Abstract
The current study aimed to evaluate the impact of putrescine foliar application and inoculation with arbuscular mycorrhizal fungi on some growth characteristics, absorption of nutrients, and post-harvest performance of Gerbera jamesonii cv. Dune. The present study was performed as a factorial trial with a completely randomized design and a total of three repetitions in the greenhouse. Experimental variants included 0, 1, 2, and 4 mM concentrations of putrescine as foliar spraying and mycorrhizal fungi, with and without mycorrhizal inoculation. The amount of mycorrhiza inoculation was 60 g per pot. Gerbera plants were transplanted into pots with or without mycorrhizal inoculation. Two weeks after the establishment of the plant and mycorrhizal fungus, foliar spraying of putrescine was performed every 15 days during a three-month period. In this experiment, morphological features such as fresh and dry weight of the root, pedicel length and diameter, the volume of the root, absorption of nutrients, including phosphorus, potassium, calcium, and nitrate, as well as post-harvest features, including relative fresh weight, solution absorption rate, and phenylalanine ammonia-lyase enzyme activity, underwent investigation. The findings demonstrated that putrescine, along with mycorrhizal fungi, had a positive effect on the growth characteristics of Gerbera, could increase nutrient absorption, and improve post-harvest indicators. Overall, these results indicated that 2 and 4 mM putrescine could positively affect growth and nutrient uptake indices, while 1 mM putrescine was more effective for post-harvest characteristics.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Gerbera jamesonii (G. jamesonii), commonly known as Gerbera, is an attractive cut flower that belongs to the prominent flowering plant family, Asteraceae. The genus Gerbera, named in honor of the German naturalist Traugott Gerber, contains 30 suitable species native to Asia and South Africa (Rymbai et al. 2017). The inflorescence is characterized by a compound or mass with three types of rays, trans, and disk florets. Gerbera flower cultivars are usually classified into standard and small sizes. Standard cultivars produce flowers 10–13 cm in diameter. Some cultivars can produce flowers up to 15 cm in diameter (Deng and Bhattarai 2018).
Polyamines (PAs) are considered a class of growth regulators in plants. There is strong evidence that PAs play an important role in physiological processes such as embryogenesis, root development, organogenesis, flower development, fruit ripening, or programmed cell death (Liu et al. 2015), as well as tolerance responses to major stresses affecting plant production. Putrescine, spermidine, and spermine are among the many PAs. Putrescine is the most common polyamine in higher plants (Collado-González et al. 2021); it is not only a signal molecule itself but also interacts with many molecules such as phytohormones and gas molecules. For example, exogenous treatment of putrescine leads to increased plant growth and maintains the post-harvest quality parameters of Lisianthus (Eustoma grandiflorum ‘Mariachi Garande White’) (Ataii et al. 2018), Rosa hybrida ‘Dolce Vita’ (Danaee and Abdossi 2018), and Dianthus caryophyllus (Karimi et al. 2017). Habba et al. (2016) showed that the foliar application of putrescine in Poplar (Populus × euramericana) significantly increased the root growth and absorption of nutrients such as nitrogen (N), phosphorus (P), and potassium (K). Yousefi et al. (2019) observed that the foliar application of putrescine has beneficial effects on various parameters of the growth of shoots and roots, and absorption of the nutrients of Rosa hybrida ‘Herbert Stevens’ plants.
Arbuscular mycorrhiza (AM) is a type of endomycorrhiza (Quilambo 2003). Symbiosis with AM fungi (AMF) has many favorable effects on plants. A consequential effect of this symbiosis may be increased uptake of mineral nutrients from the soil or soilless media, especially relatively immobile substances such as phosphate (Smith and Read 2008; Lin and Jones 2022). The symbiosis of AMF with plants can facilitate plant growth via multifarious mechanisms, including the production or regulation of the phytohormone level, an increase in plant nutrient availability and uptake, and the production of secondary metabolites (Dong et al. 2019). Microbial mechanisms for improving nutrient bioavailability and uptake include N fixation, nutrient solubilization, and expansion of root surface area (Courty et al. 2015; Halpern et al. 2015). AMF improve plant nutrient availability, especially phosphate, the depletion of which is an important plant limiting factor (Gianinazzi-Pearson et al. 2021). AMF facilitate increased uptake and transport of nutrients to plants through extensive networks of hyphae that colonize roots (Genre et al. 2020). The effects of mycorrhizal inoculation on two Lilium species (Lilium ledebourii and Lilium longiflorum) were evaluated, and it was found that plants inoculated with mycorrhizal fungi had higher root colonization and height, as well as P absorption rate, compared to control plants. Iron and zinc were more in the inoculated plants than control plants so that the concentration of zinc in the inoculated plants was in the range of 30–35 mg/kg (Arjmand Alavi et al. 2014). Beneficial mycorrhizal fungi that colonize plants can provide lasting benefits to plant growth and health during greenhouse production, extend vase life during retail, and improve landscape performance for end consumers (Paradikovic et al. 2019). The use of mycorrhizal fungus on Gazania rigens could significantly increase the number of leaves and flowers, as well as the height and dry weight of the root, compared to the control plants and led to the production of high-quality plants (Sabatino et al. 2019).
