Abstract
Dipteryx alata is an important plant species in the Brazilian Cerrado. In this work, we evaluated biochemical and physiological changes in plants exposed to the following treatments for 15 days: control (plants grown in ideal conditions, without stress), drought, flooding, and salinity. Each treatment had six replicates of one plant per pot, evaluated concerning their water relations (water potential, osmotic potential, and daily transpiration), physiological processes (chlorophyll a fluorescence, gas exchange, respiration, and photorespiration), and biochemical parameters (sugars, proline, hydrogen peroxide, malondialdehyde, and antioxidant enzymes). Drought and salinity decreased the leaf water potential and the osmotic potential, indicating osmotic adjustment, but sugars and proline did not contribute to this process. All three stresses decreased the photosynthetic rate by inducing stomatal closure. Salt stress and hypoxia, however, also altered pigment concentration and electron transport, and promoted limitations to photosynthesis. Only plants subjected to drought stress showed increased photorespiration, which although is a source of H2O2, may have helped to protect photosystems. The balance in antioxidant enzyme activities varied significantly among treatments, but membrane damage was observed only in plants grown at high salt concentrations. Although drought is commonly considered the main abiotic stress responsible for tree mortality, the vulnerability index and the hierarchical grouping of plants indicated that D. alata seedlings showed greater sensitivity to flooding and salinity stress. These results are consistent with the evolutionary history of this species and highlight the detrimental effects of land misuse on species conservation in the Cerrado Domain.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The Cerrado (Brazilian Savanna) is one of the main Brazilian domains in terms of territorial extension and species diversity. This domain has two well-defined seasons (dry and rainy) and is considered the most biodiverse savanna in the world (dos Santos et al. 2021; Lima et al. 2022). However, this great biodiversity is threatened by several factors, such as fires, low legal protection, and land use for agriculture (Sano et al. 2019; dos Santos et al. 2021). In addition, several climate models predict a significant increase in the frequency of extreme weather events (e.g., increasing heat waves, drought, waterlogging, and soil salinization) in virtually all regions covered by the Cerrado for the next decades (IPCC 2021). If these predictions are confirmed, profound changes may occur in plant communities, as species will be exposed to abiotic factors that do not naturally occur where they have evolved. Therefore, plants may not have effective defense mechanisms to cope with these new adverse conditions. In fact, although drought is identified as the main factor responsible for the widespread mortality of forest species (Senf et al. 2020), other stresses that will be magnified by changes in climate, such as flooding and salinity, also have negative consequences for plant growth, development, and survival (Analin et al. 2020; Hartman et al. 2021).
Different stressors trigger similar damage to plant cells and the most common is the increase in the generation of reactive oxygen species (ROS) (Dumanović et al. 2020). Due to its high chemical reactivity, the accumulation of ROS can lead to the oxidation of membrane proteins and lipids, inhibition of enzymatic activities, and damage to nucleic acids (Dumanović et al. 2020). Numerous defense mechanisms are activated by the cell to maintain low ROS levels, and one of the first lines of defense is the action of antioxidant enzymes, such as superoxide dismutases (SOD), catalases (CAT), ascorbate peroxidases (APX), peroxidases (POX), and glutathione reductase (GR) (Noctor et al. 2018). However, studies comparing the effectiveness of these mechanisms in species subjected to different stresses are scarce (Zandalinas and Mittler 2022).
In addition to the generation of ROS, each abiotic stress triggers changes inherent to its nature. Under drought conditions, for example, reductions in the stomatal opening, carbon assimilation capacity, and hydraulic conductivity are commonly observed (Ahluwalia et al. 2021). Water restriction also affects leaf area expansion, chlorophyll content, leaf abscission, and water and nutrient uptake (Khatun et al. 2021). Similarly, water excess can be harmful to plant development and triggers the switch from aerobic to fermentative metabolism (Hartman et al. 2021). The effects of hypoxia (i.e., low O2 concentration) on plant tissue are diverse and generally affect root permeability due to the closure of aquaporins, reduction of stomatal opening, cytosolic acidosis, and cell wall impairments (Tong et al. 2021; Lehmann et al. 2021; Khan et al. 2020). Soil salinization, in turn, exposes plants to an excess of ions in the root region, particularly sodium (Na+) and chloride (Cl−), which triggers two types of stresses: osmotic (limits water absorption) and ionic toxicity (cellular toxicity due to ions accumulation) (Vaishnav et al. 2019). Both osmotic stress and ionic toxicity are considered primary stresses and trigger a series of other cellular alterations, such as enzymatic inactivation and reductions in photosynthetic capacity (Liang et al. 2018; Analin et al. 2020).
Given the above facts, it is clear that the increase in drought, flooding, or salinization events, as a result of climate change or human activities, can cause the death and even the replacement of species within and across diverse forest communities (Menezes-Silva et al. 2019). To prevent this process, several countries have intensified the implementation of restoration programs to conserve biodiversity and mitigate the damages caused by climate change (Schmidt et al. 2019; Bieng et al. 2021). Through decree nº 8.952/2017, the Brazilian government is committed to support the restoration of native vegetation of at least 12 million hectares by 2030 (Schmidt et al. 2019). Such an action will require a large number of seedlings of native species. However, far beyond planting, it is important to understand the physiology of these seedlings to ensure their survival. Indeed, young plants tend to be more sensitive to stress and degraded areas tend to be more subject to different abiotic stresses (Grossnickle and MacDonald 2018), which can compromise the growth and survival of seedlings of native species.
In this scenario, understanding the responses of young plants to different abiotic stresses represents the first step for seeking and proposing conservation strategies. The elaboration and implementation of management and reforestation programs within the context of climate change will require extensive knowledge of the different mechanisms and strategies used by plants to overcome different stressful situations (MacKenzie and Mahony 2021). Thus, the main objective of this study was to evaluate the physiological and biochemical responses in seedlings of Dipteryx alata exposed to different abiotic stresses (drought, salinity, and hypoxia). D. alata distribution in Brazil is restricted to the Cerrado (Brazil Flora Group 2021) and was chosen because it is a key species for the conservation of this hyperdiverse domain. D. alata has a high growth rate and reproductive success, and produces fleshy pulp in the dry season, important to feed the fauna during this period (Sano 2004; dos Santos et al. 2018; Alves et al. 2020). Although this species also has great potential for reforestation of degraded areas, its use for this purpose is still very limited (Lima et al. 2022). We tested the hypothesis that different abiotic stresses will affect the physiological and biochemical aspects of the specimens at varied intensities, with the plant being more sensitive to stresses that are not frequent in their natural habitat (e.g., hypoxia and salinity).
Materials and methods
Plant material and treatments
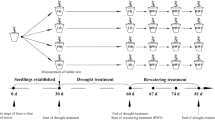
The experiment was performed in a greenhouse at the Laboratory of Ecophysiology and Plant Productivity at the Goiano Federal Institute of Education, Science, and Technology, Campus Rio Verde, Goiás, Brazil. Seedlings of Dipteryx alata (4 months old) were acquired in a forest nursery and transplanted to a substrate prepared from a mixture of Red Latosol soil (LVdf) and sand (2:1). The fertilization was carried out according to chemical analysis of the soil. The plants were grown in polyethylene pots (6 L), containing 5 kg of the substrate described above.
