Abstract
Ginsenoside Rb1 is an allelopathic self-toxic substance that can affect the growth and development of ginseng. This study investigated whether the application of exogenous Rb1 enhances the pathogenicity of ginseng by regulating the antioxidant system and endogenous hormones. Rb1 can inhibit the growth and development of Panax ginseng by inducing Fusarium oxysporum under three concentrations. At the same time, the activities of four antioxidant enzymes and the contents of three endogenous hormones in the roots of Panax ginseng decreased significantly. Compared with the control group, the incidence of ginseng in different treatment groups was significantly increased and the underground growth was significantly inhibited. In conclusion, exogenous ginsenoside Rb1 with different concentrations can enhance the pathogenicity of ginseng.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Panax ginseng is a perennial herb of Panax genus, mainly from China, South Korea and other Asian countries (Zhao et al. 2019; Zhan et al. 2021). As the main bioactive ingredients of ginseng, ginsenosides Rb1, Rd and Re have powerful pharmacological effects such as anti-tumor, anti-inflammatory, anti-diabetes, anti-oxidation, anti-allergy, neuroprotection, vasodilation and anti-cancer (Peng et al. 2012; Kim et al. 2016; Sun et al. 2016; Shi et al. 2019; Wang et al. 2019), so ginseng has been widely used in clinical for more than 2000 years (Kim. 2018; Wang et al. 2020a, b).
In the growth process of ginseng, it is necessary to interact with biological factors (microbial pathogens and pests, etc.) and abiotic factors (drought, high temperature, low temperature and salinity, etc.) (Foyer et al. 2016). These complex factors will affect the normal growth and development of ginseng, cause oxidative stress reaction, and thus lead to the phenomenon of loss of ginseng yield and quality (Cetinel et al. 2021). Soil-borne microbial pathogens are major sources of biological stress that can synergically increase the severity of negative effects with other stress sources. Fusarium oxysporum is one of the most destructive pathogens in soil. It has a high survival rate in soil under harsh environmental conditions and can cause serious rot to the roots of crops represented by ginseng, commonly known as root rot (Kim et al. 2009). According to statistics, the annual incidence of root rot disease is about 10–20%, which has become a bottleneck restricting the sustainable development of ginseng (Li et al. 2021). At the same time, studies have reported that root exudates of ginseng can also aggravate the biological stress of pathogens on ginseng (Nicol et al. 2003). At present, the known root exudates of ginseng mainly include saponins, sugars, amino acids, phenols and other primary and secondary metabolites (Sun et al. 1980). As the main member of root exudates of ginseng, the accumulation difference of ginsenosides can cause the occurrence of root rot, thus indirectly aggravating the stress effect of pathogens on ginseng (Xia et al. 2016).
Microbial pathogens, as the main biological stress factors in plant growth, are one of the main abiotic factors that affect the normal growth performance of plants. In addition, a series of physiological and biochemical changes occur in plants to counteract the potential harm caused by biological stress. The activity of antioxidant enzymes may be a good indicator of the influence of external environment on plant toxicity. MDA is one of the important indexes of membrane damage (Lukatkin et al. 2012). Once the membrane is destroyed, it is released into the extracellular environment, so MDA detection can directly assess the extent of damage to the membrane system (Peng et al. 2019). Peroxidase and catalase are mainly responsible for removing H2O2. Among them, peroxidase catalyzes the redox reaction between H2O2 and various reducing agents such as phenols, amines and alcohols, which often occurs in normal plant growth and defense reactions (Naliwajski and Skodowska 2021). Catalase is an important enzyme that removes the products of superoxide dismutase-catalyzed reactions by converting H2O2 directly to H2O and O2 (Surgun-Acar and Zemheri-Navruz 2021). In addition, endogenous plant hormones are closely related to cell differentiation in vitro, and can reflect the influence of external environment on plant toxicity through changes in plant morphology (Huang et al. 2012).
In this study, physiological indexes of ginsenoside Rb1 enhancing panax ginseng virulence were systematically analyzed, including antioxidant enzymes, soluble proteins and endogenous hormones. The results of this study will elucidate the potential physiological mechanism of saponin Rb1 regulating antioxidant system and endogenous hormones enhancing the pathogenicity of ginseng. It is hoped that this new discovery can contribute to the sustainable development of ginseng cultivation.
