Abstract
The role of protein modifications and amino acid metabolism in the dormancy breaking of pistachio (Pistacia vera L.) kernels during moist chilling (5 ºC) and warm stratification (25 ºC) were studied. Cold-stratified kernels showed germination up to 97%, while warm-stratified ones had low germination (40%). Increased protein solubility at neutral pH was accompanied by protein carbonylation in both cotyledons and embryonic axes during cold treatment, whereas these values decreased under warm incubation. Amino acid accumulation occurred in both tissues of cold- and warm-stratified kernels. Arginase activity increased in both tissues of cold-stratified kernels but significantly declined during warm treatment. While arginine decarboxylase activity of both organs increased under cold and warm stratification of pistachio kernels, ornithine aminotransferase activity declined during these periods. These results show that increased protein solubility and its carbonylation during cold stratification may induce the protein mobilization and accumulation of amino acids for their subsequent direction to the proper metabolic pathways. In this way, protein modification and arginine metabolism by arginase can be considered germination-specific events during cold stratification of kernels.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Dormancy is a current phenomenon in seeds from many temperate tree species (Derkx 2000). Dormant seeds are temporarily unable to complete germination. Pre-incubation of seeds in varying periods of moist chilling or cold stratification can alleviate dormancy and promote germination. The most common moist chilling-dependent alterations that occur in the dormant seeds include phytohormonal balance (Blake et al. 2002; Koornneef et al. 2002; Jacobsen et al. 2002; Schmitz et al. 2002) and storage reserves mobilization resulting from changes in various hydrolytic enzymes activities (Li and Ross 1990a, b; Forward et al. 2001; Bogatek et al. 2002; Andriotis et al. 2004; Nezamdoost et al. 2009; Einali and Valizadeh 2017).
Cold stratification-dependent storage protein change, which is associated with alterations in protein solubility and activation of proteolytic enzymes, is a pivotal occurrence for seed dormancy breaking because of providing free amino acids and new synthesis of germination involved proteins (Ranjan and Lewak 1995; King and Gifford 1997; Forward et al. 2001; Todd et al. 2001; Rajjou et al. 2004; Einali and Sadeghipour 2007; Vanita et al. 2008; Einali and Valizadeh 2017). Modifications of proteins frequently include carbonylation, the addition of carbonyl (aldehyde or ketone) groups to the amino acid residues (Dalle-Donne et al. 2003). It is demonstrated that carbonylation not only modifies protein solubility but also principally enhances their sensitivity to proteolytic enzymes (Dean et al. 1997; Vanita et al. 2008). However, the routes by which the amino acid products of storage protein mobilization are entered into metabolic and biosynthetic pathways or the processes through which amino acids are transported from cotyledons to the axes of stratified seeds are largely unknown. It has been found that arginine constitutes the main part of the amino acid pool and is a major residue of seed storage proteins in many pine species (King and Gifford 1997) and walnut kernels (Mapelli et al. 2001) before and during germination. Because arginine is a primary precursor of polyamines and nitric oxide biosynthesis in moist chilled seeds and other plant tissues (Guoyao and Morris 1998; Santanen and Simola 1999; Crozier et al. 2000; Urano et al. 2005), its utilization and metabolism of other products derived from protein mobilization need to the proper function of amino acid-metabolizing enzymes such as arginase, arginine decarboxylase (ADC), and ornithine aminotransferase (OAT). Therefore, the following metabolism of metabolites resulting from reserve mobilization can also be induced by cold during stratification.
Persian pistachio (Pistacia vera L.) is a commercially important small nut tree from central Asia and the Middle East. Germination of pistachio seeds has been promoted by cold stratification (Khan et al. 1999; Isfendiyaroglu and Ozeker 2001; Einali and Valizadeh 2017). Storage proteins constitute 22% of the food reserves in the pistachio kernel (Clarke et al. 1976). Considering that the amount of storage proteins in pistachio kernels might be changed during cold stratification by alterations in soluble protein composition or mobilization to amino acids (Einali and Valizadeh 2017), the importance of protein modifications and the fate of amino acids is further underlined. There is no report on the protein modifications and the activities of amino acid-metabolizing enzymes during moist chilling of Persian pistachio kernels. In the present study, the levels of protein carbonyl groups and the activities of arginase, ADC, and OAT have been determined in pistachio kernels during moist chilling and warm stratification to discover the role of protein modifications and amino acid metabolism in the dormancy breaking.
Materials and methods
Plant material and germination studies
Freshly harvested seeds of Persian pistachio (Pistacia vera L.) were acquired from the Zahedan office of Goharkuh Agro-industry Co. during September 2017 and 2018. Pistachio nuts were imbibed in tap water for 24 h and surface sterilized with 0.5% (w/v) sodium hypochlorite solution for 10 min. After four times washing with distilled water, the nuts were stratified as described by Einali and Sadeghipour (2007). Every week, lots of 100 nuts (in quadruplicate) were wrapped in two layers of moistened cheesecloth covered in polyethylene bags and incubated at 5 ºC (cold stratification) up to 6 weeks (42 d) or 25 ºC (warm stratification) up to 4 weeks (28 d) in darkness. Nuts imbibed for 24 h only served as non-stratified seeds (control). For the germination test, the cold- and non-stratified seeds were transferred into pots containing well-irrigated cocopeat, and their germination was recorded for 28 d in the culture room at 25 ºC in darkness. Seeds with an average radicle length of 10 mm, which were evident as protrusions on the cocopeat surface, were taken as germinated. The cotyledons and embryonic axes used for biochemical analyses were cut off from cold- and warm-stratified kernels that did not show any visible sign of germination.