After 30 days of acclimatization to growing conditions, the plants were subjected to the following treatments: 1—control (plants irrigated daily to maintain field capacity (FC)); 2—water deficit (WD) (gradual suspension of irrigation, until the soil reached 30% of the FC); 3—hypoxia [plants subjected to waterlogging (water kept 3 cm above the ground)]; and 4—salt (plants subjected to 100 mM NaCl). The field capacity of the soil was previously determined using the retention curve method (de Oliveira et al. 2015a, b). When irrigation is applied to the soil, all the soil pores get filled with water. After the gravitational drainage, the large soil pores are filled with both air and water, while the smaller pores are still full of water. At this stage, the soil is said to be at full field water capacity, i.e., 100% FC. At field capacity, the water and air contents of the soil are ideal for crop growth (Rai et al. 2017). For drought imposition, after irrigation suspension, the soil dried naturally and gradually until the soil moisture reached 30% of FC. The plants exposed to salinity were irrigated daily with saline solution (100 mM NaCl), and the soil was washed with running distilled water every 3 days to leach the salts, to avoid the accumulation of NaCl in the soil solution in a concentration greater than 100 mM (Aktas et al. 2006; Al-Khaliel 2010). The plants subjected to hypoxia were irrigated abundantly until the establishment of 3 cm layer of water on the top of the soil surface, thus creating a flooded environment. Water was replenished whenever necessary to maintain the water level constant. Each treatment had six replications, with each replication represented by a pot with a plant. The plants remained under these conditions for 15 days and were then used for the analysis of the traits described below.
Water relations
Predawn (Ѱpd) and midday (Ѱmd) water potential were measured using a Scholander pressure chamber (model 3005–1412, Soilmoisture Equipment Corp., Goleta, CA, USA). Measurements of Ѱpd were made between 04:00 and 05:00 a.m., while Ѱmd measurements were made between 12:00 and 13:00 h. Leaf osmotic potential (Ѱs) was measured according to Pask et al. (2012) using a vapor pressure osmometer (model 5600, VAPRO, Wescor, Logan, Utah, USA). The daily transpiration rates per unit leaf area (Ed) of whole plants were evaluated at 05:00 a.m. and at midday, using the gravimetric method, through the difference in the weight of the pots (Lima et al. 2006; Silva et al. 2013). The extraction of proline and total soluble sugars (TSS) followed the protocols proposed by Carillo and Gibbon (2011). The TSS was estimated following the protocol proposed by Dubois et al. (1951) and expressed in percentage. The proline levels were determined according to Bates (1973) and expressed as mmol g of dry weight (DW).
Physiological traits
The concentration of chloroplastic pigments was determined after the extraction with 80% acetone. Briefly, approximately 0.3 g of leaf samples were macerated in liquid nitrogen with acetone, filtered through filter paper, then read in a UV–VIS spectrophotometer (model Evolution 60S, Thermo Fisher Scientific Inc.) at wavelengths 663, 645, and 652 nm for chlorophylls a, b and total, respectively, and 470 nm for carotenoids. The results were then expressed in mg g−1 DW (Arnon 1949).
Gas exchange parameters were measured in fully expanded leaves with an infrared gas analyzer (IRGA, model LI-6800xt, Licor, Lincoln, Nebraska, USA) to determine the net rate of carbon assimilation (A, μmol CO2 m−2 s−1) and stomatal conductance (gs, mol H2O m−2 s−1). Measurements were performed between 8:00 a.m. and 10:00 a.m. under constant photosynthetically active radiation (PAR, 1000 μmol photons m−2 s−1), and at CO2 concentration (Ca) of 400 μmol mol−1, temperature (∼25 °C) and relative air humidity (48–65%). Based on the traits described above, the carboxylation efficiency (A/Ci, µmol mol−1 CO2 m−2 s−1), and the relationship between the internal and external concentrations of CO2 (Ci/Ca) were estimated. The dark respiration rate (Rdark, µmol CO2 m−2 s−1) was measured before dawn using the infrared gas analyzer mentioned above. The ratio of dark respiration to the net rate of carbon assimilation (Rdark/A) was also estimated.
For the determination of chlorophyll a fluorescence traits, first evaluations were conducted on dark-adapted leaves when reaction centers were fully opened (all oxidized primary acceptors) with minimum heat loss. Then a saturating light pulse (> 6000 μmol m−2 s−1) was applied for 0.8 s to determine the maximal fluorescence (Fm), and the potential quantum yield of photosystem II (PSII) (Fv/Fm) was calculated from these values (Van Kooten and Snel 1990). The leaf tissues were then exposed to actinic light to obtain the steady-state fluorescence (F). Subsequently, a saturating pulse was applied to obtain the maximum fluorescence in a light-adapted state (Fm’). The effective quantum yield of photosystem II (ΦPSII) was calculated according to Genty et al. (1989) and Hendrickson et al. (2004). The photochemical quenching (qP), non-photochemical quenching (qN), and the fraction of opened PSII reaction centers (qL) were also measured (Maxwell and Johnson 2000). The electron flux used for carboxylation (ETRc) and oxygenation (ETRo) of RuBisCO, as well as the photorespiration rate (PR µmol CO2 m−2 s−1) was obtained using the equations proposed by Valentine et al. (1995). The ratios ETRc/ ETRo, and PR/A were also estimated.
Biochemical analysis
Evaluation of cellular damage
For the determination of hydrogen peroxide (H2O2), leaf tissues (0.2 g) were macerated in liquid nitrogen and homogenized in 2 mL of 50 mM potassium phosphate buffer, pH 6.5, containing 1 mM hydroxylamine. After filtration, the homogenate was centrifuged at 10 000 g for 15 min at 4 ℃ (Kuo and Kao 2003). Aliquots of 150 μL of the supernatant were added to a reaction medium consisting of 250 μM FeNH4(SO4), 62.5 mM sulfuric acid, 1 mM xylenol orange, and 400 mM sorbitol (Gay and Gebicki 2000). After 30 min in the dark, the absorbance of the samples was determined at 560 nm in a UV–Vis spectrophotometer. Levels of H2O2 were estimated based on an H2O2 standard curve (0–2 μM), expressed as μg per μmol g−1 DW.
The level of lipid peroxidation was measured by estimating malondialdehyde (MDA), a decomposition product of the peroxidized polyunsaturated fatty acid composition of the membrane lipid following the method of Heath and Packer (1968). Leaf samples (0.160 g) were homogenized in 2 mL 1% (w/v) trichloroacetic acid (TCA) with 20% polyvinylpyrrolidone (PVPP), and the homogenate was centrifuged at 12,000 × g for 15 min at 4 ℃. The supernatant (500 μL) was mixed with 2 mL of TBA reagent (0.5% [w/v] of TBA in 20% TCA). The reaction mixture was heated at 95 °C for 20 min in a water bath and then quickly cooled in an ice bath and centrifuged at 3000 × g for 4 min. The absorbance of the colored supernatant was monitored at 532 and 600 nm using a UV–Vis spectrophotometer. The concentration of MDA was calculated using the molar extinction coefficient of 155 mM−1 cm−1 (Heath and Packer 1968). The concentration of MDA in leaves was expressed as nmol MDA g−1 DW.
