM, Mirzayeva SM, Sultanova NF, Aliyeva DR, Mustafayev NSh, Aliyev JA (2018) Virus-induced changes in photosynthetic parameters and peroxidase isoenzyme contents in tomato (Solanum lycopersicum L.) plants. Photosynthetica 56:841–885. https://doi.org/10.1007/s11099-017-0737-9
Islam MDT, Hossain T, Ahmed JU, Karim S (2003) Biochemical changes in tomato fruits caused by tomato yellow leaf curl virus. Bangladesh J Bot 32:81–84
Karkanis A, Ntatsi G, Lepse L, Fernández JA, Vågen IM, Rewald B et al (2018) Faba bean cultivation-revealing novel managing practices for more sustainable and competitive European cropping systems. Front Plant Sci 9:1115. https://doi.org/10.3389/fpls.2018.01115
Katoch M, Abdin MZ, Ram R, Zaidi AA (2003) An overview of diagnostics for viruses infecting gladiolus. Crop Prot 22:153–156. https://doi.org/10.1016/S0261-2194(02)00139-4
Kaur C, Kumar S, Raj SK (2013) New record of association of Bean yellow mosaic virus with mosaic disease of Vicia faba in India. Ind J Virol 24:95–96. https://doi.org/10.1007/s13337-013-0128-1
Khan M, Rauf W, Fazal-e-Habib MR, Iqbal M (2020) Screening and identification of bioactive compounds from citrus against non-structural protein 3 protease of hepatitis C virus genotype 3a by fluorescence resonance energy transfer assay and mass spectrometry. World J Hepatol 12:976–992. https://doi.org/10.4254/wjh.v12.i11.976
Kim H, Chung MS (2018) Antiviral activities of mulberry (Morus alba) juice and seed against influenza viruses. Evid Based Complement Altern Med 2018:2606583. https://doi.org/10.1155/2018/2606583
Kumar S, Chauhan PS, Agrawal L, Raj R, Srivastava A, Gupta S et al (2016) Paenibacillus lentimorbus inoculation enhances tobacco growth and extenuates the virulence of Cucumber mosaic virus. PLoS ONE 11:e0149980. https://doi.org/10.1371/journal.pone.0149980
Kumari SG, Makkouk KM (2007) Virus diseases of faba bean (Vicia faba L.) in Asia and Africa. Plant Viruses 1:93–105
Labba ICM, Frøkiær H, Sandberg AS (2021) Nutritional and antinutritional composition of fava bean (Vicia faba L., var. minor) cultivars. Food Res Int. https://doi.org/10.1016/j.foodres.2020.110038
Lichtenthaler HK, Wellbum AR (1983) Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem Soc Transactions 11:591–592. https://doi.org/10.1042/bst0110591
Luo Y, Gu Z, Han Y, Chen Z (2008) The impact of processing on phytic acid, in vitro soluble iron and Phy/Fe molar ratio of faba bean (Vicia faba L.). J Agric Food Chem 89:861–866. https://doi.org/10.1002/jsfa.3525
Maalouf F, Ahmed S, Bishaw Z (2021) Faba bean. In: Pratap A, Gupta S (Eds.), The beans and the peas From orphan to mainstream crops. Woodhead Publishing. pp 105–131 https://doi.org/10.1016/B978-0-12-821450-3.00008-1
Makkouk KM, Rizkallah L, Kumari SG, Zaki M, Abul Enien R (2003) First record of Chickpea chlorotic dwarf virus (CpCDV) affecting faba bean (Vicia faba) crops in Egypt. Plant Pathol 52:413. https://doi.org/10.1046/j.1365-3059.2003.00834.x
Mazid M, Khan TA, Khan ZH, Quddusi S, Mohammad F (2011) Occurrence, biosynthesis and potentialities of ascorbic acid in plants. Int J Plant Animal Env Sci 1(2):167–184
Mínguez MI, Rubiales D (2021) Faba bean. In: Sadras VO, Calderini DF (Eds.), Crop physiology case histories for major crops. Cambridge, MA, USA: Elsevier. pp 452–481 https://doi.org/10.1016/B978-0-12-819194-1.00015-3
Parvez MK, Tabish Rehman M, Alam P, Al-Dosari MS, Alqasoumi SI, Alajmi MF (2019) Plant-derived antiviral drugs as novel hepatitis B virus inhibitors: Cell culture and molecular docking study. Saudi Pharm J 27:389–400. https://doi.org/10.1016/j.jsps.2018.12.008
Qiu ZB, Liu X, Tian XJ, Yue M (2007) Effects of CO2 laser pretreatment on drought stress resistance in wheat. J Photochem Photobiol B 90:17–25
Radwan DEM, Fayez KA, Mahmoud SY, Lu G (2010) Modifications of antioxidant activity and protein composition of bean leaf due to Bean yellow mosaic virus infection and salicylic acid treatments. Acta Physiol Plant 32:891–904. https://doi.org/10.1007/s11738-010-0477-y