Due to the removal of soil in soilless culture systems, a wide range of beneficial organisms, including fungi and growth-promoting bacteria, are also removed, and therefore, the use of biological fertilizers such as mycorrhizal fungi is a suitable method to replace these organisms in a soilless system (Dasgan et al. 2008). Mycorrhizal fungi as one of the most important beneficial microorganisms increase the growth and development of plants by improving the absorption of nutrients (Yadav et al. 2012). On the other hand, polyamine may be an important regulatory factor in arbuscular mycorrhiza symbiosis. In addition, putrescine, the most common polyamine in higher plants, affects the growth and development, as well as the flowering and post-harvest quality of plants. Considering the above-mentioned explanations, this study sought to investigate the effect of different putrescine concentrations (0, 1, 2, and 4 mM) and mycorrhizal fungi inoculation on some traits of growth and development and nutrient absorption of Gerbera (G. jamesonii ‘Dune’).
Materials and methods
Materials and treatments of plants
For this purpose, a factorial trial was conducted with a completely randomized design under hydroponic conditions. It included a total of three repetitions each containing three pots, and each pot consisted of one plant. The Gerbera plants were transferred to the cultivation place (Urmia University). They were prepared as the tissue culture obtained from mother plants of the Dutch Company Floris and cultivated in modern farms in Iran. The Gerbera cultivar (i.e., G. jamesonii cv. Dune) having semi-full and orange flowers, as well as medium-to-large size and a dark center was cultivated in the present study. The plants were grown in plastic pots whenever they had 4–6 leaves (volume, height, and diameter of the pot of 7 L, 19 cm, and 24 cm, respectively). It should be noted that the soilless growing medium, which was obtained from Green Azin Company, Tabriz, Iran, contained perlite (30%), peat moss (65%), and cocopeat (5%).
The potting medium was inoculated with a combination of three fungi, including Rhizophagus fasciculatus, Diversispora versiformis, and Funneliformis mosseae. Inoculation was applied at the time of plant planting by placing 60 g of inoculum (soil, spores, hyphae, and infected roots) in the root zone of half plants (inoculated plants). Non-AM plants received the same weight as the autoclaved inoculum. Furthermore, the main inoculum was obtained from the Microbial Bank of the Department of Soil Sciences, Urmia University.
In this experiment, putrescine (Sigma-Aldrich) foliar spraying was used at the concentrations of 0, 1, 2, and 4 mM two weeks after planting and establishing the Gerbera plant, once every 15 days, for three months.
Growing conditions
The growing conditions in the greenhouse were light intensity of 400–500 μmol.m–2.s−1, day/night temperature of 20–25/13–16 °C, and a relative humidity of 60 ± 5%. The plants were fertilized three times a week based on the constitution of the nutrient solution mentioned in Rakbar et al. 2022.
Biometric measurements
Morphological characteristics were calculated two weeks following the ultimate treatments. Moreover, the pedicle length and its diameter were precisely determined by a ruler and a digital caliper (Mitutoyo, Japan), respectively. Additionally, the roots were cleansed with water. In addition, a digital scale (METTLER, PJ300) with a 0.0001 g accuracy was utilized to compute the roots’ fresh weight. To estimate the dry weight of the roots, the samples were placed in an oven at a temperature of 72 °C for 72 h and then measured with a digital scale. A cylinder containing 500 cc of water was used to measure the root volume. Further, the root was placed in a cylinder, followed by writing down the volume of water that came up and counting the volume of the root.
Cut flower characteristics
When 2–3 stamen rows of bisexual disk florets matured, the flowers were harvested by pulling the stems from the plants (Geraspolus and Chebli 1999). The stems were cut to a length of 45 cm and immediately transported to the laboratory. After recording the initial fresh weight, the flowers were placed in glass vases with 500 ml of distilled water. The flowers were then kept under controlled conditions (photoperiod of 14 h at photosynthetically activated radiation of 15 μmol.m−2.s−1 provided by fluorescent lamps, temperature of 20 ± 3 °C, and relative humidity of 65–70%). Changes in fresh weight and solution absorption were expressed as relative fresh weight (RFW) and relative solution uptake at different times after harvesting (the 1st, 6th, and 12th day).
Relative fresh weight
The fresh weight of each cut stem was measured on the 1st, 6th, and 12th days of the experiment. RFW was calculated using the method of Joyce and Jones (1992) and expressed in g per g of the initial fresh weight per day by the following formula:
The amount of relative solution uptake
The cut flowers of Gerbera were placed in vessels that consisted of 500 ml of distilled water. Moreover, the level of solution uptake was determined as the ratio of the solution absorbed to the flower and the initial fresh weight of the flower stem and represented in ml g−1 initial fresh weight day−1 by the following formula (Alaey 2011):
Phenylalanine ammonia-lyase activity (PAL) assay
The method of Kang and Saltveit (2002) was used to prepare the plant extract in order to measure the activity of the PAL enzyme. The reaction mixture containing 50 mM phosphate buffer (pH 7), 10 mM phenylalanine, 0.4 ml distilled water, and 0.1 ml enzyme extract was mixed and incubated at 37 °C for one hour. The reaction time was stopped by adding 6 M hydrochloric acid, and the samples’ absorbance was determined by a spectrophotometer (HALO DB-20, Dynamica, England) at 260 nm (D’cunha et al. 1996). PAL enzyme activity was expressed according to the acid kinematic standard by the following formula:
Measurement of the amount of leaf elements
Measurement of leaf nitrate (NO3.2−)
For this purpose, 0.1 g of ground leaf samples was suspended in 10 ml of distilled water and kept for one hour at 45 °C and filtered through Whatman No. 40 filter paper. The samples were extracted and analyzed immediately or within 24 h after extraction at 4 °C. A volume of 0.1 ml of the previous extract was thoroughly mixed with 0.4 ml of salicylic acid solution in a 30 ml tube. After 20 min at room temperature, 9.5 ml of 2N NaOH solution was added slowly. The mixture was cooled to room temperature, followed by reading the color intensity at a 410 nm wavelength by applying a spectrophotometer. Nitrate–N in plant tissue is expressed as mg NO3−.g−1 dry weight by the following formula (Cataldo et al. 1975):
Measurement of leaf phosphorus, potassium, and calcium
The colorimetric method was employed to measure the amount of plant P based on the method of Ohyama et al. (1991). To this end, ammonium molybdate, ammonium vanadate, and phosphorus solution standards were provided, and then the samples were read via a spectrophotometer at a 470 nm wavelength. After calculation, the amount of P was expressed as a percentage. The K content of plants was estimated by applying a flame photometer with the method presented by Ohyama et al. (1991). Ca content was calculated by titration with 0.01 M ethylenediaminetetraacetic acid (EDTA). Finally, the amount of the consumed EDTA was recorded, and then the amount of Ca ion was measured using the following formula (Ghazan Shahi 2006):
V = 5, N = 0.01, VEDTA = 0.5.