Extraction and measurement of soluble protein, free amino acid, proline, and arginine
Total soluble proteins were extracted from cotyledonary or axial tissue (1 g) with 3 mL of extraction buffer containing 100 mM cold potassium phosphate buffer (pH 4.5, 7.5, 9), 0.4 M sucrose, 1 mM EDTA, 10 mM KCl, 1 mM MgSO4, 1 mM phenylmethylsulfonyl fluoride (PMSF), 70 mM 2-mercaptoethanol, and 1% (w/v) polyvinylpolypyrrolidone (PVPP). The homogenate was filtered through four layers of cheesecloth and then centrifuged at 12,000 g for 10 min at 4 ºC. The supernatant was used to estimate total soluble protein (TSP) by the Bradford (1976) method.
Amino acids were extracted from cotyledonary or axial tissue (1 g) with 5 mL of 80% (v/v) ethanol at 70 ºC for 10 min. The homogenate was centrifuged at 2000 g for 10 min, the supernatant was pooled into a flask, and the pellet was re-extracted four times with the same volume of 80% ethanol as above. The ethanolic extract was concentrated by evaporation and mixed with chloroform (1:5; v/v) to isolate pigments and lipids from the extract. The aqueous phase was used for the determination of amino acid content. Total free amino acid content was determined by the ninhydrin method of Yemm and Cocking (1955), using glycine as the standard. Proline content was determined according to the method of Bates et al. (1973) using a calibration curve of proline. Arginine concentration was determined according to the spectrophotometric method of Sastry and Tummuru (1984) using a standard curve of arginine.
Measurement of protein carbonyl groups
Protein carbonyl groups were determined according to the method of Levine et al. (1994) with some changes. Cotyledonary or axial tissue (1 g) was homogenized in 3 mL of cold homogenization buffer containing 100 mM potassium phosphate buffer (pH 7.0), 0.4 M sucrose, 1 mM EDTA, 10 mM KCl, 1 mM MgSO4, 70 mM 2-mercaptoethanol, 1 mM PMSF, and 2% (w/v) PVPP. The homogenate was filtered through four layers of cheesecloth and centrifuged at 12,000 g for 15 min at 4 ºC. The supernatant was mixed with 1% (w/v) streptomycin sulfate and incubated for 20 min at room temperature in darkness. The supernatant was divided into two parts. One part was mixed with 500 µl of 10 mM 2,4-dinitrophenylhydrazine (DNPH) in 2 M HCl, while another part, which was incubated with 500 µl of 2 M HCl (without DNPH), served as blank. Both parts were incubated for 30 min at 37 °C in the dark. Proteins were precipitated with 500 µl of 20% (w/v) trichloroacetic acid (TCA) for 10 min. The resultant pellets were washed three times with 1 mL of 1:1 (v/v) ethanol: ethyl acetate solution. The washed pellets were dissolved in 1 mL of 6 M guanidine hydrochloride in 2 M HCl, and the absorbance was measured at 375 nm. Protein carbonyl group concentration was expressed as nmol mg−1 protein assuming an extinction coefficient (ε375) of 22 (mM cm)−1 for DNPH.
Extraction and assay of arginase activity
Arginase was extracted from 1 g of cotyledonary or axial tissues according to the method of Goldraij and Polacco (1999) with some modifications. The extraction was carried out with 3 mL of cold extraction buffer containing 100 mM potassium phosphate buffer (pH 7.5), 0.4 M sucrose, 1 mM EDTA, 10 mM KCl, 1 mM MgSO4, 1 mM PMSF, 50 mM 2-mercaptoethanol, and 1% (w/v) PVPP. The homogenate was filtered through four layers of cheesecloth and centrifuged at 12,000 g for 15 min at 4 ºC. The supernatant was used to determine arginase (EC 3.5.3.1) activity.
Arginase activity was assayed according to the amount of liberated ornithine as described by Roubelakis and Kliewer (1978). The enzyme activation process was carried out by mixing the crude enzymatic extract with 50 mM MnSO4 at 35 ºC for 15 min (Greenberg 1955). Arginase reaction mixture (1 mL) containing 170 mM arginine (pH 9.5) and enzyme extract was incubated for 30 min at 30 ºC. The reaction was terminated by adding 0.7 mL of 20% (w/v) TCA to 0.5 mL of the reaction mixture. The produced ornithine content was determined by the ninhydrin method of Chinard (1952), using ornithine as the standard.
Extraction and assay of ADC activity
Cotyledonary or axial tissues (1 g) were extracted with 3 mL of cold extraction buffer containing 100 mM potassium phosphate buffer (pH 7.5), 0.4 M sucrose, 1 mM EDTA, 10 mM KCl, 1 mM MgSO4, 1 mM PMSF, 0.05 mM pyridoxal phosphate (PLP), 50 mM 2-mercaptoethanol, 0.1% (v/v) Triton X-100, and 1% (w/v) PVPP. The homogenate was filtered through four layers of cheesecloth, incubated at 4 ºC for 1 h, and centrifuged at 12,000 g for 15 min at 4 ºC. The 12,000 g supernatant was used to determine ADC (EC 4.1.1.19) activity by measuring the produced agmatine due to ADC action according to the method of Goldschmidt and Lockhont (1971). The assay mixture (1 mL) contained 50 mM potassium phosphate buffer (pH 7.0), 25 mM arginine, 0.025 mM PLP, and aliquots from the enzyme extract. The mixture was incubated for 30 min at 37 ºC. The reaction was stopped by adding 0.2 mL of 10% (w/v) TCA. The liberated agmatine was extracted from the mixture of arginine and agmatine by a differential extraction procedure described by Cohn and Shore (1961) and quantified by the method of Goldschmidt and Lockhont (1971) using a calibration curve of agmatine.