Determination of enzyme activity
To determine the activities of superoxide dismutases (SOD, EC 1.15.1.1), ascorbate peroxidases (APX, EC 1.11.1.11), peroxidases (POX, EC 1.11.1.7), and glutathione reductase (GR, EC 1.6.4.2), 0.3 g of leaf tissue were ground into a fine powder using a mortar and pestle with liquid nitrogen. The powder was then homogenized in an ice bath in 2 mL of a solution containing 0.1 M potassium phosphate buffer, pH 6.8, 0.1 mM ethylenediaminetetraacetic acid (EDTA), and 2% polyvinylpolypyrrolidone (PVP). The homogenate was centrifuged at 12,000×g for 15 min at 4 ℃, and the supernatant was used as a crude enzyme extract (Peixoto et al. 1999; Carlberg and Mannervik 1985). The activity of SOD was determined by measuring its ability to photochemically reduce p-nitrotetrazole blue (NBT). After 5 min of light exposure, the light was interrupted, and the production of formazan blue, which resulted from the photoreduction of NBT, was monitored by the increase in absorbance at 595 nm in the UV–Vis spectrophotometer. The reaction mixture for the control samples was kept in darkness for 10 min, and the absorbance was measured at 595 nm. The values obtained were subtracted from the values obtained from the samples of the replications of each treatment exposed to light. One unit of SOD was defined as the amount of enzyme necessary to inhibit NBT photoreduction by 50% DW (Giannopolitis and Ries 1977). APX activity was measured by ascorbate oxidation rate at 290 nm using a UV–Vis spectrophotometer for 3 min at 25 °C. The POX activity was measured at 420 nm in a UV–Vis spectrophotometer for 1 min at 25 ℃. The GR activity was determined with the decrease in absorbance at 340 nm using a UV–Vis spectrophotometer for 1 min at 30 ℃. The following molar extinction coefficients were used: 2.8 mM−1 cm−1 (APX; Peixoto et al. 1999), 2.47 mM−1 cm−1 (POX; Peixoto et al. 1999) and 6.22 mM−1 cm−1 (GR; Carlberg and Mannervik 1985). The enzymatic activities were expressed as min−1 mg−1 DW.
Vulnerability index (VI)
Vulnerability to different stresses was calculated based on the percentage of changes in physiological parameters compared to the ideal condition (control). Five parameters were used in the calculation: Fv/Fm, ɸPSII, A, Clh totals, and MDA. These traits were chosen since they represent plant fitness and the final result of key physiological processes (Chl totals represent the set of chlorophylls; Fv/Fm and ɸPSII represent the efficiency of the photochemical process; A represents the effective carbon fixation; and MDA represents the result of all the mechanisms of production and elimination of ROS). For each parameter, the percentage change was calculated using the following formula (Valladares et al. 2000; Couso and Fernández 2012):
where Mmax is the treatment with the highest values (control) and Mmin is the treatment with the lowest values (stress condition).
Then to calculate the VI, the percentage change average was taken for the five parameters evaluated in each of the repetitions.
Statistical analysis
The experimental design was completely randomized, with six replicates. The data were subjected to Fisher’s univariate analysis of variance. Treatment means were then compared by the Newman–Keuls test (α = 0.05). The analyses were performed using the software SISVAR v.5.6 (Ferreira 2018). Graphs were generated using Sigma Plot software (Systat Software v.10.0). Treatment means were clustered based on the Euclidean distance for Ward’s method of hierarchical clustering. The definition of the groups was performed considering the sum of the inertia of each group and the greatest relative loss of inertia (Lê et al. 2008). A principal component analysis (PCA) was also performed to verify the results of the clustering of means and to measure the importance of variables in the total variability. To avoid biased results due to the difference in the scale of variables, the multivariate analyses were performed with the standardized version of the treatment means, to zero mean, and unit standard deviation. A biplot method was used to represent the result of PCA. The multivariate analysis was performed in R v. 4.2.0 (R Core Team 2020).
Results
Water relations
The exposure of Dipteryx alata seedlings to contrasting abiotic stresses for 15 days affected the water relations of the plants (Fig. 1). Concerning the predawn water potetial (Ѱpd), significant reductions were observed in all stresses but more intense in plants exposed to NaCl (Fig. 1A). The midday water potential (Ѱmd) was more negative in plants subjected to both drought and salinity than in control and hypoxia treatments. Leaf osmotic potential (Ѱs), in turn, decreased in all treatments compared to control (Fig. 1C), while the daily transpiration rates per unit leaf area decreased only in plants submitted to drought (Fig. 1D). All stresses decreased total soluble sugars (TSS) in comparison to control plants (Fig. 1E), whereas no statistical difference was observed for proline (Fig. 1F).
Water relations and osmotic adjustment parameters in Dipteryx alata plants under full irrigation (control, light blue bar) or exposed to water deficit (brown bar), salinity (yellow bar) or hypoxia (dark blue bar). The graphs show the predawn (Ѱpd—A) and midday (Ѱmd—B) water potential, leaf osmotic potential (Ѱs—C), the daily transpiration rates per unit leaf area (Ed—D), total soluble sugars (TSS—E), and proline (F). Means followed by the same letter did not differ significantly from each other (P ≤ 0.05; Newman–Keuls test)
Photosynthetic pigments
The levels of chlorophylls a and b were reduced in salt and hypoxia treatments, while the water deficit remained statistically equal to the control (Fig. 2A, B). The concentration of total chlorophylls decreased under all stresses, but such reductions were more accentuated in seedlings submitted to salt stress and hypoxia (Fig. 2C). Conversely, the different stresses had no significant impact on the carotenoid content of D. alata seedlings (Fig. 2D).
Variation in the concentration of pigments chlorophyll a (A), chlorophyll b (B), total chlorophylls (C), and carotenoids (D) in Dipteryx alata plants under full irrigation (control, light blue bar) or exposed to water deficit (brown bar), salinity (yellow bar) or hypoxia (dark blue bar). Means followed by the same letter did not differ significantly from each other (P ≤ 0.05; Newman–Keuls test)
Chlorophyll a fluorescence
The potential quantum yield of PSII (Fv/Fm) did not change in any of the treatments analyzed (Fig. 3A). In contrast, the effective quantum yield of PSII (ΦPSII) was lower in salt and hypoxia treatments than in the control (Fig. 3B). Both the photochemical quenching coefficient (qP) and the fraction of opened PSII reaction centers (qL) decreased only in seedlings exposed to hypoxia, whereas significant differences in the non-photochemical quenching (qN) were not observed among treatments (Fig. 3C, E).
Chlorophyll a fluorescence parameters in Dipteryx alata plants under full irrigation (control, light blue bar) or exposed to water deficit (brown bar), salinity (yellow bar) or hypoxia (dark blue bar). The graphs show the potential quantum yield of photosystem II (PSII) (Fv/Fm—A), effective quantum yield of photosystem II (ΦPSII—B), photochemical quenching (qP—C), the fraction of PSII centers that are open (qL—E), and non-photochemical quenching (qN—D). Means followed by the same letter did not differ significantly from each other (P ≤ 0.05; Newman–Keuls test)
Gas exchange
Exposure to abiotic stresses affected all parameters of gas exchange (Fig. 4). The net carbon assimilation rate (A) decreased in all treatments when compared to control plants, especially under hypoxia (Fig. 4A). A sharp drop was observed in stomatal conductance (gs) after exposure to the different stresses when compared to control plants, with no statistical difference among drought, salinity, and hypoxia (Fig. 4B). The Ci/Ca ratio decreased only in plants subjected to drought (Fig. 4C), while A/Ci decreased in plants cultivated under salt and hypoxia (Fig. 4D). The exposure of D. alata plants to stress did not change dark respiration rate (Rdark), although an increase in dark respiration/photosynthesis (Rdark/A) ratio in plants submitted to hypoxia and salt stress was observed when compared to the control plants (Table 1).