Materials and methods
Overview and experimental design of the study area
The experiment was conducted in the Medicinal Plant Resource Garden of Changchun University of Chinese Medicine (43° 891014″ N, 125º 317505″ E) in Changchun, Jilin Province on April 25, 2021. Four treatments were used in pot experiment: (1) only F. oxysporum was added into the soil of the new forest as the control group (CK); (2) on the basis of F. oxysporum, 0.3 µg/kg ginsenoside Rb1 was poured into the new forest soil (Rb1-0.3); (3) on the basis of F. oxysporum, 3 µg/kg ginsenoside Rb1 was poured into the new forest soil (Rb1-3); (4) on the basis of F. oxysporum, 30 µg /kg ginsenoside Rb1 was poured into the new forest soil (Rb1-30).
Experimental materials
The test material was 2-year-old ginseng plants of the ginseng species ‘Ma Ya’, purchased in April 2021 from the ginseng market in Wanliang Town, Fusong County, Jilin Province (512.2 m above sea level (Latitude 42˚4438" N, Longitude 127˚ 3098" E). Ginseng was planted in round-bottomed pots with 6 ginseng plants per pot and 9 kg of soil per pot. The cultivated soil was taken from the new forest soil of Baixi Forest Farm, Fusong County, Jilin Province. The surface leaf litter was removed and the soil of 10–20 cm layer was collected for reserve. Ginsenoside control products were purchased from Shanghai Baoman Biotechnology Co., LTD., protein (TP) assay kit, Malondialdehyde (MDA) kit, Superoxide Dismutase (SOD), Catalase (MDA) kit. CAT kit and Peroxidase (POD) kit were purchased from Nanjing Jiancheng Co., LTD. Plant Gibberellin (GA) ELISA Kit, Plant Hormone Abscisic acid (ABA) ELISA Kit Plant indole-3-acetic acid (IAA) ELISA Kit were purchased from Chemical Control Research Center of China Agricultural University.
Experimental instrument
The microplate meter used in this study is M200pro produced by Tecan of Switzerland, the high-speed refrigerated centrifuge is CR30NX produced by Ebender China Co., LTD, and the oscillation incubator is ZQZY-85CNS produced by Shanghai Zhichu Instrument Co., LTD. The digital display constant temperature water bath is HH-8 produced by Changzhou Zhibo Instrument Manufacturing Co., LTD., the vernier caliper is 532-101 NM13 produced by Shanghai First Precision Instrument, and the analytical balance is MS-TS produced by Mettler Toledo International Trade (Shanghai) Co., LTD.
Morphological determination and growth analysis of ginseng
The experiment was carried out on September 16, 2021, and the underground growth of ginseng was recorded during harvesting. Six plants were randomly selected from each treatment and labeled. The root diameter, main root length and fibrous root number of ginseng were measured, and the fresh weight of ginseng was weighed. Use a ruler to measure taproot length and a vernier caliper to measure root thickness.
Determination of physiological resistance of ginseng
Fresh ginseng roots (0.5 g) were ground in 5 ml of PBS (0.05 mol/l, pH 7.8) in an ice bath. The content of malondialdehyde (MDA) was measured by a kit (Nanjing Jiancheng Institute of Biological Engineering, Nanjing, China). The absorbance of the reaction solution was measured at 530 nm. This value was used to calculate the MDA content. The crude extract of the enzyme was prepared by reference to a modified version of the method of Ba et al. (Ba et al. 2013). The samples were ground into a homogenate and transferred to a centrifuge tube. Buffer was then added to a final volume of 5 ml. The samples were then centrifuged at 10,000 rpm for 10 min at 4 °C. SOD, POD and CAT activities were measured using commercial assay kits (Nanjing Jiancheng Biologicals), specifically, SOD activity was determined by the SWT-1 method, where SOD was expressed as one unit of enzyme activity per g of plant tissue in the reaction system at 50% SOD inhibition; CAT was determined by the ammonium molybdate method, where CAT activity was measured as one unit of activity per milligram of H2O2 per second. Colorimetric method was used to determine POD, and POD activity was defined as the amount of enzyme catalyzing micrograms of substrate per minute per milligram of plant tissue protein at 37 °C.