Extraction and assay of OAT activity
OAT (EC 2.6.1.13) activity was extracted and assayed as described by Kim et al. (1994). The tissue (1 g) was extracted with 3 mL of cold extraction buffer containing 100 mM potassium phosphate buffer (pH 7.5), 0.4 M sucrose, 1 mM EDTA, 10 mM KCl, 1 mM MgSO4, 1 mM PMSF, 0.2 mM PLP, 50 mM 2-mercaptoethanol, 0.2% (v/v) Triton X-100, and 1% (w/v) PVPP. The homogenate was filtered through four layers of cheesecloth and incubated at 4 ºC for 1 h. The filtrate was then centrifuged (12,000 g for 15 min at 4 ºC), and the supernatant was used for assaying the enzyme activity. The reaction mixture in the final volume of 1 mL comprised 50 mM potassium phosphate buffer (pH 7.0), 35 mM ornithine, 5 mM α-ketoglutarate, 0.05 mM PLP, and aliquots from the enzyme extract. The mixture was incubated at 37 ºC for 20 min. The reaction was terminated by adding 0.3 mL of 3 N perchloric acid, and the produced pyrroline-5-carboxylate (P5C) by OAT activity was determined spectrophotometrically using a standard calibration curve of P5C (Kim et al. 1994).
Statistical analysis
All data except the germination test were presented as the mean ± standard deviations (SD) of at least three separate experiments. Germination test results were expressed as the mean ± SD of four independent experiments. Analysis of variance (ANOVA) at P < 0.05 with a Duncan multiple comparisons post hoc test was used to determine the statistically significant differences between treatments.
Results
Effect of cold stratification on germination of pistachio kernels
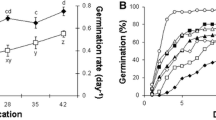
The germination percentage and rate of non-stratified seeds after incubating at 25 ºC for 28 d were obtained up to 40% and 0.1 d−1, respectively. Cold-stratified seeds displayed a significant increase in both percentage and rate of germination compared to non-stratified seeds (Fig. 1a). The maximum germination percentage of 97% was found for cold-stratified seeds for 21 d, and higher stratification periods did not affect the germination, while the maximum germination rate (0.3 d−1) was obtained for kernels that cold-stratified for 42 d (Fig. 1a). The time course of germination of pistachio kernels showed that the maximum germination percentage of non-stratified seeds occurred after 22 d incubating at 25 ºC, but it was reduced to 10 d for cold-stratified seeds for 42 d (Fig. 1b).
Germination percentage (GP) and germination rate (GR) (a) and time course of germination at 25 ºC (b) of non-stratified (control) and cold-stratified pistachio kernels. Results are the mean ± SD of four independent experiments, each consisting of 100 seeds. Different letters (a–d for GP and w–z for GR) display significant differences between the various treatments at P < 0.05 according to the Duncan test
Effect of stratification on the solubility of proteins extracted with different pHs in pistachio kernels
Cold stratification increased protein solubility extracted with neutral pH in cotyledonary tissue of pistachio kernels but did not change the solubility of proteins extracted with acid and alkaline pHs (Fig. 2a). The solubility of proteins extracted with acid pH in axial tissue did not significantly affected by cold treatment, while the solubility increased with neutral and alkaline pHs (Fig. 2b). In contrast, warm stratification increased protein solubility extracted with acid and alkaline pHs in both cotyledonary and axial tissues of pistachio kernels, but significantly decreased or did not change the solubility of proteins at neutral pH (Fig. 2c, d).
Changes in the solubility of proteins extracted with different pHs in cotyledonary (a, c) and axial (b, d) tissues of cold- (a, b) and warm-stratified (c, d) pistachio kernels. Each value is mean ± SD of three separate experiments. Different letters indicate significant differences between the various treatments at P < 0.05 according to the Duncan test
Soluble protein carbonylation of cold- and warm-stratified pistachio kernels
A similar pattern of soluble protein carbonylation was found in cotyledonary and axial tissues of kernels during cold stratification (Fig. 3a). The concentration of protein carbonyl groups was unchanged up to 14 d after cold stratification but increased drastically after 21 d and returned to the initial level on the 28th day. However, the protein carbonylation level in cotyledons was much higher than in axial tissues after 21 d. It increased after 35 and 42 d in cotyledons and 35 d in axes tissues in response to cold treatment (Fig. 3a). Warm stratification increased the concentration of protein carbonyl groups in cotyledons after 7 and 14 d, but decreased or did not change in axial tissues (Fig. 3b). However, the concentration of soluble protein carbonyl groups drastically reduced in both cotyledonary and axial tissues after 21 and 28 d of warm treatment (Fig. 3b).