Statistical analysis
Plants were treated based on a two-factor factorial design with three repetitions. Data management and analysis were performed using SAS, version 9.2 (North Carolina State University). Eventually, the means underwent a comparison by applying Tukey’s multi-domain method at a significance level of 5%.
Results
Pedicel length and diameter
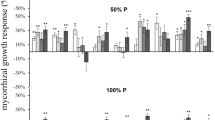
As shown in Fig. 1, pedicel length increased with an increase in the putrescine concentration, but at each level of putrescine, the pedicel length of inoculated plants was greater than that of non-inoculated plants. Based on Fig. 2a, putrescine treatment (at all concentrations) significantly increased the pedicel diameter compared to the control plants. Likewise, the inoculated plants demonstrated significantly greater pedicel diameter than the non-inoculated groups (Fig. 2b).
Root fresh and dry weight
Based on data related to the fresh and dry weights of the plant roots, the concentration of putrescine 2 or 4 mM with the inoculation of mycorrhizal fungi was associated with a significant increase in the fresh weight compared to the control plants (Fig. 3). Accordingly, no significant difference was observed in the fresh weight of the roots of non-inoculated plants with the use of putrescine. Mycorrhizal fungi increased the dry weight of roots and represented a noticeable difference in comparison to non-inoculated plants (Fig. 4).
Root volume
By analyzing the amount of root volume under the conditions of this research, plants treated with 2 or 4 mM putrescine demonstrated the highest increase in the root volume. Nonetheless, there was no significant difference between the control plants and plants sprayed with 1 mM putrescine (Fig. 5a). Finally, mycorrhizal fungi increased root volume and represented a considerable difference in comparison with non-inoculated plants (Fig. 5b).
Nutrient uptake
Phosphorus
In terms of the impact of mycorrhiza and putrescine treatments on the P amount, the interaction impact of mycorrhiza inoculation and putrescine foliar spraying could increase the level of leaf P compared to the control (Fig. 6). Interestingly, the foliar application of putrescine failed to increase the P content of non-inoculated plants.
Calcium
Based on the results (Fig. 7), the use of putrescine on inoculated Gerbera plants had a definite effect on the amount of leaf Ca and caused a significant increase compared to the control. No increase in Ca content was observed in non-inoculated plants using putrescine.
Potassium
The obtained data (Fig. 8) revealed that with the application of putrescine, the concentration of leaf K in non-inoculated plants did not differ significantly, while it increased in inoculated plants. In general, at all concentrations of putrescine (1, 2, and 4 mM), plants inoculated with AMF had higher K content than non-inoculated plants.
Nitrate
By analyzing the amount of nitrate under the conditions of the current study, the highest increase in nitrate percentage was found in plants treated with 2 mM putrescine and mycorrhiza. The lowest amount of nitrate (0.009 mg. g−1 DW) was related to the control treatment without the inoculation of mycorrhizal fungi (Fig. 9).
Post-harvest indices
Relative fresh weight
Regarding the impact of various concentrations of putrescine and mycorrhiza inoculation on the RFW of Gerbera plants, the data indicated (Table 1) that the increase in RFW was associated with putrescine treatments (1 mM) and mycorrhiza inoculation 6 and 12 days after harvest. On day 12, the lowest RFW (0.63 g. g−1 FW0) belonged to the control condition)with no mycorrhizal fungi inoculation).
Relative adsorbed solution
The data represented that the spraying of putrescine, along with mycorrhizal inoculation, increases the absorption of the solution by the flower, indicating that plant transpiration is less than absorption, which delays the wilting of the flower and prolongs the vase life (Table 1). In inoculated plants, all concentrations of putrescine increased the absorbed solution 6 and 12 days after harvest compared to non-inoculated plants.
Phenylalanine ammonia-lyase
According to the activity of PAL, the enzyme activity increased during the vase life period of Gerbera cut flowers so that on days 6 and 12, the enzyme activity level demonstrated a noticeable difference with day 1. The greatest amount of PAL activity (54.94 u/mg) belonged to the 2 mM putrescine treatment with mycorrhizal fungi inoculation on day 12, and it was significantly different from the control on all days. The lowest activity of PAL was associated with the control (with no mycorrhizal fungi inoculation), the details of which are provided in Table 2.
Discussion
Based on the findings of the present study, mycorrhizal fungi inoculation and putrescine treatment could increase the growth and development of Gerbera and nutrient absorption. PAs are considered a group of growth regulators that play an essential role in the biochemical and physiological processes of plants. They are related to the regulation of enzyme activity, DNA replication, gene transcription, cell division, and membrane stability, and have a wide range of biological functions in plant growth and development (Chen et al. 2019). It has been also demonstrated that the chemical or genetic depletion of putrescine is lethal for many organisms, not only for plants, suggesting that putrescine may play a vital role in growth and development (Kusano et al. 2008). Therefore, in the present study, the increase in pedicel length and diameter could be due to the effect of PAs on cell division and cell length increase.