Root survival rate
Six ginseng strains were randomly selected from each treatment to investigate the incidence of root disease in underground part of ginseng, and the incidence area of each ginseng root was graded. Disease severity classification standard and disease severity index refer to Rahman et al.’s method (Rahman and Punja 2006): A indicated that there was no visible root spot, B indicated that the diameter of root spot was 0.9 mm, C indicated that the root spot was 1–4.0 mm, D indicated that the root spot was 4.1–7.0 mm, E indicated that the root spot was larger than 7.0 mm, and F indicated that the root spot infected the whole root. The calculation formula is as follows:
where XA, XB, XC, XD, XE, and XF represent the numbers of plants with rotting severity in Grades A, B, C, D, E and F, respectively.
Data processing and analysis
Excel 2019 was used for data recording, sorting and chart making, and SPSS 23.0 was used for data analysis. (IBM, New York, USA). One-way ANOVA and Duncan’s New Complex Range method were used to test the significance of the difference between the mean values of all treatments. In statistical analysis, P < 0.05 was considered as significant.
Results
Growth and development of ginseng
Compared with CK group, the main root length, root diameter and fibrous root number of ginseng treated with different concentration of saponin Rb1 were significantly reduced (Fig. 1). Among them, Rb1-3 group had extremely significant effect (P < 0.01), and Rb1-30 group had significant difference in main root length and fibrous root number compared with the control group (P < 0.05), but there was no significant difference in root diameter among different treatment groups (P > 0.05).
Physiological indexes of ginseng
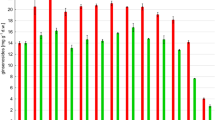
Compared with the control group, the soluble protein content of ginseng treated with all three concentrations of ginsenoside Rb1 was significantly different, with the highest soluble protein content (74.99 µg/ml) in the Rb1-3 treated group (Fig. 2). At concentrations of 0.3, 3, and 30 µg/kg, Rb1 induced changes in the antioxidant protection system of ginseng root tissues through the induction of Fusarium oxysporum (Fig. 3). In brief, it was observed that the CAT enzyme, POD enzyme and SOD enzyme activities of ginseng roots treated with the three concentrations of ginsenosides were significantly reduced compared to the control group, and the MDA content was also significantly decreased, with the lowest activity of all three enzymes at a ginsenoside Rb1 concentration of 3 ug/kg, as evidenced by a CAT enzyme activity of only 0.12 U/mgprot (Fig. 3A) The activity of POD enzyme was 4.35 U/mgprot (Fig. 3B), the activity of SOD enzyme was 14.09 U/mgprot (Fig. 3C), and the MDA content was only 0.45 nmol/mgprot (Fig. 3D).
Effect of different treatments on CAT, SOD, POD activities and MDA content of ginseng roots. A Changes in CAT activity. B Changes in SOD activity. C Changes in POD activity. D Changes in MDA content. Each value represents the mean of three replicates per treatment ± SD. Different lowercase letters indicate significant differences between treatments (P < 0.05)
Effects on endogenous hormones of ginseng
Compared with CK group, the endogenous hormone ABA content of ginseng root treated with three concentrations of ginsenosides showed significant differences. The endogenous hormone ABA in ginseng roots had the lowest ABA content (308.86 ng/g) at a concentration of 3 µg/kg. When the concentration of Rb1 was 0.3 µg/kg, the highest ABA content was 401.73 ng/g (Fig. 4A.). Compared with CK group, the contents of endogenous hormone IAA in ginseng root treated with three concentrations of ginsenosides showed significant differences. As shown in Fig. 4B, the endogenous hormone IAA content of ginseng roots had the lowest IAA content at a concentration of 3 µg/kg, with a concentration of 60.07 nmol/g, and the highest IAA content at a concentration of 70.63 nmol/g when the Rb1 concentration was 0.3 µg/kg. Compared with CK group, the content of endogenous hormone GA in ginseng root treated with three concentrations of ginsenosides showed significant differences. The GA content of ginseng root endogenous hormone was the lowest at a concentration of 3 µg/kg with a concentration of 372.69 pmol/g; the highest GA content was found when the concentration of Rb1 was 0.3 ug/kg with a concentration of 459.13 pmol/g (Fig. 4C).