Concentration of carbonyl groups of proteins in cotyledonary and axial tissues during cold (a) and warm (b) stratification of pistachio kernels. The values are mean ± SD of three separate experiments. Different letters (a–c for cotyledon and w–x for axis) indicate significant differences between the various treatments at P < 0.05 according to the Duncan test
Changes in concentrations of amino acids in cold- and warm-stratified pistachio kernels
The total free amino acid concentration of cotyledons increased significantly up to 35 d after cold stratification but severely fell to lower than the control level after 42 d (Fig. 4a). The amino acid levels of axial tissues increased gradually during cold stratification and reached about 122 and 350% of control after 35 and 42 d, respectively (Fig. 4a). Total free amino acid accumulated in both cotyledonary and axial tissues after 14 d of warm stratification (Fig. 4b). However, the amino acid content of the cotyledons returned to control level on day 21 and re-increased on day 28 while remained accumulated in axial tissues (Fig. 4b).
Changes in total free amino acid (a, b), proline (c, d), and arginine (e, f) contents in cotyledonary and axial tissues during cold (a, c, e) and warm (b, d, f) stratification of pistachio kernels. Values are mean ± SD of three separate experiments. Significant differences between the various treatments were calculated at P < 0.05 according to the Duncan test and addressed by different letters (a–e for cotyledon and v–z for axis)
Proline content increased significantly in both cotyledonary and axial tissues in a concomitant manner with the duration of cold stratification (Fig. 4c). However, proline concentration in axes was much higher than cotyledons after 42 d of cold treatment. Similarly, the proline content of cotyledonary and axial tissues highly increased following warm stratification (Fig. 4d). While cotyledon proline concentration reached a maximum level after 28 d of warm treatment, it decreased in axial tissues but remained higher than the control level.
Arginine concentration of cotyledonary and axial tissues was positively affected by both cold and warm treatments (Fig. 4e, f). It increased significantly after 7 d of cold treatment and reached about 400% of control in both tissues after 42 d (Fig. 4e). Arginine concentrations in both cotyledons and axes tissue increased similarly, reaching more than 400% of control after 28 d of warm stratification (Fig. 4f).
Arginase activity in cold- and warm-stratified pistachio kernels
Arginase activity of cotyledons decreased significantly following cold treatment up to 21 d after stratification but drastically increased in cotyledonary tissues of cold-stratified kernels for higher periods (Fig. 5a). Axis arginase activity did not change up to 14 d of cold treatment, but enhanced significantly for periods of 21 to 35 d and then dropped to a higher level than the control after 42 d (Fig. 5a). In contrast, arginase activity in the cotyledonary and axial tissues of warm-stratified kernels decreased significantly during the warm incubation period (Fig. 5b). While cotyledons arginase activity declined after 14 d of warm treatment, it fell in axial tissues after 7 d and then returned to control level on the 14th day and decreased thereafter (Fig. 5b).
Changes in arginase activity in cotyledons and axes of pistachio kernels during cold (a) and warm (b) stratification. Data are mean ± SD obtained from three independent analyses. Statistically significant differences between the various treatments were obtained at P < 0.05 according to the Duncan test and shown by different letters (a–d for cotyledon and w–z for axis)
Changes in ADC activity of cold- and warm-stratified pistachio kernels
ADC activity in cotyledonary tissues of cold-stratified kernels remained unchanged up to 14 d after cold stratification but significantly increased in the cotyledons that received higher cold periods (Fig. 6a). The activity of ADC in axial tissues displayed a slight increase but was not significant up to 14 d and increased significantly after 21 d of cold stratification. However, a drastic decrease was found in the axes ADC activity of cold-stratified kernels on days 28 and 35 with a return to the maximum level of activity after 42 d (Fig. 6a). Warm stratification increased the ADC activity in both cotyledonary and axial tissues of pistachio kernels (Fig. 6b). The maximum activity of ADC in cotyledons and axes tissue of warm-stratified kernels was obtained on days 14 and 7, respectively. However, for higher warm incubation periods, enzyme activity in the kernels decreased to higher levels than the control (Fig. 6b).
Changes in arginine decarboxylase activity in cotyledons and axes of pistachio kernels during cold (a) and warm (b) stratification. Results are mean ± SD obtained from three independent analyses. Different letters (a–c for cotyledon and w–y for axis) were used to indicate statistically significant differences between the various treatments at P < 0.05 according to the Duncan test
Changes in OAT activity of cold- and warm-stratified pistachio kernels
OAT activity of cotyledonary and axial tissues showed fluctuations during cold stratification (Fig. 7a). The activity of OAT in cotyledons increased significantly following exposure of kernels to cold treatment, but returned to the initial level after 14 d and re-increased on days 21 and 28, which subsequently lowered to the control level after 35 and 42 d. Inversely, axial OAT activity decreased significantly during the 14 d after cold treatment, but increased drastically on the 21st day and then declined to a lower level than the control (Fig. 7a). The OAT activity of the cotyledons enhanced significantly 7 d after incubation in warm conditions, but decreased to the control level on days 14 and 21 and reached lower levels after 28 d. However, warm stratification decreased the activity of OAT in axial tissues of pistachio kernels (Fig. 7b).