In this research, spraying G. jamesonii with putrescine had a significant effect on root growth in inoculated plants. These effects may be related to the known role of PAs in regulating cell division and differentiation in the root apex and during lateral and adventitious root formation (Yu et al. 2016). In addition to being a signaling molecule, putrescine can interact with gaseous molecules, phytohormones, and the like (González-Hernández et al. 2022). Overall, putrescine was affirmatively associated with gene expression for biosynthesis of indole acetic acid (IAA) (Anwar et al. 2015). Auxin biosynthesis, transport, and its signaling play a vital role in root growth and development control. The consistent role of auxin in root development has identified it as an important regulator (Shivani et al. 2013). The Pas–auxin interaction has been reported in root formation and growth in two sweet orange (Citrus sinensis L. Osb.) cultivars (Mendes et al. 2011). Furthermore, S-adenosyl methionine (SAM) is a precursor of ethylene and PAs biosynthesis (Couee et al. 2004), and ethylene also participates in auxin biosynthesis. Therefore, it is possible that PAs also indirectly regulates root growth and development through interaction with ethylene and auxin. Our results are consistent with those of Abbasi et al. (2017) demonstrating that foliar application of putrescine increased plant growth and root and shoot biomass.
The increase in the dry weight of the plant shoots and other growth traits are related to the high absorption of P in the plant and better root colonization and establishment. AMF inoculation can significantly increase the concentration of various macronutrients and micronutrients, increasing the production of photosynthate and thus the accumulation of biomass (Chen et al. 2017; Mitra et al. 2019). AMF can enhance the uptake of mineral nutrients in nearly all plants, particularly phosphate (Nell et al. 2010). Following this research, experimental tests focusing on AMF-inoculated tomato plants revealed an increase in N, K, Ca, and P contents and leaf area, representing an increase in plant growth (Balliu et al. 2015). AMF establish a symbiosis with roots in order to obtain essential nutrients from the host plant, providing mineral nutrients (e.g., N, P, K, Ca, Zn, and S). Moreover, they produce fungal structures such as arbuscules, helping in the exchange of compounds of carbon and P and inorganic minerals, eventually providing important vigor to host plants (Prasad et al. 2017). Hence, they can noticeably increase the P concentration in shoot and root systems (Al-Hmoud and Al-Momany 2017). An increase in the photosynthetic activity and other leaf functions is directly attributed to an improvement in the growth frequency of AMF inoculum, which is directly associated with N, K, P, and carbon uptake, moving to roots and promoting tuber growth (Begum et al. 2019). N increases photosynthesis by increasing leaf thylakoids and stroma protein. K plays a vital role in the production of proteins and hydrocarbons in plants. It provides the pressure potential necessary for growth by producing high vacuole turgor in expanded cells. The appropriate amount of absorbed P can improve root growth, thus enhancing the quantity and quality of flowers (Khalaj et al. 2019). Mycorrhizal fungi also stimulate rooting by producing auxin, cytokinin, and gibberellins, causing hormonal changes and activating the root meristem (Pons et al. 2020). As a result, the absorption of nutrients increases in the presence of mycorrhiza; thus, improving these nutritional conditions and other beneficial effects of mycorrhizal fungi can improve plant growth and performance characteristics. Probably, putrescine and mycorrhiza have caused more expansion of the root due to their effect on plant growth regulators and absorption of nutrients, thus increasing its yield. The ability of the root to absorb water and nutrients increases the growth of the shoot and root; as a result, the biomass of the plant has increased, and it has gained a greater ability to produce shoots and flowers.
Studies demonstrated that PAs play a role in the process of root growth, and the application of exogenous PAs enhances the structure of the root through increasing the percentage of hairy and thin roots. These alterations can enhance nutrient absorption and increase their concentrations in the plant. On the other hand, PAs can function as a source of N for plants and improve plant growth (Rezvanipour et al. 2016). The dry matter of the plant increased with an increase in N content (Fekri 1999). It was reported that in plants treated with putrescine, the physiological efficiency of these plants improved due to the increase in the efficiency of the roots in the absorption of macronutrient elements (Habba et al. 2016). According to a previous study, the effect of polyamine on the growth rate is because it helps absorb minerals such as N, P, and K from the soil (Abbasi et al. 2017).
The results of many previous studies emphasize the increase of P and K absorption by the inoculation of mycorrhizal fungi. The effect of mycorrhizal fungi on the absorption of elements by plant roots is noticeable and depends on the type of plant element and plant species. Evidence suggests that mycorrhizae prevent nutrient absorption (Arjmand Alavi et al. 2014). In contrast, some reports indicate that inoculation with mycorrhizal fungi increases nutrient uptake (Perner et al. 2007). Roots inoculated with mycorrhizal fungi develop the networks of hyphae that allow the hyphae to absorb nutrients directly from the rhizosphere. Mycorrhiza can also release glomalin, which is part of the soil structure, into the rhizosphere and improve the structure of the rhizosphere and better absorb water and nutrients from the rhizosphere (Bi et al. 2018). Considering that putrescine and mycorrhiza increase root efficiency in the plant rhizosphere, they increase the plant’s ability to better absorb nutrients. Based on the results of the present study, putrescine and mycorrhiza could increase the absorption of P, K, Ca, and nitrate.