Effects of different treatments on the ABA, IAA and GA content in ginseng roots. A Changes in ABA contents. B Changes in IAA contents. C Changes in GA contents. Each value represents the mean of three replicates per treatment ± SD. Different lowercase letters indicate significant differences between treatments (P < 0.05)
Incidence and severity of ginseng
The incidence rate of CK group was the lowest, only 33.3%, and the ginseng disease severity index of this group was 1.67. The incidence of ginseng treated with ginsenoside Rb1 was significantly higher than that of CK group, and the highest incidence was Rb1-30 group, which had an incidence of 100% and disease severity index of 4.16. The incidence of ginseng in Rb1-3 group was 66.7%, and the disease severity index was 4. Although the incidence of ginseng in Rb1-0.3 group was not as high as that in the first two groups, the incidence also reached 50%, and the ginseng disease severity index in this group was 3 (Table 1).
Underground growth of ginseng
Figure 5 shows the underground root growth of ginseng treated with CK and three concentrations of ginsenosides. It was found that the ginseng in the CK group, the root growth was better than that in the three treatment groups, as shown by the thicker roots, more number of fibrous roots and almost no disease spots on the ginseng epidermis in this group. It can be seen from the Fig. 5 that Rb1 inhibited the growth of ginseng roots by inducing Fusarium oxysporum at the concentrations of 0.3, 3, and 30 µg/kg, with the most severe inhibition of underground growth in the ginseng treated by the Rb1-30 group.
Discussion
Ginseng metabolites with high activity can significantly affect the growth and quality of ginseng (Li et al. 2010). Compared with the control group, the growth of ginseng was inhibited in treatment groups with different concentrations. Among them, Fusarium oxysporum showed the strongest induction ability when Rg1 concentration was 3 ug/kg, which effectively restricted the growth and development of ginseng. This can be verified by the growth index characteristics of the group, and the growth index of the group showed significant differences compared with the control group (P < 0.05). Compared with the control group, the taproot length, root diameter and fibrous root number in this group decreased by 33.34%, 18.25% and 38.22%, respectively. In addition, most ginseng in this group showed disease spots, and its growth was obviously inhibited.
Studies have shown that antioxidant enzyme activity is closely related to plant resistance to stress and is an important index to study allelopathy of plants (Sathiyaraj et al. 2014). As an intermediary, H2O2 is often used to study the mechanism of oxidative stress on cell damage (Sohn et al. 2013). CAT and POD are two indicators of oxidative stress in higher plants. They usually play a synergistic role to promote the decomposition of H2O2 in cells and prevent it from further producing highly toxic hydroxyl and oxygen free radicals, thus protecting the antioxidant system (Song et al. 2007; Chung et al. 2016). The content of MDA can reflect the degree of membrane peroxidation and indirectly reflect the degree of cell damage and stress resistance of plants (Wang et al. 2014; Liang et al. 2018; Zhang et al. 2021). SOD is an antioxidant metal enzyme existing in organisms, and its activity is generally positively correlated with the production of excess superoxide (Islam et al. 2021). The physiological resistance test results showed that MDA content and SOD, CAT and POD activities of Rb1-0.3 and Rb1-30 groups were higher than those of Rb1-3 group, indicating that the stress resistance of these two groups was stronger than that of Rb1-3 group, that is, the degree of stress suffered by ginseng was greater than that of Rb1-3 group. It can be seen that several physiological indexes studied in this experiment were lower than those in CK group, which may be because ginseng was sampled at the harvesting stage, and ginsenoside Rb1 induced Fusarium oxysporum, then the low-toxicity H2O2 was converted into H2O in ginseng mainly through the synergistic effect of CAT and POD. The disproportionation reaction led by SOD reached a dynamic balance, and after a long test cycle, the activity values of antioxidant enzymes in these treatment groups were all lower than those in CK group.