Changes in ornithine aminotransferase activity in cotyledons and axes of pistachio kernels during cold (a) and warm (b) stratification. Results are mean ± SD obtained from three separate analyses. Statistically significant differences between the various treatments were calculated according to the Duncan test at P < 0.05 and addressed by different letters (a–c for cotyledon and w–z for axis)
Discussion
As described previously, pistachio kernels are not deeply dormant (Einali and Valizadeh 2017). In the present study, a germination percentage of 40% was found for non-stratified seeds. However, both germination percentage and rate of pistachio kernels were positively affected by cold stratification, as obtained for different seeds (Lin et al. 1994; Andriotis et al. 2004; Kaur et al. 2006; Sanchez-Zamora et al. 2006; Einali and Sadeghipour 2007; Pipinis et al. 2020). Because no sign of germination was found in pistachio kernels during 42 d of cold stratification at 5 ºC, all the biochemical changes reported in this study resulted from dormancy removal, not germination.
Warm-stratified pistachio seeds at 25 ºC started to germinate after 5 d of incubation, but most of them decayed after 28 d. Therefore, warm stratification studies on kernels were carried out up to 28 d of incubation. To prevent interference by germination or post-germination processes, kernels were exclusively selected for analyses that did not show any sign of germination.
pH-driven solubilization of proteins was found for storage proteins of pistachio kernels during stratification (Fig. 2). This agrees with previous reports on the effect of pH on protein solubility (Jiang et al. 2010; Vilg and Undeland 2017). Because increased solubility of proteins occurs before storage protein mobilization (Yano et al. 2001; Wong et al. 2004), changes in protein solubility may be related to dormancy release. Proteins have a variety of amino acids with acidic or basic groups on their side chains, so the effect of pH on the solubility of protein occurs because of the ionization of these groups in its structure. Such changes reflect alterations in the conformation of proteins and express the function of the enzymes at an optimum pH. Increased solubility of protein at neutral pH during cold stratification can show the optimum pH for the activity of enzymes involved in the dormancy breaking. Failure to change the solubility of proteins at neutral pH may be related to the low competence of warm-stratified seeds for germination.
Protein carbonylation was started in pistachio kernels after 21 d of cold stratification. However, it increased up to 14 d after warm incubation and decreased thereafter (Fig. 3). It shows that protein modification may play an essential role in the dormancy removal of pistachio kernels. In accordance with this suggestion, the role of protein carbonylation in apple seeds dormancy alleviation (Debska et al. 2013) or regulation of germination in sunflower seedlings (Oracz et al. 2007) and Arabidopsis thaliana (Job et al. 2005; Rajjou et al. 2006) has been detected. The pattern of protein carbonylation in pistachio kernels was nearly similar to amino acid accumulation during both cold and warm stratification (Fig. 3, 4a, b). It is in agreement with the suggestion stating that protein carbonylation not only changes protein solubility but mainly also increases their susceptibility to proteolytic enzymes (Dean et al. 1997; Vanita et al. 2008). Therefore, changes in both protein solubility and amino acid accumulation during stratification are dependent on the pattern of protein carbonylation.
Cold stratification of pistachio kernels enhanced the accumulation of amino acids in cotyledonary and axial tissues (Fig. 4). Amino acid accumulation during cold stratification has also been reported for other species (Dawidowicz-Grezegorezewska 1989, King and Gifford 1997; Einali and Sadeghipour 2007). Decreased amino acid concentration of cotyledonary tissues after 35 and 42 d of cold treatment was associated with its increase in axial tissues. It can be due to the transport of amino acids from cotyledons to embryonic axes. Such amino acid transport was also reported for loblolly pine (King and Gifford 1997) and walnut kernels (Einali and Sadeghipour 2007) during stratification. Amino acid accumulation was associated with increased proteolytic activity in pistachio kernels (Einali and Valizadeh 2017). However, it also accumulated during warm stratification, expressing that protein mobilization to amino acids is not a prerequisite for increasing germination, but subsequent metabolism of amino acids may be involved. Thus, the conversion of amino acids into transportable forms (Canovas et al. 2007) or utilizable for radicle protrusion by the suitable and sequential activity of amino acid-metabolizing enzymes including arginase (King and Gifford 1997) and ADC seems to be necessary for dormancy release.
The role of proline in the dormancy breaking of pomegranate seeds (Shalimu et al. 2016) and promoting germination in Arabidopsis thaliana (Hare et al. 2003) has been documented. However, the proline accumulation in pistachio kernels occurred in both cotyledonary and axial tissues during cold and warm stratification. Because proline is an important molecule in stress conditions (Shalimu et al. 2016), its accumulation during warm stratification of pistachio kernels may be attributed to the oxidative stress caused by warm incubation. Because of the higher proline concentration compared to free amino acid contents in cotyledonary tissues of pistachio kernels after 42 d of cold stratification, it is unlikely that increased proline during stratification is due to protein mobilization. Thus, an increase in the activity of proline-synthesizing enzymes during the stratification period might be imagined. Specific evidence obtained from Arabidopsis thaliana confirms that the increase in proline content before and during germination is due to de novo synthesis from glutamate rather than the mobilization of storage proteins (Hare et al. 2003).
Arginine accumulation in both cotyledonary and axial tissues of pistachio kernels during stratification may be related to the mobilization of arginine-rich storage proteins. The large influx of arginine into the free amino acid pool was also found in several pine species following imbibition (King and Gifford 1997). Since the concentration of arginine in the cotyledons and embryonic axes is almost the same during cold and warm stratification, there is no transport of free arginine between these organs. It raises the possibility that arginine metabolites and not free arginine may be transported from cotyledons to the embryonic axes. It is in contrast to loblolly pine, in which arginine is exported from the megagametophyte to the seedling without metabolic interconversions (King and Gifford 1997).