Water balance is the main factor in determining the quality of cut flowers. Cut flowers lose more water and wilt when transpiration exceeds water absorption. The inability of flowers to absorb water is one of the reasons for the flourishing of flowers, which may be due to the blockage of vessels (Ahmadi Majd et al. 2021; Fanourakis et al. 2016, 2021). Gerbera cut flowers are susceptible to drooping. During the vase life, the neck bends and the flow of water to the flower is almost blocked. The resistance to drooping depends on the strength and density of the stems and pedicels, which is determined by the absorption of the solution. Previous studies indicated that PAs increase water absorption. The proposed mechanism for PAs can be the role of these compounds in reducing evaporation from the tissue of cut flowers, as well as reducing their respiration, which prevents the weight loss and wrinkling of cut flowers and maintains their quality (Successful and Zamani Bahramabadi 2015).
In the present study, it was found that putrescine and mycorrhizal fungi affect PAL activity in plants. This activity is influenced by several factors, including light, temperature, growth regulators, inhibitors of RNA and protein synthesis, wounding, mineral nutrition, and stimulus treatment (Mohr and Cahill 2001). PAL can also be activated by fungal stimuli and is the first and most important allosteric enzyme of phenylpropanoid metabolism. The phenylpropanoid pathway catalyzed by PAL leads to various derivatives such as phenolics, lignin, suberin, and the like (Abd Elbar et al. 2019). Therefore, increasing PAL activity increases lignin biosynthesis. The occurrence of pedicel bending during the vase life is one of the major problems after harvesting Gerbera cut flowers, threatening flower producers and consumers. In general, the bent neck of cut flowers is associated with a poor lignification mechanism during pedicel elongation (Cinotti et al. 2005). A decrease in lignin content weakens the stiffness of the stem and the vascular tissue that supports water and minerals in the xylem, thereby reducing the mechanical strength of real flowers, and disrupts the transport of water and minerals to the flower, leading to pedicel bending (Soe et al. 2022).
Based on the findings of this study, it is recommended that in addition to the use of mycorrhizal fungi, the use of 2 and 4 mM putrescine is beneficial for growth and nutrient absorption. Some studies clearly confirmed the relationship between PAs and improved plant growth and development due to their effects on cell division and differentiation (e.g., Khan et al. 2008; Qing-Sheng and Zou 2009). However, it depends on the plant species and the type of polyamine (Liu et al. 2006). In this research, 2 and 4 mM putrescine were effective not only in the growth and development of Gerbera but also in plant–fungus interactions, root growth, and absorption of nutrients, including N, by the roots. An increase in leaf nutrient content by PAs has also been observed in other horticultural plants, including gladiolus, pepper, and trifoliate orange seedlings (Nahed et al. 2009; Shawky 2003; Wu et al. 2010).
In this study, the foliar spraying of 1-mM putrescine was useful for improving the post-harvest quality of Gerbera cut flowers. In accordance with these results, previous literature demonstrated that the foliar application of PAs at low concentrations is effective at bud stages (Upfold and Van Staden 1991). When PAs are sprayed, first, they accumulate in the petals and are successively transported to the flower stem, where they probably delay the progression of senescence by combining with various cell wall components (Bagni and Tassoni 2006). The results of this research are consistent with the findings of Bagni and Tassoni (2006), indicating that spraying high concentrations of PAs has a toxic effect on the appearance of flowers, but does not have adverse effects on cut flowers in vase water.
Conclusion
The current study evaluated the impact of mycorrhizal fungi and putrescine on some growth and post-harvest features of G. jamesonii ‘Dune.’ According to the results of the present research, putrescine and mycorrhiza improved the growth indicators and better absorption of elements by the roots, as well as maintaining the water balance and freshness of Gerbera cut flowers. According to the obtained results, 2 and 4 mM putrescine had a positive effect on growth and element absorption indicators, but 1 mM putrescine was more effective after harvest. The findings of this study can be highly useful for Gerbera growers in producing high-quality cut flowers that last longer.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Abbasi NA, Ali I, Hafiz IA, Khan AS (2017) Application of polyamines in horticulture: a review. Int J Biosci 10(5):319–342. https://doi.org/10.12692/ijb/10.5.319-342
Abd Elbar OH, Farag RE, Shehata SA (2019) Effect of putrescine application on some growth, biochemical and anatomical characteristics of Thymus vulgaris L. under drought stress. Ann Agric Sci 64(2):129–137. https://doi.org/10.1016/j.aoas.2019.10.001