The growth process of higher plants is affected by a variety of complex factors, such as environmental conditions (such as light, temperature, and humidity), enzymes, proteins and endogenous plant hormones, all play a particularly important role (Kim et al. 2014). As a growth inhibitor, ABA has some physiological effects on increasing stress resistance. The trend of ABA content in different treatment groups was Rb1-3 < Rb1-30 < Rb1-0.3, which was consistent with the trend of antioxidant enzyme activity of ginseng. Ginsenoside Rb1 could enhance the induction of Fusarium oxysporum and inhibit the elongation and growth of ginseng roots and other organs, affect differentiation, and induce the appearance of ginseng disease spots. Under allelopathic stress, IAA index was sensitive. The trend of IAA content in different treatment groups was Rb1-3 < Rb1-30 < Rb1-0.3. These results were consistent with the trend of ABA results and the change of ABA content also caused a certain degree of growth and metabolism inhibition in physiology. The content trend of GA in different treatment groups was Rb1-3 < Rb1-30 < Rb1-0.3. The results showed that the pathogenicity of Fusarium oxysporum induced by ginseng was different with the concentration of ginsenoside Rb1, and the endogenous hormone GA of ginseng might be combined with other hormone indexes to adapt to the change of environment by balancing hormone homeostasis.
Conclusion
In this study, dynamic differences in the content of active saponin Rb1 at different concentrations in soil could affect the changes of enzyme activity in plants and restrict the normal growth of ginseng by causing oxidative stress damage. Specifically, under the induction of ginsenoside Rb1 at three different concentrations, CAT can enhance the destruction of ginseng tissues and cells, while SOD and POD can protect the bacteria from the influence of ginsenoside Rb1. It was beneficial to the infection of Fusarium oxysporum, enhanced the damage of Fusarium oxysporum to the root of ginseng and hindered the healthy growth of ginseng. After testing found that ginseng saponin Rb1 in vitro after induction of F. oxysporum caused some pathogen, host interactions in the changes of antioxidant enzyme activity and endogenous hormone, calculated the Rb1 hinder ginsenosides of ginseng normal growth and development of the new mechanism, prevention and control of this in the future for us to develop new ideas, ginseng root rot also provide guarantee for the sustainable development of ginseng industry.
Author contribution statement
NY, the first author of this article, is mainly responsible for the experimental part of the paper and the writing of the article. XM, YZ and EW are mainly responsible for the processing of experimental data and the drawing of graphs in the text. CC is mainly responsible for the funding of papers and experiments. QL is mainly responsible for determining the content of the experiment and checking the articles.
Data availability
The entire data used in this study are all available within the text of this paper.
References
Ba QS, Zhang GS, Wang JS et al (2013) Relationship between metabolism of reactive oxygen species and chemically induced male sterility in wheat (Triticum aestivum L.). Can J Plant Sci 93:675–681. https://doi.org/10.4141/cjps2012-280
Cetinel A, Gokce A, Erdik E et al (2021) The effect of Trichoderma citrinoviride treatment under salinity combined to Rhizoctonia solani infection in strawberry (Fragaria x ananassa Duch.). Agron 11:1589–1607. https://doi.org/10.3390/agronomy11081589
Chung SI, Kang MY, Lee SC (2016) In vitro and in vivo antioxidant activity of aged ginseng (Panax ginseng). Preven Nutr Food Sci 21:24–30. https://doi.org/10.3746/pnf.2016.21.1.24
Foyer CH, Rasool B, Davey JW et al (2016) Cross-tolerance to biotic and abiotic stresses in plants: a focus on resistance to aphid infestation. J Exp Bot 67:2025–2037. https://doi.org/10.1093/jxb/erw079