Although the accumulation of free arginine occurred under both cold and warm conditions, the arginase activity increased in cotyledonary and axial tissues of cold-stratified kernels (Fig. 4 e, f; 5). The correlation between arginine content and arginase activity of cold-stratified seeds can indicate that the subsequent metabolism of arginine is an essential requirement for breaking of dormancy of pistachio kernels by cold treatment. The lack of correlation between these two in warm-stratified kernels represents the prevention of arginine metabolism and influencing germination. These suggestions are in agreement with the arginase activity of imbibed loblolly pine (King and Gifford 1997) and cold-stratified and warm-incubated walnut kernels (Zarei-Ghadikolaee et al. 2010).
Increased ADC activity during cold and warm stratification of pistachio kernels (Fig. 6) showed that arginine metabolism might proceed through multiple pathways. Therefore, it is likely that arginine accumulated under warm incubation may likely be used in other metabolic pathways that utilize this amino acid, such as polyamine biosynthesis. The directing of arginine to the polyamine biosynthesis pathway during warm stratification of walnut kernels despite decreasing arginase activity has been reported (Zarei-Ghadikolaee et al. 2010). In fact, induction of ADC activity in cotyledons and embryonic axes of stratified seeds indicates biosynthesis of polyamines, including agmatine and putrescine. Simultaneous induction of arginase and ADC activities was also determined in germinating yellow lupin (Borek et al. 2001). However, the contribution of polyamine production from various metabolic pathways may differ in cold- and warm-stratified kernels. Considering the decrease in arginase activity and the increase in ADC activity in the cotyledonary and axial tissues of warm-stratified kernels, it can be suggested that most polyamines produced under warm conditions are synthesized by arginine catabolism through ADC activity instead of arginase.
OAT activity of cotyledons and embryonic axes of pistachio kernels showed an increase only in the mid-period of cold stratification, while decreased thereafter and during warm incubation as well (Fig. 7). Therefore, most of the ornithine produced by arginase activity in cold-stratified kernels might metabolize to synthesize polyamines (Majumdar et al. 2016). P5C originated from OAT activity can convert to glutamate in mitochondria or proline in cytosol and chloroplast (Szabados and Savoure 2009). However, the discrepancy between proline accumulation and OAT activity in cotyledons and embryonic axes of cold- and warm-stratified pistachio kernels may point to the fact that this amino acid is produced from glutamate rather than the ornithine pathway. The dramatic decrease of arginase activity in warm-stratified kernels further confirms this suggestion.
Earlier studies on some arboraceous species point to the protein mobilization during cold stratification as a prerequisite of dormancy breaking and germination (Bogatek et al. 1989; Andriotis et al. 2004). Our results showed that amino acids accumulated in cold- and warm-stratified pistachio kernels. Thus, subsequent amino acid metabolism may be involved during seed dormancy breaking by cold treatment. The present results suggest that protein modifications and amino acid metabolism may be considered a determinant factor for promoted germination competence of pistachio kernels due to cold treatment. While the solubility of protein at neutral pH and carbonylated protein level decreased under warm incubation, the increased protein solubility during moist chilling of pistachio kernels, which was associated with protein carbonylation, may induce the protein mobilization and accumulation of amino acids for their following direction to the appropriate metabolic pathways to stimulate germination. Arginine metabolism by arginase rather than ADC during cold stratification complies with the demand of pistachio kernels for nitrogen and subsequently stimulates germination. However, the downregulation of arginase despite the high activity of ADC during warm stratification may perturb the nitrogen metabolism of kernels and affect germination. Thus, lower germination of warm-incubated (40%) versus cold-stratified (97%) kernels may be correlated with decreased protein solubility, lower protein carbonylation, and the downfall of arginase activity. Low activity of OAT under both treatments showed that proline in pistachio kernels might be synthesized by glutamate instead of the ornithine pathway. Therefore, as depicted before (Zarei-Ghadikolaee et al. 2010), our results confirm that cold stratification has an important role in the metabolism of arginine as a rich source of nitrogen during dormancy removal.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article.