Leaf gas exchange parameters in Dipteryx alata plants under full irrigation (control, light blue bar) or exposed to water deficit (brown bar), salinity (yellow bar) or hypoxia (dark blue bar). The parameters included net photosynthesis rate (A, μmol CO2 m−2 s−1) in (A), stomatal conductance to water vapor (gs, mol H2O m−2 s−1) in (B), relationship between the internal and external concentrations of CO2 (Ci/Ca in (C), and carboxylation efficiency (A/Ci, µmol mol−1 CO2 m−2 s.−1) in (D). Means followed by the same letter did not differ significantly from each other (P ≤ 0.05; Newman–Keuls test)
Plants submitted to water restriction showed an increase in photorespiration rate (PR), while in the salt-treated plants, PR remained equal to the control and, in hypoxia, the photorespiration decreased (Fig. 5A). The PR/A ratio (Fig. 5B) increased in all stresses compared to the control (Fig. 5B). The electron flux used for carboxylation (ETRc) (Fig. 5C) was higher in control plants followed by plants exposed to water deficit, with the lowest values observed for plants under salt and flooding; whereas the highest values of electron flux used for oxygenation (ETRo) were observed in drought-treated plants (Fig. 5D). Only the flooded plants showed lower ETRo than the control. The ETRc/ETRo ratio (Fig. 5E) was lower in all stresses compared to control plants.
Changes in the photorespiration parameters in Dipteryx alata plants under full irrigation (control, light blue bar) or exposed to water deficit (brown bar), salinity (yellow bar) or hypoxia (dark blue bar). The graphs show the photorespiration rate (PR µmol CO2 m−2 s.−1—A), the ratio between photorespiration and photosynthesis (Rp/A—B), the electron flux used for carboxylation (ETRc—C) or oxygenation (ETRo—D), and the ratio between the carboxylation rate and the oxygenation rate of Rubisco (ETRc/ETRo—E). Means followed by the same letter did not differ significantly from each other (P ≤ 0.05; Newman–Keuls test)
Cell damage and antioxidant metabolism
Plants submitted to water deficit and high concentrations of NaCl showed an increase in ROS, evidenced by the higher concentration of H2O2 (Fig. 6A). In turn, an increase in the concentration of MDA was only observed in the salt treatment compared to the control (Fig. 6B). The occurrence of cellular damage activated the antioxidant defense system, although plant response varied according to the sort of stress. The activity of the enzymes superoxide dismutases (SOD) increased considerably in the drought and hypoxia-treated plants (Fig. 6C), while a reduction in glutathione reductase (GR) activity was observed only in plants under water restriction (Fig. 6D). Peroxidases (POX) had their activity increased in treatments with water deficit and hypoxia (Fig. 6E), but APX increased in plants exposed to salinity and flooding (Fig. 6F).
Cell damage and antioxidant metabolism in Dipteryx alata plants under full irrigation (control, light blue bar) or exposed to water deficit (brown bar), salinity (yellow bar) or hypoxia (dark blue bar). The parameters investigated include hydrogen peroxide (H2O2—A), malondialdehyde (MDA—B), superoxide dismutase (SOD—C), glutathione reductase (GR—D), peroxidase (POX—E) and ascorbate peroxidase (APX—F). Means followed by the same letter did not differ significantly from each other (P ≤ 0.05; Newman–Keuls test)
Vulnerability index (VI)
The VI varied according to the nature of the stress, being similar in plants submitted to salt and hypoxia treatments and considerably lower in plants exposed to drought (Fig. 7A). The differential values of VI allowed the separation of three clusters by hierarchical cluster analysis, with plants exposed to salinity and hypoxia forming a single group, while control and drought formed separate groups (Fig. 7B).
Multivariate analysis
The impact of different stresses on D. alata was further investigated using a PCA, in which the first two components explained 81.4% of the total variation (Fig. 8). The first and second components explained 54.7% and 26.7% of the total variation, respectively. Analyzing the main patterns of variability, high stomatal conductance, high water potential, and high concentration of sugars were characteristics correlated with plants grown in an ideal water regime (control). Antioxidant enzymes were strongly correlated with flooding (GR and APX) and salt excess (SOD and POX). ROS and MDA concentrations were also important for the differentiation of plants exposed to NaCl. The water deficit treatment was marked by high photorespiration rate and proline levels, traits that were positively and strongly correlated. In addition, the physiological parameters were also compared among stress treatments, excluding the control. We observed once again that parameters that indicate the maintenance of plant fitness, such as high photosynthesis and high concentration of pigments, occurred in plants subjected to water deficit (Fig. 9).
Principal component analyses (PCA) with the mean values of physiological traits in plants of Dipteryx alata exposed to full irrigation (control) or to different abiotic stresses (water deficit, salinity or hypoxia). The figure shows the variation in trait scores along the first two PCA axes with the percentages of explained variation given
Schematic figure representing the physiological and biochemical changes triggered in Dipteryx alata after exposure to abiotic stresses. Up arrows indicate increase and down arrows indicate decrease in evaluated parameters. Only the parameters that were different between the stresses are represented in the figure (P ≤ 0.05; Newman–Keuls test)
Discussion
Understanding different responses of the same species to the main abiotic stressors is extremely relevant when considering species from threatened domains such as the Cerrado. Although Cerrado plants face seasonal droughts, salinization (Hunke et al. 2015; Vilela et al. 2020) and hypoxia (Bogarín et al. 2022) have become increasingly common in this domain due to incorrect land use and climate change. Exposing plants to new abiotic factors can result in greater sensitivity, which, in turn, can trigger species replacement and/or compromise reforestation programs. Here, we observed that, although drought is commonly considered the main abiotic stress affecting plant physiology (Menezes‐Silva et al. 2019), D. alata seedlings showed greater sensitivity to salt stress and flooding. These results probably reflect the species’ adaptive strategies and are possibly related to the habitat in which the species have evolved.
Water balance in plants under different abiotic conditions
The Ψw is quite sensitive to environmental variations and usually decreases in plants subjected to drought, salinity, and hypoxia (Lin et al. 2018; Vaishnav et al. 2019; Tan et al. 2018). In D. alata seedlings, substantial reductions in Ψw were observed, especially in plants treated with NaCl and drought (Fig. 1A and B), reflecting the decline in soil water potential due to salt accumulation or lower water availability (Távora et al. 2001; Lacerda 2016). Despite this finding, Ψpd remained higher in plants exposed to drought probably as a result of the rehydration capacity when transpiration ceases, indicating good functioning of the xylem conducting vessels (García-Tejera et al. 2021; Venturas et al. 2017). In fact, plants under drought showed lower daily transpiration (Fig. 1D), which is crucial to survival in water-limited environments (Vilagrosa et al. 2012).
In general, species exposed to flooding also tend to have a drop in plant water status, as observed here for Ψpd, which often involves inhibition of aquaporin activity in the membrane of root cells (Tan et al. 2018). At midday, however, the water potential of plants under hypoxia was similar to that of control plants. Several studies obtained similar results, finding no relationship between flooding and reductions in Ψw even when plants sensitive to flooding were studied (Blanke and Cooke 2004; Herrera 2013; Oliveira et al. 2015a, b). Our results indicate that the impairment of water absorption may not be inherent to flooding stress or that plants presented strategies to deal with this constraint, as discussed below.
Maintaining an adequate flow of water through the soil–plant–atmosphere continuum requires the existence of a decreasing gradient of water potential, which can be a challenge for plants in saline or water-deficient soils due to their low soil water potential (Silva et al. 2022; Venturas et al. 2017). D. alata seedlings exposed to NaCl and drought showed reduced osmotic potential (Fig. 1C), indicating osmotic adjustment. This strategy helps to maintain a gradient that favors the absorption of water and, therefore, collaborates with the maintenance of high water potential and leaf turgor (Babita et al. 2010; Askari and Ehsanzadeh 2015). It is interesting to note that plants submitted to hypoxia also had lower osmotic potential, although in this case, there was no decrease in soil water potential. It is likely that the osmotic adjustment in these plants might have helped to maintain the water uptake, counterbalancing the inhibition of aquaporin activity. Alternatively, the production of compatible osmolytes in plants exposed to flooding may be related to other aspects of cellular metabolism. GABA, for example, is a compatible osmolyte that helps to restore the membrane potential under hypoxic conditions (Wu et al. 2021). Although the drop in osmotic potential in plants exposed to salt and drought may simply be a consequence of the lower water content in the cells, the data obtained for plants under hypoxia point to an effective capacity of D. alata seedlings to produce a higher amount of osmotically compatible molecules.