Huang WL, Lee CH, Chen YR (2012) Levels of endogenous abscisic acid and indole-3-acetic acid influence shoot organogenesis in callus cultures of rice subjected to osmotic stress. Plant Cell Tissue Org Cult. https://doi.org/10.1007/s11240-011-0038-0
Islam MJ, Ryu BR, Azad MOK et al (2021) Exogenous putrescine enhances salt tolerance and ginsenosides content in Korean ginseng (Panax ginseng Meyer) Sprouts. Plants Basel 10:1–22. https://doi.org/10.3390/plants10071313
Kim DH (2018) Gut microbiota-mediated pharmacokinetics of ginseng saponins. J Ginseng Res 42:255–263. https://doi.org/10.1016/j.jgr.2017.04.011
Kim JH, Kim SG, Kim MS et al (2009) Different structural modifications associated with development of ginseng root rot caused by Cylindrocarpon destructans. Plant Pathol J 25:1–5. https://doi.org/10.5423/PPJ.2009.25.1.001
Kim YH, Ahn IO, Khan AL et al (2014) Regulation of endogenous gibberellins and abscisic acid levels during different seed collection periods in Panax ginseng. Hortic Environ Biotechnol 55:166–174. https://doi.org/10.1007/s13580-014-0146-y
Kim JK, Tabassum N, Uddin MR et al (2016) Ginseng: a miracle sources of herbal and pharmacological uses. Orient Pharm Exp Med 16:243–256. https://doi.org/10.1007/s13596-016-0246-6
Lei FJ, Fu JF, Zhou RJ et al (2017) Chemotactic response of Ginseng bacterial soft-rot to Ginseng root exudates. Saudi J Biol Sci. https://doi.org/10.1016/j.sjbs.2017.05.006
Li D, Li C, Sun H et al (2010) Effects of drought on soluble protein content and protective enzyme system in cotton leaves. Front Agric China 4:56–62. https://doi.org/10.1007/s11703-010-0102-2
Li Q, Zhan Y, Xie HZ et al (2021) Potential causes and recovery of soil sickness from the Panax ginseng cultivation. Allelopath J 54(1):105–120
Liang S, Xu X, Lu Z (2018) Effect of azoxystrobin fungicide on the physiological and biochemical indices and ginsenoside contents of ginseng leaves. J Ginseng Res. https://doi.org/10.1016/j.jgr.2017.02.004
Lukatkin AS, Brazaityte A, Bobinas È et al (2012) Chilling injury in chilling-sensitive plants: a review. Zemdirb Agric 99:111–124
Naliwajski M, Skodowska M (2021) The relationship between the antioxidant system and proline metabolism in the leaves of cucumber plants acclimated to salt stress. Cells 10:609–624. https://doi.org/10.3390/cells10030609
Nicol RW, Yousef L, Traquair JA et al (2003) Ginsenosides stimulate the growth of soilborne pathogens of American ginseng. Phytochem 64:257–264. https://doi.org/10.1016/s0031-9422(03)00271-1
Peng L, Sun S, Xie LH et al (2012) Ginsenoside re: pharmacological effects on cardiovascular system. Cardiovasc Ther 30:e183–e188. https://doi.org/10.1111/j.1755-5922.2011.00271.x
Peng JN, Wang K, Feng TY et al (2019) The effect of (1S,2R-((3-bromophenethyl)amino)-N-(4-chloro-2-trifluoromethylphenyl) cyclohexane-1–sulfonamide) on Botrytis cinerea through the membrane damage mechanism. Molecules 25:1–11. https://doi.org/10.3390/molecules25010094
Rahman M, Punja ZK (2006) Influence of iron on cylindrocarpon root rot development on Ginseng. Phytopathol 96:1179–1187. https://doi.org/10.1094/PHYTO-96-1179
Sathiyaraj G, Srinivasan S, Kim YJ et al (2014) Acclimation of hydrogen peroxide enhances salt tolerance by activating defense-related proteins in Panax ginseng C.A Meyer. Mol Biol Rep 41:3761–3771. https://doi.org/10.1007/s11033-014-3241-3
Shi ZY, Zeng JZ, Wong A (2019) Chemical structures and pharmacological profiles of Ginseng saponins. Molecules 24:2443–2457. https://doi.org/10.3390/molecules24132443
Sohn SH, Kim SK, Kim YO et al (2013) A comparison of antioxidant activity of korean white and red ginsengs on H2O2-induced oxidative stress in HepG2 hepatoma cells. J Ginseng Res 37:442–450. https://doi.org/10.5142/jgr.2013.37.44