Ahmadi-Majd M, Rezaei Nejad A, Mousavi-Fard S, Fanourakis D (2021) Deionized water as vase solution prolongs flower bud opening and vase life in cut carnation and rose through sustaining an improved water balance. Eur J Hortic Sci 86:682–693. https://doi.org/10.17660/eJHS.2021/86.6.12
Alaey M (2011) Study the effect of salicylic acid in pre- and post-harvest treatment on physiochemical characteristics and vase-life of Rose hybrid L. Black Magic. Ph.D. Thesis. Faculty of Agriculture Tehran University, Iran. (In Persian)
Al-Hmoud G, Al-Momany A (2017) Effect of four mycorrhizal products on squash plant growth and its effect on physiological plant elements. Adv Crop Sci Tech 5:260. https://doi.org/10.4172/2329-8863.1000260
Anwar R, Mattoo AK, Handa AK (2015) Polyamine interactions with plant hormones: crosstalk at several levels. Polyamines. Springer, Tokyo, pp 267–302
Arjmand Alavi M, Ehteshami M, Hatamzadeh A (2014) The effect of bulb inoculation with four species of mycorrhiza on the quantitative and qualitative yield of two species of lilies. Iran Seed Sci Res 1(2):57–65 (In Persian)
Ataii D, Naderi R, Khandan Mir Koohi A (2018) The effect of putrescine pre-harvest treatment on quantitative, qualitative and post-harvest characteristics of cut flowers of (Eustoma grandiflorum cv. Miarichi Grand White). Hortic Sci Iran 48(2):229–242 (In Persian)
Bagni N, Tassoni A (2006) The role of polyamines in relation to flower senescence. Floricult Ornam Plant Biotechnol 1536(1):855–856
Balliu A, Sallaku G, Rewald B (2015) AMF Inoculation enhances growth and improves the nutrient uptake rates of transplanted, salt-stressed tomato seedlings. Sustainability 7:15967–15981. https://doi.org/10.3390/su71215799
Begum N, Qin C, Ahanger MA, Raza S, Khan MI, Ashraf M, Ahmed N, Zhang L (2019) Role of arbuscular mycorrhizal fungi in plant growth regulation: implications in abiotic stress tolerance. Front Plant Sci 10:1068. https://doi.org/10.3389/fpls.2019.01068
Bi Y, Zhang Y, Zou H (2018) Plant growth and their root development after inoculation of arbuscular mycorrhizal fungi in coal mine subsided areas. Int J Coal Sci Technol 5(1):47–53. https://doi.org/10.1007/s40789-018-0201-x
Cataldo DA, Haroon M, Schrader LE, Young VL (1975) Rapid colorimetric determination of nitrat in plant tissue by nitration of salicylic acid. Soil Sci Plant Anal 6:71–80. https://doi.org/10.1080/00103627509366547
Chen S, Zhao H, Zou C, Li Y, Chen Y, Wang Z et al (2017) Combined Inoculation with multiple arbuscular mycorrhizal fungi improves growth, nutrient uptake and photosynthesis in cucumber seedlings. Front Microbiol 8:25–16. https://doi.org/10.3389/fmicb.2017.02516
Chen D, Shao Q, Yin L, Younis A, Zheng B (2019) Polyamine function in plants: metabolism, regulation on development, and roles in abiotic stress responses. Front Plant Sci 9:1–13. https://doi.org/10.3389/fpls.2018.01945
Cinotti A, Ferrante A, Serra G, Tognoni F (2005) Stem bending study of cut Gerbera jamesonii H. Bolus flowers. Cult Protette 34:131–136
Collado-González J, Piñero MC, Otálora G, López-Marín J, del Amor FM (2021) Effects of different nitrogen forms and exogenous application of putrescine on heat stress of cauliflower: photosynthetic gas exchange, mineral concentration and lipid peroxidation. Plant 10:152–170. https://doi.org/10.3390/plants10010152
Couee I, Hummel I, Sulmon C, Gouesbet G, Amrani AE (2004) Involvement of polyamines in root development. Plant Cell Tiss Organ Cult 76:1–10. https://doi.org/10.1023/A:1025895731017
Courty PE, Smith P, Koegel S, Redecker D, Wipf D (2015) Inorganic nitrogen uptake and transport in beneficial plant root-microbe interactions. Crit Rev Plant Sci 34(1–3):4–16. https://doi.org/10.1080/07352689.2014.897897
D’cunha GB, Satyanarayan V, Nair PM (1996) Purification of phenyl alanine ammonialyase from Rhodotorulag lutinis. Phytochem 42:17–20. https://doi.org/10.1016/0031-9422(95)00914-0
Danaee E, Abdossi V (2018) Effect of different concentrations and application methods of polyamines (Putrescine, Spermine, Spermidine) on some morphological, physiological, and enzymatic characteristics and vase life of rosa hybrida cv. ‘Dolce Vita’ cut flower. J Ornament Plant 8(3):171–182
Dasgan HY, Kusvuran S, Ortas I (2008) Responses of soilless grown tomato plants to arbuscular mycorrhizal fungal (Glomus fasciculatum) colonization in recycling and open systems. Afr J Biotech 7:3606–3613
Deng ZH, Bhattarai K (2018) Chapter 17. Gerbera. University of Florida, IFAS, Department of Environmental Horticulture, Gulf Coast Research
Dong CJ, Wang LL, Li Q, Shang QM (2019) Bacterial communities in the rhizosphere, phyllosphere and endosphere of tomato plants. PLoS One 14:e0223847. https://doi.org/10.1371/0223847
Fanourakis D, Giday H, Li T, Kambourakis E, Ligoxigakis EK, Papadimitriou M, Strataridaki A, Bouranis D, Fiorani F, Heuvelink E, Ottosen C-O (2016) Antitranspirant compounds alleviate the mild-desiccation-induced reduction of vase life in cut roses. Postharvest Biol Technol 117:110–117. https://doi.org/10.1016/j.postharvbio.2016.02.007