No increase in proline concentration was observed in any of the analyzed treatments (Fig. 1F). A similar response was observed in D. alata exposed to drought (Alves 2020), indicating that proline does not participate in the defense mechanisms of this species and that other osmocompatible solutes may have contributed to osmotic adjustment (Servillo et al. 2011; Alves 2020). In the case of plants exposed to salt stress, it is likely that Na+ and Cl− ions accumulated in tissues, which also contribute to the lower solute potential (Silva et al. 2009). Carbohydrates are another group of molecules commonly pointed out as important in osmotic adjustment (Macêdo et al. 2019). In our study, total soluble sugars (TSS) were reduced in leaves of plants exposed to the stresses, which may be the result of three factors: (i) soluble sugar translocation to roots to promote organ growth and water uptake (Xu et al. 2015); (ii) greater metabolic demand to supply the defense mechanisms; and (iii) damage to the photosynthetic process as a result of exposure to stressors. To verify the extent of these mechanisms in D. alata, we evaluated both the carbon balance and the antioxidant enzymatic machinery, as discussed in the following sections.
Physiological adjustments in Dipteryx alata subjected to stressful conditions
Photosynthesis integrates the physiological status of the plant and is, therefore, often used to assess plant fitness (Alves 2020). This key process was affected at different levels in D. alata subjected to stresses. In general, plants exposed to drought showed fewer damages than plants subjected to other stresses. One example is the maintenance of pigment concentration (Fig. 2), considered a robust trait in stress tolerance (Carvalho et al. 2015; Ma et al. 2020). The reduction of photosynthetic pigments under salt stress and flooding may be a consequence of decreased chlorophyll synthesis, induction of chlorophyllase activity, disturbance in thylakoid membranes, and/or impaired absorption of nutrients such as iron (Latif and Mohamed 2016; Bose et al. 2017; Maswada et al. 2018; Hussain et al. 2021; Seymen 2021).
Changes in photosynthetic pigments were reflected in chlorophyll a fluorescence parameters (Fig. 3). In all treatments, the Fv/Fm values remained constant (Fig. 3A), close to the values considered optimal for most species (around 0.75–0.85), suggesting the absence of damage to proteins and PSII components (Baker 2008; Murchie and Lawson 2013). Low ɸPSII values, on the other hand, were observed in the salt and hypoxia treatments (Fig. 3B). In the case of salt stress, the reduction in ɸPSII was not accompanied by changes in qP (Fig. 3C). This result indicates that the lower flow of electrons through the PSII was due to the low efficiency in light absorption and transfer from the antenna complex to the reaction center (Cousins et al. 2002), probably as a consequence of the decrease in pigments concentration. In hypoxia treatment, ɸPSII, qP, and qL were considerably reduced (Fig. 3B–D), indicating that in addition to the lower efficiency in light absorption and transfer, the availability of electron acceptors in PSII was also impacted (Cousins et al. 2002; Liu et al. 2012).
All the stresses resulted in decreases in the net carbon assimilation rate due to diffusive limitations (Fig. 4 A and B). Stomatal closure is one of the first plant responses to water imbalances and is essential to decrease water loss to the atmosphere, although it also reduces CO2 influx to the Rubisco carboxylation reaction (Baillie and Fleming 2020; Hussain et al. 2021). However, daily transpiration (Fig. 1D) was lower only in plants subjected to drought, and it is possible that, in addition to changes in gs, plants in this treatment could also have other responses that delay water loss, such as changes in leaf area, stomatal anatomy, and/or in the cuticle composition (Machado et al. 2021). In plants exposed to salt and hypoxia, the Ci/Ca ratio remained similar to the control (Fig. 4C), suggesting that biochemical limitations also affected the photosynthetic process in these treatments (Bai et al. 2008; Osakabe et al. 2014). This hypothesis is reinforced by the drop in the A/Ci ratio, which indicates the efficiency of the carboxylase activity of the Rubisco (Silva et al. 2022).
In addition to affecting photosynthesis, stressful events can also limit de-assimilation processes, such as respiration and photorespiration, with consequent changes in carbon balance (Hasibeder et al. 2015). Surprisingly, none of the treatments affected nocturnal respiration, although the Rdark/A ratio was higher in plants exposed to salinity and flooding due to the marked drops in carbon assimilation. Respiratory metabolism is flexible, with alternative routes in response to adverse stress conditions (Jethva et al. 2022), which were probably activated in D. alata plants. Photorespiration increased in plants exposed to drought due to redirecting of electrons to oxidation reactions (Fig. 5A–E). These high photorespiratory rates were important for separating this treatment from the other stresses in the PCA (Fig. 8). Photorespiratory metabolism is important under stress conditions, and although it is a source of H2O2, it contributes greatly to preventing the accumulation of ROS in the plant cells (Voss et al. 2013; Sunil et al. 2019). In addition, photorespiration is a major sink for reducing equivalents as well as ATP to regenerate acceptors for the primary reactions, connecting cyclic electron flow pathways (Foyer et al. 2012; Voss et al. 2013) and helping to protect the PSI and PSII (Huang et al. 2012).
Biochemical changes and vulnerability to stress
The imposition of drought, salinity, and hypoxia required adjustments of physiological and biochemical processes for maintaining cellular homeostasis (Fig. 6). Maintenance of ROS levels involves the participation of enzymes capable of scavenging these radicals, such as superoxide dismutases (SOD), ascorbate peroxidases (APX), peroxidases (POX), and glutathione reductase (GR) (Farnese et al. 2016). In this study, increases in SOD activity were observed in plants under drought and hypoxia. This enzyme acts as the first line of defense against oxidative stress converting the superoxide anion (O2.−) into H2O2, which can be eliminated from the cell by the action of specific enzymes (Farnese et al. 2016). Similar alterations were also observed for POX, indicating the activation of defense mechanisms to eliminate H2O2 in plants subjected to water stress and hypoxia, while plants treated with NaCl showed higher activity of APX only. The joint action of these enzymes maintained constant concentrations of H2O2 in the hypoxia treatment. Although increases in ROS concentration have been observed in both salt and water deficit treatments, MDA levels increased only in plants treated with NaCl. It is likely that the higher concentration of H2O2 in plants under drought is correlated with the higher photorespiratory rates in this treatment. Indeed, under drought conditions, photorespiration can increase H2O2 production by up to 70% (Noctor et al. 2002). In photorespiration, however, ROS production predominantly occurs in the peroxisomes instead of the chloroplasts, protecting Calvin cycle enzymes, and is usually associated with the activation of specific defense mechanisms, such as the action of catalase (Noctor et al. 2002; Scheibe and Beck 2011; Voss et al. 2013).