Song NH, Yin XL, Chen GF et al (2007) Biological responses of wheat (Triticum aestivum) plants to the herbicide chlorotoluron in soils. Chemosphere 78:1779–1787. https://doi.org/10.1016/j.chemosphere.2007.03.023
Sun Y, Liu Y, Chen K (2016) Roles and mechanisms of ginsenoside in cardiovascular diseases- progress and perspectives. Sci China-Life Sci 59:292–298. https://doi.org/10.1007/s11427-016-5007-8
Sun J, Yang J, Zhao SY et al (2023) Root exudates influence rhizosphere fungi and thereby synergistically regulate Panax ginseng yield and quality. Front Microbiol 14:1–13. https://doi.org/10.3389/fmicb.2023.1194224
Surgun-Acar Y, Zemheri-Navruz F (2021) Exogenous application of 24-epibrassinolide improves manganese tolerance in Arabidopsis thaliana L. via the modulation of antioxidant system. J Plant Growth Regul 41:546–557. https://doi.org/10.1007/s00344-021-10320-7
Wang M, Jiao L, Li B et al (2014) Antioxidant activities of the oligosaccharides from the roots, flowers and leaves of Panax ginseng C A Meyer. Carbohydr Polym. https://doi.org/10.1016/j.carbpol.2014.02.035
Wang C, Liu J, Deng J et al (2020a) Advances in the chemistry, pharmacological diversity, and metabolism of 20(R)-ginseng saponins. J Ginseng Res 44:14–23. https://doi.org/10.1016/j.jgr.2019.01.005
Wang CM, Liu J, Deng JQ et al (2020b) Advances in the chemistry, pharmacological diversity, and metabolism of 20(R)-ginseng saponins. J Ginseng Res 4:14–23. https://doi.org/10.1016/j.jgr.2019.01.005
Xia PG, Guo HB, Zhao HG et al (2016) Optimal fertilizer application for Panax notoginseng and effect of soil water on root rot disease and saponin contents. J Ginseng Res 40:38–46. https://doi.org/10.1016/j.jgr.2015.04.003
Yu SE, Mwesige B, Yi YS et al (2018) Ginsenosides: the need to move forward from bench to clinical trials. J Ginseng Res 43:1–7. https://doi.org/10.1016/j.jgr.2018.09.001
Zhan Y, Xie HZ, Yan N (2021) Ginseng: history, cultivation, industry and future prospects. Allelop J 54(1):79–92
Zhang T, Chen CB, Chen YQ et al (2021) Changes in the leaf physiological characteristics and tissue-specific distribution of ginsenosides in Panax ginseng during flowering stage under cold stress. Front Bioeng Biotechnol. https://doi.org/10.3389/fbioe.2021.637324
Zhao B, Lv CN, Lu JC (2019) Natural occurring polysaccharides from Panax ginseng C A Meyer: a review of isolation, structures, and bioactivities. Intern J Biolog Macromol. https://doi.org/10.1016/j.ijbiomac.2019.03.229
Acknowledgements
This work was financially supported by grants from National Natural Science Foundation of China (82204558), Key R & D plan of science and Technology Department of Jilin Province (20220204078YY), National Natural Science Foundation of China (82073969), Jilin Province Major Science and Technology Special Project (20200504003YY), Jilin Province Natural Science Foundation Project (YDZJ202101ZYTS015), and Changchun Science and Technology Development Plan Project (21ZGY13). These grants were received by Professor Changbao Chen and played an important role in deciding to publish and prepare manuscripts.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No conflict of interest exits in the submission of this manuscript, and all the authors have approved the manuscript and agreed to submit it to Sustainability. The author would like to declare on behalf of his co-authors that the work described was original research that has not been published previously, and we confirm that neither the manuscript nor any part of its content is currently being considered or published in another journal.
Additional information
Communicated by V. `P. Singh.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yan, N., Miao, X., Zhan, Y. et al. Study on the mechanism of Rb1 regulating antioxidant defense system and endogenous hormones leading to increased virulence of Panax ginseng. Acta Physiol Plant 46, 43 (2024). https://doi.org/10.1007/s11738-024-03648-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-024-03648-6