Fanourakis D, Papadopoulou E, Valla A, Tzanakakis VA, Nektarios PA (2021) Partitioning of transpiration to cut flower organs and its mediating role on vase life response to dry handling: a case study in chrysanthemum. Postharvest Biol Technol 181:111636. https://doi.org/10.1016/j.postharvbio.2021.111636
Fekri M (1999) Effects of nitrogen, potassium and boron on leaf nutrient concentration, yield, quality and budding of pistachio trees. Doctoral dissertation, University of Tehran, Tehran, p 110 (In Persian)
Genre A, Lanfranco L, Perotto S, Bonfante P (2020) Unique and common traits in mycorrhizal symbioses. Nat Rev Microbiol 18:649–660. https://doi.org/10.1038/s41579-020-0402-3
Geraspolus D, Chebli B (1999) Effects of pre- and post-harvest calcium applications on the vase-life of cut Gerberas. J Hortic Sci Biotechnol 74:78–81. https://doi.org/10.1080/14620316.1999.11511076
Ghazan Shahi J (2006) Soil and plant analysis. Motarjem Press, Mashhad, p 311
Gianinazzi-Pearson V, Maldonado-Mendoza I, Lopez-Meyer M, Weidmann S, Harrison MJ (2021) Arbuscular mycorrhiza. In: Mathesius U, Journet EP, Sumner LW (eds.) The Medicago truncatula Handbook; Noble Research Institute, Ardmore, OK, 2006; Available online: http://www.noble.org/MedicagoHandbook
González-Hernández AI, Scalschi L, Troncho P, García-Agustín P, Camañes G (2022) Putrescine biosynthetic pathways modulate root growth differently in tomato seedlings grown under different N sources. J Plant Physiol 268:1–10. https://doi.org/10.1016/j.jplph.2021.153560
Habba EE, Abdel Aziz NG, Sarhan AMZ, Arafa AMS, Youssefi NM (2016) Effect of putrescine and growing media on vegetative growth and chemical constituents of Populus euramericana plants. J Innov Pharm Biol Sci 3(1):61–73
Halpern M, Bar-Tal A, Ofek M, Minz D, Muller T, Yermiyahu U (2015) The use of biostimulants for enhancing nutrient uptake. Adv Agron 130:141–174
Joyce DC, Jones PN (1992) Water balance of the foliage of cut Geraldton waxflower. Post Harvest Biol Technol 2:31–39. https://doi.org/10.1016/0925-5214(92)90025-K
Kang HM, Saltveit ME (2002) Chilling tolerance of maize, cucumber and rice seedling leaves and roots and differentially affected by salicylic acid. Plant Physiol 115:571–576. https://doi.org/10.1034/j.1399-3054.2002.1150411.x
Karimi M, Akbari F, Heidarzade A (2017) Protective effects of polyamines on regulation of senescence in spray carnation cut flowers (Dianthus caryophyllus’Spotlight’). Acta Agric Slov 109(3):509–515. https://doi.org/10.14720/aas.2017.109.3.03
Khalaj MA, Kumar S, Roosta HR (2019) Evaluation of nutrient uptake and flowering of Gerbera in response of various growing media. World J Environ Biosci 8(4):12–18
Khan AS, Singh Z, Abbasi NA, Swinny EE (2008) Pre-or post-harvest application of putrescine and low temperature storage affect fruit ripening and quality of ‘Angeline’ plum. J Sci Food Agric 88:1686–1695. https://doi.org/10.1002/jsfa.3265
Kusano T, Berberich T, Tateda C, Takahashi Y (2008) Polyamines: essential factors for growth and survival. Plant 228(3):367–381. https://doi.org/10.1007/s00425-008-0772-7
Lin Y, Jones ML (2022) Evaluating the growth-promoting effects of microbial bio stimulants on greenhouse floriculture crops. HortSci 57(1):97–109. https://doi.org/10.21273/HORTSCI16149-21
Liu JH, Honda C, Moriguchi T (2006) Involvement of polyamines in floral and fruit development. Jpn Agric Res q 40:51–58. https://doi.org/10.6090/jarq.40.51
Liu JH, Wang W, Wu H, Gong X, Moriguchi T (2015) Polyamines function in stress tolerance: from synthesis to regulation. Front Plant Sci 6:827. https://doi.org/10.3389/fpls.2015.00827
Mendes AFS, Cidade LC, Otoni WC, Soares-Filho WS, Costa WGC (2011) Role of auxins, polyamines and ethylene in root formation and growth in sweet orange. Biol Plant 55:375–378. https://doi.org/10.1007/s10535-011-0058-y
Mitra DU, Navendra U, Panneerselvam S, Ansuman AN, Ganeshamurthy AN, Divya J (2019) Role of mycorrhiza and its associated bacteria on plant growth promotion and nutrient management in sustainable agriculture. Int J Life Sci Appl Sci 1:1–10
Mohr PG, Cahill DM (2001) Relative roles of glyceollin, lignin and the hypersensitive response and the influence of abscisic acid in compatible and incompatible interactions of soybeans with Phytophthora sojae. Physiol Mol Plant Pathol 58:31–41. https://doi.org/10.1006/pmpp.2000.0306
Nahed GA, Lobana ST, Soad MMI (2009) Some studies on the effect of putrescine, ascorbic acid and thiamine on growth, flowering and some chemical constituents of gladiolus plants ‘Nubaria.’ Ozean J Appl Sci 2:169–179
Nell M, Wawrosch C, Steinkellner S, Vierheilig H, Kopp B, Lössl A (2010) Root colonization by symbiotic arbuscular mycorrhizal fungi increases sesquiterpenic acid concentrations in Valeriana officinalis L. Planta Med 76:393–398. https://doi.org/10.1055/s-0029-1186180