References
Andriotis VME, Smith B, Ross JD (2004) Phytic acid mobilization is an early response to chilling of the embryonic axes from dormant oilseed of hazel (Corylus avellana L.). J Exp Bot 56:537–545
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207
Blake PS, Taylor JM, Finch-Savage WE (2002) Identification of abscisic acid, indol-3-acetic acid, jasmonic acid, indole-3-acetonitrile, methyl jasmonate and gibberellins in developing, dormant and stratified seeds of ash (Fraxinus excelsior). Plant Growth Regul 37:119–125
Bogatek R, Zarska-Maciejewska B, Sinska I, Lewak S (1989) The embryonic axis controls lipid catabolism in cotyledons of apple seeds during germination. Physiol Plant 76:557–562
Bogatek R, Come D, Corbineau F, Ranjan R, Lewak S (2002) Jasmonic acid affects dormancy and sugar catabolism in germinating apple embryos. Plant Physiol Biochem 40:167–173
Borek S, Morkunas I, Ratajczak W, Ratajczak L (2001) Metabolism of amino acids in germinating yellow lupin seeds. III. Breakdown of arginine in sugar-starved organs cultivated in vitro. Acta Physiol Plant 23:141–148
Bradford M (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Canovas FM, Avila C, Canton R, Canas RA, Torre FDL (2007) Ammonium assimilation and amino acid metabolism in conifers. J Exp Bot 58:2307–2318
Chinard FP (1952) Photometric estimation of proline and ornithine. J Biol Chem 199:91–95
Clarke JA, Brar GS, Procopiou J (1976) Fatty acid, carbohydrate and amino acid composition of pistachio (Pistacia vera) Kernels. Plant Food Hum Nutr 25:219–225
Cohn VH, Shore PA (1961) A microfluorometric method for the determination of agmatine. Anal Biochem 2:237–241
Crozier A, Kamiya Y, Bishop G, Yokota T (2000) Biosynthesis of hormones and elicitor molecules. In: Buchanan BB, Gruissem W, Jones RL (eds) Biochemistry and molecular biology of plants. American Society of Plant Biologists Inc, Rockville, pp 911–915
Dalle-Donne I, Giustarini D, Colombo R, Rossi R, Milzani A (2003) Protein carbonylation in human diseases. Trends Mol Med 9:169–176
Dawidowicz-Grzegorzewska A (1989) Degradation of protein and lipid bodies during dormancy removal in apple seeds. J Plant Physiol 135:43–51
Dean RT, Fu S, Stocker R, Davies MJ (1997) Biochemistry and pathology of radical-mediated protein oxidation. Biochem J 324:1–18
Dębska K, Krasuska U, Budnicka K, Bogatek R, Gniazdowska A (2013) Dormancy removal of apple seeds by cold stratification is associated with fluctuation in H2O2, NO production and protein carbonylation level. J Plant Physiol 170:480–488
Derkx MPM (2000) Pre-treatment at controlled seed moisture content as an effective means to break dormancy in tree seeds. In: Viemont JD, Crabbe J (eds) Dormancy in plants. CAB International, Wallingford, pp 69–78
Einali AR, Sadeghipour HR (2007) The alleviation of dormancy in walnut kernels by moist chilling is independent from storage protein mobilization. Tree Physiol 27:519–525
Einali A, Valizadeh J (2017) Storage reserve mobilization, gluconeogenesis, and oxidative pattern in dormant pistachio (Pistacia vera L.) seeds during cold stratification. Trees 31:659–671
Forward BS, Tranbarger TJ, Misra S (2001) Characterization of proteinase activity in stratified Douglas-fir seeds. Tree Physiol 21:625–629
Goldraij A, Polacco JC (1999) Arginase is inoperative in developing soybean embryos. Plant Physiol 119:297–303
Goldschmidt MC, Lockhart BM (1971) Simplified rapid procedure for determination of agmatine and other guanidino-containing compounds. Anal Chem 43:1475–1479
Greenberg DM (1955) Enzymes of protein metabolism. Methods Enzymol 2:368–374
Guoyao WU, Morris SM (1998) Arginine metabolism: nitric oxide and beyond. Biochem J 336:1–17
Hare PD, Cress WA, van Staden J (2003) A regulatory role for proline metabolism in stimulating Arabidopsis thaliana seed germination. Plant Growth Regul 39:41–50
Isfendiyaroglu M, Ozeker E (2001) The relations between phenolic compounds and seed dormancy in Pistacia spp. In: Ak BE (ed) XIGREMPA seminar on pistachios and almonds. Zaragoza, CIHEAM, pp 227–232
Jacobsen JV, Pearce DW, Poole AT, Pharis RP, Mander LN (2002) Abscisic acid, phaseic acid and gibberellin contents associated with dormancy and germination in barley. Physiol Plant 115:428–441
Jiang J, Xiong YL, Chen J (2010) pH Shifting alters solubility characteristics and thermal stability of soy protein isolate and its globulin fractions in different pH, salt concentration, and temperature conditions. 58:8035–8042
Job C, Rajjou L, Lovigny Y, Belghazi M, Job D (2005) Patterns of protein oxidation in Arabidopsis seeds and during germination. Plant Physiol 138:790–802
Kaur R, Sharma N, Kumar K, Sharma DR, Sharma SD (2006) In vitro germination of Walnut (Juglans regia L.) embryos. Sci Hort 109:385–388
Khan J, Rauf MA, Ali Z, Rashid H, Khattack MR (1999) Different stratification techniques on seed germination of pistachio cv. Wild Pak J Biol Sci 2:1412–1414
Kim HR, Rho HW, Park JW, Park BH, Kim JS, Lee MW (1994) Assay of Ornithine aminotransferase with ninhydrin. Anal Biochem 223:205–207
King JE, Gifford DJ (1997) Amino acid utilization in seeds of loblolly pine during germination and early seedling growth. Plant Physiol 113:1125–1135
Koornneef M, Bentsink L, Hilhorst H (2002) Seed dormancy and germination. Curr Opin Plant Biol 5:33–36
Levine RL, Williams JA, Stadtman ER, Shacker E (1994) Carbonyl assays for determination of oxidatively modified proteins. Methods Enzymol 233:346–357