Conclusion
The analysis of the physiological and biochemical traits revealed that D. alata seedlings were more susceptible to the stresses triggered by flooding and NaCl as shown by the highest vulnerability index (Fig. 7A). This result was corroborated by the PCA since the traits that indicate damage were more strongly associated with plants in the salt and hypoxia treatments (Fig. 8). Consequently, salt- and hypoxia-treated plants formed a single group in hierarchical clustering, while plants subjected to drought showed greater similarities with the control group (Fig. 7B). The comparison among stresses, illustrated in Fig. 9, demonstrated the main physiological and biochemical characteristics inherent to their nature, pointing out crucial responses for the survival of D. alata in environments with water scarcity and greater vulnerability in flooded and saline environments. These data are surprising when we consider that water deficit is the main abiotic factor responsible for the mortality of forest species, but they are fully consistent with the evolutionary history of D. alata. In Brazil, this species is restricted to the Cerrado Domain, which is characterized by having a dry season that lasts from 3 to 7 months a year (Brazil Flora Group 2021). Thus, throughout its evolutionary history, D. alata was constantly exposed to cyclic drought events. Functional traits of plants are typically correlated with the environment where the species occurs and are determinants of plant performance in the climatic conditions of that region (Li et al. 2017). However, the misuse of land in the Cerrado regions, associated with the advance of climate change, has exposed plants of this domain to unfavorable conditions that they are not naturally adapted to, such as high temperatures, salinity, and even flooding. The results obtained in this study indicate that these changes can have harmful effects on D. alata conservation and probably on other Cerrado species, which may cause the loss and replacement of species and compromise the success of reforestation programs.
Data availability
Data are available under request.
References
Ahluwalia O, Singh PC, Bhatia R (2021) A review on drought stress in plants: Implications, mitigation and the role of plant growth promoting rhizobacteria. Environ Dev Sustain 5:100032
Aktas H, Abak K, Cakmak I (2006) Genotypic variation in the response of pepper to salinity. Sci Hortic 110(3):260–266
Al-Khaliel AS (2010) Effect of salinity stress on mycorrhizal association and growth response of peanut infected by Glomus mosseae. Plant Soil Environ 56(7):318–324
Alves RD, Menezes-Silva PE, Sousa LF, Loram-Lourenço L, Silva ML, Almeida SE, Silva GG, Souza LP, Fernie AR, Farnese FS (2020) Evidence of drought memory in Dipteryx alata indicates differential acclimation of plants to savanna conditions. Sci Rep 10(1):16455
Analin B, Mohanan A, Bakka K, Challabathula D (2020) Cytochrome oxidase and alternative oxidase pathways of mitochondrial electron transport chain are important for the photosynthetic performance of pea plants under salinity stress conditions. Plant Physiol Biochem 154:248–259
Arnon DI (1949) Copper enzymes in isolates chloroplasts. polyphenoloxidade in Beta vulgaris. Plant Physiol. 24(1):1–15
Askari E, Ehsanzadeh P (2015) Osmoregulation-mediated differential responses of field-grown fennel genotypes to drought. Ind Crops Prod 76:494–508
Babita M, Maheswari M, Rao LM, Shanker AK, Rao DG (2010) Osmotic adjustment, drought tolerance and yield in castor (Ricinus communis L.) hybrids. Environ. Exp. Bot. 69(3):243–249
Bai J, Xu DH, Kang HM, Chen K, Wang G (2008) Photoprotective function of photorespiration in Reaumuria soongorica during different levels of drought stress in natural high irradiance. Photosynthetica 46(2):232–237
Baillie AL, Fleming AJ (2020) The developmental relationship between stomata and mesophyll airspace. New Phytol 225(3):1120–1126
Baker NR (2008) Chlorophyll fluorescence: a probe of photosynthesis in vivo. Anais Annual Rev Plant Biol 59:89–113
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39(1):205–207
Bieng MAN, Oliveira MS, Roda JM, Boissière M, Herault B, Guizol P, Villalobos R, Sist P (2021) Relevance of secondary tropical forest for landscape restoration. For Ecol Manag 493:119265
Blanke MM, Cooke DT (2004) Effects of flooding and drought on stomatal activity, transpiration, photosynthesis, water potential and water channel activity in strawberry stolons and leaves. Plant Growth Regul 42(2):153–160
Bogarín MR, Reis LK, Laura VA, Pott A, Szabo JK, Garcia LC (2022) Morphological and phenological strategies for flooding tolerance in Cerrado and Pantanal trees: implications for restoration under new legislation. Restor. Ecol. 31(1):e13660
Bose J, Munns R, Shabala S, Gilliham M, Pogson B, Tyerman SD (2017) Chloroplast function and ion regulation in plants growing on saline soils: lessons from halophytes. J Exp Bot 68(12):3129–3143
Brazil Flora Group (2021) Brazilian Flora 2020 project—Projeto Flora do Brasil 2020. v393.274 Instituto de Pesquisas Jardim Botanico do Rio de Janeiro. Dataset/Checklist. https://doi.org/10.15468/1mtkaw
Carillo P, Gibon Y (2011) Protocol: extraction and determination of proline. PrometheusWiki. p 1–5.
Carlberg I, Mannervik B (1985) Glutathione reductase. Meth Enzymol 113(1):484–490
Carvalho JDFC, Crusiol LGT, Perini LJ, Sibaldelli RNR, Ferreira LC, Marcelino-Guimarães FC, Nepomuceno AL, Neumaier N, Farias JRB (2015) Phenotyping soybeans for drought responses using remote sensing techniques and non-destructive physiological analysis. Global Sci Technol 08:1–1
Cousins AB, Adam NR, Wall GW, Kimball BA, Pinter PJ Jr, Ottman MJ, Leavitt SW, Webber AN (2002) Photosystem II energy use, non-photochemical quenching and the xanthophyll cycle in Sorghumbicolor grown under drought and free-air CO2 enrichment (FACE) conditions. Plant Cell Environ 25(11):1551–1559
Couso LL, Fernández RJ (2012) Phenotypic plasticity as an index of drought tolerance in three Patagonian steppe grasses. Ann Bot 110(4):849–857
de Oliveira RA, Ramos MM, de Aquino LA (2015a) Irrigation management. In: Sugarcane. Academic Press, New York
dos Santos KDG, de Melo Ferreira W, Silva KLF, Coimbra RR, Souza CC (2018) Germination and initial development of Dipteryx alata Vogel (Fabaceae) in two “Cerrado” areas: a step toward its use in reforestation programs. Rev Bras Bot 41(2):415–424
dos Santos GL, Pereira MG, Delgado RC, Magistrali IC, da Silva CG, de Oliveira CMM, Laranjeira JPB, da Silva TP (2021) Degradation of the brazilian Cerrado: interactions with human disturbance and environmental variables. For Ecol Manag 482:118875
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith FAJN (1951) A colorimetric method for the determination of sugars. Nature 168(4265):167–167
Dumanović J, Nepovimova E, Natić M, Kuča K, Jaćević V (2020) The significance of reactive oxygen species and antioxidant defense system in plants: a concise overview. Front Plant Sci 11:552969
Farnese FS, Menezes-Silva PE, Gusman GS, Oliveira JA (2016) When bad guys become good ones: the key role of reactive oxygen species and nitric oxide in the plant responses to abiotic stress. Front Plant Sci 7:471
Ferreira DF (2018) Sisvar: a computer statistical analysis system. Cienc e Agrotec 35(6):1039–1042
Foyer CH, Neukermans J, Queval G, Noctor G, Harbinson J (2012) Photosynthetic control of electron transport and the regulation of gene expression. J Exp Bot 63(4):1637–1661
García-Tejera O, López-Bernal Á, Orgaz F, Testi L, Villalobos FJ (2021) The pitfalls of water potential for irrigation scheduling. Agric Water Manag 243:106522
Gay C, Gebicki JM (2000) A critical evaluation of the effect of sorbitol on the ferric–xylenol orange hydroperoxide assay. Anal Biochem 284(2):217–220
Genty B, Briantais JM, Baker NR (1989) The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta - Gen Subj 990(1):87–92
Giannopolitis CN, Ries SK (1977) Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol 59(2):309–314
Grossnickle SC, MacDonald JE (2018) Why seedlings grow: influence of plant attributes. New for 49(1):1–34
Hartman S, Sasidharan R, Voesenek LA (2021) The role of ethylene in metabolic acclimations to low oxygen. New Phytol 229(1):64–70
Hasibeder R, Fuchslueger L, Richter A, Bahn M (2015) Summer drought alters carbon allocation to roots and root respiration in mountain grassland. New Phytol 205(3):1117–1127
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 125(1):189–198
Hendrickson L, Furbank RT, Chow WS (2004) A simple alternative approach to assessing the fate of absorbed light energy using chlorophyll fluorescence. Photosynth Res 82(1):73–81
Herrera A (2013) Responses to flooding of plant water relations and leaf gas exchange in tropical tolerant trees of a black-water wetland. Front Plant Sci 4:106
Huang W, Yang SJ, Zhang SB, Zhang JL, Cao KF (2012) Cyclic electron flow plays an important role in photoprotection for the resurrection plant Paraboea rufescens under drought stress. Planta 235(4):819–828
Hunke P, Roller R, Zeilhofer P, Schröder B, Mueller EN (2015) Soil changes under different land-uses in the Cerrado of Mato Grosso. Brazil Geoderma Reg 4:31–43
Hussain S, Hussain S, Ali B, Ren X, Chen X, Li Q, Saqib M, Ahmad N (2021) Recent progress in understanding salinity tolerance in plants: Story of Na+/K+ balance and beyond. Plant Physiol Biochem 160:239–256
IPCC (2021) Summary for Policymakers. In: Masson-Delmotte V, Zhai P, Pirani A, Connors SL, Péan C, Berger S, Caud N, Chen Y, Goldfarb L, Gomis MI, Huang M, Leitzell K, Lonnoy E, Matthews JBR, Maycock TK, Waterfield T, Yelekçi O, Yu R, Zhou B (eds) Climate Change 2021 The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge.