Radwana DEM, Lu G, Fayez KA, Mahmoud S (2008) Protective action of salicylic acid against bean yellow mosaic virus infection in Vicia faba leaves. J Plant Physiol 165:845–857. https://doi.org/10.1016/j.jplph.2007.07.012
Rohani B, Habibi MK, Mosahebi G (2008) Nodule infection by Bean yellow mosaic virus in Vicia faba and molecular characterization of it. Commun Agric Appl Biol Sci 73:303–306 (PMID:19226767)
Savicka M, Škute N (2010) Effects of high temperature on malondialdehyde content, superoxide production and growth changes in wheat seedlings (Triticum aestivum L.). Ekologija 56:26–33. https://doi.org/10.2478/v10055-010-0004-x
Schwinghamer MW, Nicholas AH, Schilg MA (2009) Three aphid vectors of faba bean (Vicia faba) viruses in northern New South Wales and occurrence of Acyrthosiphon pisum-transmitted isolates of Soybean dwarf virus. Austral Plant Pathol 38:262–269. https://doi.org/10.1071/AP09001
Serraj R, Sinclair TR (2002) Osmolyte accumulation: can it really help increase crop yield under drought conditions? Plant Cell Environ 25:333–341. https://doi.org/10.1046/j.1365-3040.2002.00754.x
Shaik R, Ramakrishna W (2014) Machine learning approaches distinguish multiple stress conditions using stress-responsive genes and identify candidate genes for broad resistance in rice. Plant Physiol 164:481–495. https://doi.org/10.1104/pp.113.225862
Shalitin D, Wolf S (2000) Cucumber mosaic virus infection affects sugar transport in melon plants. Plant Physiol 123:597–604
Singh AK, Bhardwaj R, Singh IS (2014) Assessment of nutritional quality of developed faba bean (Vicia faba L.) lines. J AgriSearch 1:96–101. https://doi.org/10.1007/s11738-010-0477-y
Tecsi LI, Maule AJ, Smith AM, Leegood RC (1996) A spatial analysis of physiological changes associated with infection of cotyledons of marrow plants with cucumber mosaic virus. Plant Physiol 111:975–985. https://doi.org/10.1104/pp.111.4.975
Towo E, Matuschek E, Svanberg U (2006) Fermentation and enzyme treatment of tannin sorghum gruels: effects on phenolic compounds, phytate and in vitro accessible iron. Food Chem 94:369–376. https://doi.org/10.1016/j.foodchem.2004.11.027
Wang C-J, Yang W, Wang C, Gu C, Niu D-D (2012) Induction of drought tolerance in cucumber plants by a consortium of three plant growth-promoting rhizobacterium strains. PLoS ONE. https://doi.org/10.1371/journal.pone.0052565
Whitham SA, Yang C, Goodin MM (2006) Global impact: elucidating plant responses to viral infection. Mol Plant Microbe Interact 19:1207–1215. https://doi.org/10.1094/MPMI-19-1207
Younes HA, El-Aziz MHA, Zeid AH (2021) Detection of Bean yellow mosaic potyvirus in infected faba bean plants (Vicia faba L.) from Northern Egypt. Arch Phytopathol Plant Prot 54:19–20. https://doi.org/10.1080/03235408.2021.1932285
Acknowledgements
The authors are thankful to the Director, CSIR-NBRI, Lucknow, for the facilities. Author C. Kaur is thankful to the University Grants Commission, India for her fellowship.
Funding
The authors are thankful to the Director, CSIR-National Botanical Research Institute, Lucknow, India for laboratory facilities and financial support.
Author information
Authors and Affiliations
Contributions
S.K.R conceived the experiment. C.K, R.R, and A.S and A.N did all the experiments. A.N and A.L analyzed and interpreted the biochemical and specific phenolic-related results. S.K and S.K.R wrote the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Communicated by M. Capuana.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kaur, C., Srivastava, A., Raj, R. et al. Physio-biochemical and nutritional alterations in faba bean due to bean yellow mosaic virus infection. Acta Physiol Plant 46, 54 (2024). https://doi.org/10.1007/s11738-024-03681-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-024-03681-5