Ohyama T, Ito M, Kobayashi K, Araki S, Yasuyoshi S, Sasaki O, Yamazaki T, Sayoma K, Tamemura R, Izuno Y, Ikarashi T (1991) Analytical procedures of N, P and K content in plant and manure materials using H2SO4-H2O2 Kjeldahl digestion method. Bulletin of the faculty of agriculture, Niigata University. Food Agric Organ United Nation 43:111–120
Paradikovic N, Teklic T, Zeljkovic S, Lisjak M, Spoljarevic M (2019) Biostimulants research in some horticultural plant species–a review. Food Energy Secur 8:e00162. https://doi.org/10.1002/fes3.162
Perner H, Schwarz D, Bruns Ch, Mäder M, George E (2007) Effect of arbuscular mycorrhizal colonization and two levels of compost supply on nutrient uptake and flowering of pelargonium plants. Mycorrhiza 17:469–474. https://doi.org/10.1007/s00572-007-0116-7
Pons S, Fournier S, Chervin Ch, Bécard G, Rochange S, Frey NFD, Pagès VP (2020) Phytohormone production by the arbuscular mycorrhizal fungus Rhizophagus irregularis. PLoS One 15(10):e0240886. https://doi.org/10.1371/0240886
Prasad R, Bhola D, Akdi K, Cruz C, Sairam KVSS, Tuteja N et al (2017) Introduction to mycorrhiza: historical development. In: Varma A, Prasad R, Tuteja N (eds) Mycorrhiza. Springer, Cham, pp 1–7. https://doi.org/10.1007/978-3-319-53064-2_1
Qing-Sheng WU, Zou Y (2009) The effect of dual application of arbuscular mycorrhizal fungi and polyamines upon growth and nutrient uptake on trifoliate orange (Poncirus trifoliate) Seedlings. Not Bot Horti Agrobot Cluj Napoca 37:95–98. https://doi.org/10.15835/nbha3723237
Quilambo OA (2003) The vesicular-arbuscular mycorrhizal symbiosis. Afr J Biotechnol 2:539–546. https://doi.org/10.5897/AJB2003.000-1105
Rakbar S, Jabbarzadeh Z, Barin M (2022) Effect of exogenous putrescine on flower growth, post-harvest quality and root mycorrhizal development of gerbera (Gerbera jamesonii cv. Dune) cut flowers. S Afr J Bot 150:641–650. https://doi.org/10.1016/j.sajb.2022.08.002
Rezvanipour S, Hatamzadeh A, Elahinia SA, Asghari HR (2016) Exogenous polyamines improve mycorrhizal development and growth and flowering of Freesia hybrida. J Hortic Res 23(2):17–25 (In Persian)
Rymbai H, Jha AK, Talang HD, Assumi SR, Deshmukh NA, Roy AR (2017) Evaluation of new Gerbera (Gerbera jamesonii Bolus) genotypes under fan and pad polyhouse. J Ornament Hortic 20(3&4):126–131
Sabatino L, D’anna F, Torta L, Ferrara G, Iapichino G (2019) Effects of arbuscular mycorrhizal fungi on Gazania rigens pot plant cultivation in a mediterranean environment. Not Bot Horti Cluj-Napoca Agrobot 47(1):221–226. https://doi.org/10.15835/nbha47111272
Shawky NBT (2003) Physiological studies on the effect of salinity, ascorbic acid and putrescine on sweet pepper plant, vol 21. Ph. D. thesis, Fac. Agric., Cairo University, Egypt, pp 1070–1071
Shivani S, Sharma I, Kaur N, Pati PK (2013) Auxin: a master regulator in plant root development. Plant Cell Rep 32(6):741–57. https://doi.org/10.1007/s00299-013-1430-5
Smith SE, Read DJ (2008) Mycorrhizal symbiosis, 3rd edn. Academic Press, San Diego, CA, p 803
Soe MT, Naing AH, Kim SR, Kim ChK (2022) Characterizing the effects of different chemicals on stem bending of cut snapdragon flower. Plant Method 18(4):1–10. https://doi.org/10.1186/s13007-021-00835-1
Upfold SJ, Van Staden J (1991) Polyamines and carnation flower senescence: endogenous levels and the effect of applied polyamines on senescence. Plant Grow Regul 10:355–362. https://doi.org/10.1007/BF00024594
Wu QS, Zou YN, He XH (2010) Exogenous putrescine, not spermine or spermidine, enhances root mycorrhizal development and plant growth of trifoliate orange (Poncirus trifoliata) seedlings. Int J Agric Biol 12:576–580. https://doi.org/10.2306/scienceasia15131874.2010.36.254
Yadav K, Singh N, Aggarwal A (2012) Arbuscular mycorrhizal (AM) technology for the growth enhancement of micropropagated Spilanthes acmella Murr. Plant Protect Sci 48:31–36. https://doi.org/10.17221/21/2011-PPS
Yousefi F, Jabbarzadeh Z, Amiri J, Rasouli-Sadaghiani MH (2019) Response of roses (Rosa hybrida L. ‘Herbert Stevens’) to foliar application of polyamines on root development, flowering, photosynthetic pigments, antioxidant enzymes activity and NPK. Sci Rep 9(16025):1–11. https://doi.org/10.1038/s41598-019-52547-1
Yu Y, Jin C, Sun C, Wang J, Ye Y, Zhou W, Lu L, Lin X (2016) Inhibition of ethylene production by putrescine alleviates aluminum-induced root inhibition in wheat plants. Sci Rep 6(1):18888. https://doi.org/10.1038/srep18888
Funding
No funding was received for this work.
Author information
Authors and Affiliations
Contributions
SR and ZJ conducted physiological analysis and wrote the manuscript. MB prepared mycorrhiza and conducted leaf element analysis.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest.
Additional information
Communicated by P. K. Nagar.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rakbar, S., Jabbarzadeh, Z. & Barin, M. Impact of putrescine and arbuscular mycorrhizal fungi on nutrient uptake, growth, and post-harvest performance of Gerbera (Gerbera jamesonii cv. Dune) cut flowers. Acta Physiol Plant 46, 45 (2024). https://doi.org/10.1007/s11738-024-03674-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-024-03674-4