Jethva J, Schmidt RR, Sauter M, Selinski J (2022) Try or die: Dynamics of plant respiration and how to survive low oxygen conditions. Plants 11(2):205
Khan MIR, Trivellini A, Chhillar H, Chopra P, Ferrante A, Khan NA, Ismail AM (2020) The significance and functions of ethylene in flooding stress tolerance in plants. Environ Exp Bot 179:104188
Khatun M, Sarkar S, Era FM, Islam AM, Anwar MP, Fahad S, Datta R, Islam AA (2021) Drought stress in grain legumes: effects, tolerance mechanisms and management. Agronomy 11(12):2374
Kuo MC, Kao CH (2003) Aluminum effects on lipid peroxidation and antioxidative enzyme activities in rice leaves. Biol Plant 46(1):149–152
Lacerda, J.J., 2016. Estresse salino e seus efeitos no crescimento inicial de clones de Eucalyptus (Dissertação—Mestrado em Agronomia, Área de Concentração em Fitotecnia). Vitória da Conquista—BA: UESB. 122p.
Latif HH, Mohamed HI (2016) Exogenous applications of moringa leaf extract effect on retrotransposon, ultrastructural and biochemical contents of common bean plants under environmental stresses. S Afr J Bot 106:221–231
Lê S, Josse J, Husson F (2008) FactoMineR: an R package for multivariate analysis. J Stat Softw 25:1–18
Lehmann J, Jørgensen ME, Fratz S, Müller HM, Kusch J, Scherzer S, Navarro-Retamal C, Mayer D, Böhm J, Konrad KR, Terpitz U, Dreyer I, Thomas D, Mueller TD, Sauer M, Hedrich R, Geiger D, Maierhofer T (2021) Acidosis-induced activation of anion channel SLAH3 in the flooding-related stress response of Arabidopsis. Curr Biol 31(16):3575–3585
Li J, Cang Z, Jiao F, Bai X, Zhang D, Zhai R (2017) Influence of drought stress on photosynthetic characteristics and protective enzymes of potato at seedling stage. J Saudi Soc Agric Sci 16(1):82–88
Liang W, Ma X, Wan P, Liu L (2018) Plant salt-tolerance mechanism: a review. Biochem Biophys Res Commun 495(1):286–291
Lima JRS, Antonino ACD, Andrade AP, Souza C, Soares WA, Souza ES, Silva IF (2006) Comparação da sonda de nêutrons e de sensores tipo TDR para a determinação dos componentes do balanço hídrico no solo e evapotranspiração do feijão caupi. Agro Tec 27(1):21–29
Lima DC, da Rocha AM, Noguera NH, do Nascimento, R. D. P., (2022) A review on Brazilian baru plant (Dipteryx alata Vogel): morphology, chemical composition, health effects, and technological potential. Future Foods. https://doi.org/10.1016/j.fufo.2022.100146
Lin C, Gentine P, Huang Y, Guan K, Kimm H, Zhou S (2018) Diel ecosystem conductance response to vapor pressure deficit is suboptimal and independent of soil moisture. Agric for Meteorol 250:24–34
Liu M, Qi H, Zhang ZP, Song ZW, Kou TJ, Zhang WJ, Yu JL (2012) Response of photosynthesis and chlorophyll fluorescence to drought stress in two maize cultivars. Afr J Agric Res 7(34):4751–4760
Ma Y, Dias MC, Freitas H (2020) Drought and salinity stress responses and microbe-induced tolerance in plants. Front Plant Sci 11:591911
Macêdo ÉC, Zonta JH, Melo YL, Melo ASD, Silva DCD, Andrade WLD (2019) Changes in osmoregulatory metabolism of cotton genotypes during water deficit and recovery period. Rev Bras Eng Agr Amb 23(8):607–613
Machado R, Loram-Lourenço L, Farnese FS, Alves RDFB, Sousa LF, Silva FG, Filho SCV, Torres-Ruiz JM, Cochard H, Menezes-Silva PE (2021) Where do leaf water leaks come from? Trade-offs underlying the variability in minimum conductance across tropical savanna species with contrasting growth strategies. New Phytol 229(3):1415–1430
MacKenzie WH, Mahony CR (2021) An ecological approach to climate change-informed tree species selection for reforestation. For Ecol Manag 481:118705
Maswada HF, Djanaguiraman M, Prasad PVV (2018) Seed treatment with nano-iron (III) oxide enhances germination, seeding growth and salinity tolerance of sorghum. J Agron Crop Sci 204(6):577–587
Maxwell K, Johnson GN (2000) Chlorophyll fluorescence-a practical guide. J Exp Bot 51(345):659–668
Menezes-Silva PE, Loram-Lourenço L, Alves RDFB, Sousa LF, Almeida SEDS, Farnese FS (2019) Different ways to die in a changing world: consequences of climate change for tree species performance and survival through an ecophysiological perspective. Ecol Evol 9(20):11979–11999
Murchie EH, Lawson T (2013) Chlorophyll fluorescence analysis: a guide to good practice and understanding some new applications. J Exp Bot 64(13):3983–3998
Noctor G, Veljovic-Jovanovic SONJA, Driscoll S, Novitskaya L, Foyer CH (2002) Drought and oxidative load in the leaves of C3 plants: a predominant role for photorespiration? Ann Bot 89(7):841–850
Noctor G, Reichheld JP, Foyer CH (2018) ROS-related redox regulation and signaling in plants. Semin Cell Dev Biol Academic Press 80:3–12
Oliveira ASD, Ferreira CS, Graciano-Ribeiro D, Franco AC (2015b) Anatomical and morphological modifications in response to flooding by six Cerrado tree species. Acta Bot Brasilica 29:478–488
Osakabe Y, Osakabe K, Shinozaki K, Tran LSP (2014) Response of plants to water stress. Front Plant Sci 5:86
Pask AJD, Pietragalla J, Mullan DM, Reynolds MP (2012) Physiological Breeding II: A Field Guide to Wheat Phenotyping. In: Pierre CS, Arce VT (eds.), Osmotic adjustment. D.F., CIMMYT, México. p 21–24.