Li L, Ross JD (1990a) Lipid mobilization during dormancy breakage in oilseed of Corylus avellana. Ann Bot 66:501–505
Li L, Ross JD (1990b) Starch synthesis during dormancy breakage in oilseed of Corylus avellana. Ann Bot 66:507–512
Lin CH, Lee LY, Tseng MJ (1994) The effect of stratification and thidiazuron treatment on germination and protein synthesis of Pyrus serotina Rehd cv. Niauli Ann Bot 73:515–523
Majumdar R, Barchi B, Turlapati SA, Gagne M, Minocha R, Long S, Minocha SC (2016) Glutamate, ornithine, arginine, proline, and polyamine metabolic interactions: The pathway is regulated at the post-transcriptional level. Front Plant Sci 7:78
Mapelli S, Brambilla I, Bertani A (2001) Free amino acids in walnut kernels and young seedlings. Tree Physiol 21:1299–1302
Nezamdoost T, Tamaskani F, Abdolzadeh A, Sadeghipour HR (2009) Lipid mobilization, gluconeogenesis and aging related processes in walnut (Juglans regia L.) kernels during moist chilling and warm incubation. Seed Sci Res 19:91–101
Oracz K, El-Maarouf Bouteau H, Farrant JM, Cooper K, Belghazi M, Job C, Job D, Corbineau F, Bailly C (2007) ROS production and protein oxidation as a novel mechanism for seed dormancy alleviation. Plant J 50:452–465
Pipinis E, Stampoulidis A, Milios E, Kitikidou K, Akritidou S, Theodoridou S, Radoglou K (2020) Effects of seed moisture content, stratification and sowing date on the germination of Corylus avellana seeds. J for Res 31:743–749
Rajjou L, Gallardo K, Debeaujon I, Vandekerckhove J, Job C, Job D (2004) The effect of α-amanitin on the Arabidopsis seed proteome highlights the distinct roles of stored and neosynthesized mRNA during germination. Plant Physiol 134:1598–1613
Rajjou L, Belghazi M, Huguet R, Robin C, Moreau A, Job C, Job D (2006) Proteomic investigation of the effect of salicylic acid on Arabidopsis seed germination and establishment of early defense mechanisms. Plant Physiol 141:910–923
Ranjan R, Lewak S (1995) Interaction of jasmonic and abscisic acid in the control of lipase and proteases in germinating apple embryos. Physiol Plant 93:421–426
Roubelakis KA, Kliewer WM (1978) Enzymes of Krebs-Henseleit in Vitis vinifera L. Plant Physiol 62:344–347
Sanchez-Zamora MA, Cos-Terrer J, Frutos-Tomas D, Garcia-Lopez R (2006) Embryo germination and proliferation in vitro of Juglans regia L. Sci Hort 108:317–321
Santanen A, Simola LK (1999) Metabolism of L [U-14C]-arginine and L [U-14C]-ornithine in maturing and vernalised embryos and megagametophyte of Picea abies. Physiol Plant 107:433–440
Sastry CSP, Tummuru MK (1984) Spectrophotometric determination of arginine in proteins. Food Chem 15:257–260
Schmitz N, Abrams SR, Kermode AR (2002) Changes in ABA turnover and sensitivity that accompany dormancy termination of yellow-cedar (Chamaecyparis nootkatensis) seeds. J Exp Bot 53:89–101
Shalimu D, Sun J, Baskin CC, Baskin JM, Sun L, Liu Y (2016) Changes in oxidative patterns during dormancy break by warm and cold stratification in seeds of an edible fruit tree. AoB PLANTS 8:plw024
Szabados L, Savoure A (2009) Proline: a multifunctional amino acid. Trends in Plant Sci 15:89–97
Todd CD, Cooke JEK, Mullen RT, Gifford DJ (2001) Regulation of loblolly pine (Pinus taeda L.) arginase in developing seedling tissue during germination and post-germinative growth. Plant Mol Biol 45:555–565
Urano K, Hobo T, Shinozaki K (2005) Arabidopsis ADC genes involved in polyamine biosynthesis are essential for seed development. FEBS Lett 579:1557–1564
Vanita J, Werner K, Huber SC (2008) Cytokinin inhibits the proteasome-mediated degradation of carbonylated proteins in Arabidopsis leaves. Plant Cell Physiol 49:843–852
Vilg JV, Undeland I (2017) pH-driven solubilization and isoelectric precipitation of proteins from the brown seaweed Saccharina latissima—effects of osmotic shock, water volume and temperature. J Appl Phycol 29:585–593
Wong JH, Cai N, Tanaka CK, Vensel WH, Hurkman WJ, Buchanan BB (2004) Thioredoxin reduction alters the solubility of proteins of wheat starchy endosperm: an early event in cereal germination. Plant Cell Physiol 45:407–415
Yano H, Wong JH, Cho MJ, Buchanan BB (2001) Redox changes accompanying the degradation of seed storage proteins in germination rice. Plant Cell Physiol 42:879–883
Yemm EW, Cocking EC (1955) The determination of amino acids with Ninhydrin. Analyst 80:209–213
Zarei Ghadikolaee M, Abdolzadeh A, Sadeghipour HR (2010) Arginase, glutamine synthetase and glutamate dehydrogenase activities in moist chilled and warm incubated walnut (Juglans regia L.) kernels. Trees 24:425–433
Acknowledgements
The authors would like to thank the Deputy of Research at the University of Sistan and Baluchestan for monetary contributions in the form of a grant for the M.Sc. research project.
Author information
Authors and Affiliations
Contributions
MS carried out most of the laboratory research. VA completed the laboratory research and performed additional tests. AE designed the experiment, provided all the technical support during the laboratory work, analyzed data and wrote the manuscript. All authors have read and approved the submitted manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Weijun Zhou.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shahnavazi, M., Azarpeykan, V. & Einali, A. Protein carbonylation and arginine utilization in cold- and warm-stratified pistachio (Pistacia vera L.) kernels. Acta Physiol Plant 46, 57 (2024). https://doi.org/10.1007/s11738-024-03673-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-024-03673-5