Peixoto PHP, Cambraia J, Sant’Anna R, Mosquim PR, Moreira MA (1999) Aluminum effects on lipid peroxidation and on the activities of enzymes of oxidative metabolism in sorghum. Rev. Bras. Fisiol. Veg. 11(3):137–143
R Core Team (2020) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Rai RK, Singh VP, Upadhyay A (2017) Planning and evaluation of irrigation projects: methods and implementation. Academic press, New York
Sano EE, Rodrigues AA, Martins ES, Bettiol GM, Bustamante MM, Bezerra AS, Couto AF Jr, Vasconcelos V, Schüler J, Bolfe EL (2019) Cerrado ecoregions: a spatial framework to assess and prioritize Brazilian savanna environmental diversity for conservation. J Environ Manage 232:818–828
Sano SM, Ribeiro JF, De Brito MA (2004) Baru: Biologia e uso. Embrapa Cerrado, Planaltina-DF. p 52.
Scheibe R, Beck E (2011) Drought, desiccation, and oxidative stress. Plant desiccation tolerance. Springer, Berlin, Heidelberg, pp 209–231
Schmidt IB, De Urzedo DI, Piña-Rodrigues FCM, Vieira DLM, De Rezende GM, Sampaio AB, Junqueira RGP (2019) Community-based native seed production for restoration in Brazil–the role of science and policy. Plant Biol 21(3):389–397
Senf C, Buras A, Zang CS, Rammig A, Seidl R (2020) Excess forest mortality is consistently linked to drought across Europe. Nat Commun 11(1):1–8
Servillo L, Giovane A, Balestrieri ML, Cautela D, Castaldo D (2011) Proline derivatives in fruits of bergamot (Citrus bergamia Risso et Poit): presence of N-methyl-L-proline and 4-hydroxy-L-prolinebetaine. J Agric Food Chem 59(1):274–281
Seymen M (2021) How does the flooding stress occurring in different harvest times affect the morpho-physiological and biochemical characteristics of spinach? Sci Hortic 275:109713
Silva END, Silveira JAG, Rodrigues CRF, Lima CSD, Viégas RA (2009) Contribuição de solutos orgânicos e inorgânicos no ajustamento osmótico de pinhão-manso submetido à salinidade. Pesqui Agropecu Bras 44:437–445
Silva PE, Cavatte PC, Morais LE, Medina EF, DaMatta FM (2013) The functional divergence of biomass partitioning, carbon gain and water use in Coffea canephora in response to the water supply: implications for breeding aimed at improving drought tolerance. Environ Exp Bot 87:49–57
Silva AAD, Rubio ZCC, Linhares PCA, Silva KR, Pimentel GV, Marchiori PER (2022) Genotypic variation of sugarcane for salinity tolerance: Morphological and physiological responses. Cienc Agrotec 46:1–12
Sunil B, Saini D, Bapatla RB, Aswani V, Raghavendra AS (2019) Photorespiration is complemented by cyclic electron flow and the alternative oxidase pathway to optimize photosynthesis and protect against abiotic stress. Photosynth Res 139(1):67–79
Tan X, Xu H, Khan S, Equiza MA, Lee SH, Vaziriyeganeh M, Zwiazek JJ (2018) Plant water transport and aquaporins in oxygen-deprived environments. J Plant Physiol 227:20–30
Távora FJAF, Ferreira RG, Hernandez FFF (2001) Crescimento e relações hídricas em plantas de goiabeira submetidas a estresse salino com NaCl. Rev Bras Frutic 23(2):441–446
Tong C, Hill CB, Zhou G, Zhang XQ, Jia Y, Li C (2021) Opportunities for improving waterlogging tolerance in cereal crops-physiological traits and genetic mechanisms. Plants 10(8):1560
Vaishnav A, Shukla AK, Sharma A, Kumar R, Choudhary DK (2019) Endophytic bacteria in plant salt stress tolerance: current and future prospects. J Plant Growth Regul 38(2):650–668
Valentini R, Epron D, De Angelis P, Matteucci G, Dreyer E (1995) In situ estimation of net CO2 assimilation, photosynthetic electron flow and photorespiration in Turkey oak (Q. cerris L.) leaves: diurnal cycles under different levels of water supply. Plant Cell Environ. 18(6):631–640
Valladares F, Wright SJ, Lasso E, Kitajima K, Pearcy RW (2000) Plastic phenotypic response to light of 16 congeneric shrubs from a Panamanian rainforest. Ecology 81(7):1925–1936
Van Kooten O, Snel JF (1990) The use of chlorophyll fluorescence nomenclature in plant stress physiology. Photosynth Res 25(3):147–150
Venturas MD, Sperry JS, Hacke UG (2017) Plant xylem hydraulics: what we understand, current research, and future challenges. J Integr Plant Biol 59(6):356–389
Vilagrosa A, Chirino E, Peguero-Pina JJ, Barigah TS, Cochard H, Gil-Pelegrin E (2012) Xylem cavitation and embolism in plants living in water-limited ecosystems. Plant responses to drought stress. Springer, Berlin, Heidelberg, pp 63–109
Vilela F, de Souza AR (2020) Fatores que condicionam a salinização do solo cultivado com arroz. Anais do 3° Simpósio de TCC, das faculdades FINOM e Tecsoma. p 188–204.
Voss I, Sunil B, Scheibe R, Raghavendra AS (2013) Emerging concept for the role of photorespiration as an important part of abiotic stress response. Plant Biol 15(4):713–722
Wu Q, Su N, Huang X, Cui J, Shabala L, Zhou M, Min Yu, Shabala S (2021) Hypoxia-induced increase in GABA content is essential for restoration of membrane potential and preventing ROS-induced disturbance to ion homeostasis. Plant Commun 2(3):100188
Xu W, Cui K, Xu A, Nie L, Huang J, Peng S (2015) Drought stress condition increases root to shoot ratio via alteration of carbohydrate partitioning and enzymatic activity in rice seedlings. Acta Physiol Plant 37(2):1–11
Zandalinas SI, Mittler R (2022) Plant responses to multifactorial stress combination. New Phytol 234(4):1161–1167
Acknowledgements
This study was funded by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (grant nos. 207920/2017-6 and 432264/2018-3 to PEM-S; 309733/2021-9 to ARS; and 314719/2020-2 to FSF), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—CAPES (grant to LFS), and PROPPI (Pró-Reitoria de Pesquisa, Pós-Graduação e Inovação do Instituto Federal Goiano).
Author information
Authors and Affiliations
Contributions
FSF: designed the experiments and supervised the project. LFS, APSA, MCAPS, and SESA: cultivated the plants and conducted the experiments. LFS, AAS, and LLL: executed the hydraulic and physiological measurements. LFS and RDFBA: carried out the biochemical analyses. ARS: supervised the statistical analyses. FSF: wrote the manuscript with the support of LFS, PEMS, PFB, ACC, and ARS.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial or non-financial interests that are directly or indirectly related to the work submitted for publication.
Additional information
Communicated by A. Piotrowska-Niczyporuk.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sousa, L.F., Menezes-Silva, P.E., Batista, P.F. et al. Environmental vulnerability index in Dipteryx alata: influence of abiotic stresses on central processes of plant physiology and metabolism. Acta Physiol Plant 46, 31 (2024). https://doi.org/10.1007/s11738-024-03660-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-024-03660-w