Abstract
The distribution of woody species in flooded environments depends on the duration of stress as well as seed germination strategies and plant morphophysiological adaptations. Allophylus edulis is a tree that occurs in temporarily or permanently flooded areas in several countries of South America. In this paper, we evaluate seed germination, growth parameters, photosynthetic pigment contents and chlorophyll a fluorescence in young plants to understand the tolerance of the specie to flood events. The evaluations were performed in non-flooded (NFL) and flooded (FL) plants in a temporal scale that included short (up to 30 days) and long (up to 90 days) flood periods. A short flooding (15 days) may favor germination but maintaining stress for 60 days makes the seeds unviable. Although 71.4% of the FL plants survived up to 90 days of flooding, injuries such as chlorosis and foliar abscission appeared. An increase in stem height and diameter was only observed in NFL plants; whereas, FL plants showed a growth inhibition. At 90 days, NFL and FL plants presented total dry mass of 18.35 ± 1.57 g and 1.93 ± 0.62 g, respectively. The photosynthetic performance indexes indicated acclimatization of the plants on the third day of flooding, but the stress induced a progressive decline in the parameters, signaling damages to the photosystem II. Both seeds and young plants of A. edulis tolerate short periods of flooding, but prolonged floods make the seeds unfeasible and damages the photosynthetic apparatus, leading to death of the plants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Periodic or permanent floods exert strong selective pressure on the species, influencing aspects like the composition and structure of the tree flora in forest formations (Silva et al. 2007). Flooding causes a number of changes in the soil, such as decreased or disappeared O2, accumulation of CO2, and formation of toxic compounds affecting various processes of plant development (Kozlowski 1997).
Flood events are challenging in all life stages of the plant, especially during the seed germination and seedlings initial development (Zúñiga-feest et al. 2017). The seeds and seedlings of some species are not tolerant to flooding although adult plants of the same species tolerant stress (da Paz et al. 2017). The oxygen is less available to the embryos in flooded soils, preventing or delaying the germination of seeds of various species (Kozlowski 1997). To withstand flooding events, seeds may have morphological adaptations that improve their fluctuation (Parolin et al. 2004); physical dormancy (Lucas et al. 2012; da Paz et al. 2017); physiological dormancy (Scarano et al. 2003) and energy reserve compounds (Ferreira et al. 2009).
Flooding of the root system can cause a number of consequences to the plant such as increased shoot biomass to the detriment of the root (Liu et al. 2015; Yu et al. 2015), reduced or inhibited growth (Bailey-Serres and Voesenek 2008; Colmer and Voesenek 2009), decreased water and nutrient uptake (Colmer and Voesenek 2009; Liu et al. 2015), leaf fall (Parolin et al. 2004), minor chlorophyll content and decreased photosynthetic performance (Junior et al. 2015). The decrease in photosynthetic activity during flooding has been related to several factors such as decreased stomatal conductance (Medina et al. 2009), lower photosynthetic enzyme activities (Pezeshki 2001), lower chlorophyll content (Parolin et al. 2004), photosystem II damage and photosynthetic electron transport (Reginfo et al. 2005). The stress duration influences the damage to the structure and activity of the photosystem II and the development of photosynthetic adjustments in the plant (Larré et al. 2013).
The occurrence of some species in a predominantly flooded area is a strong but inconclusive indication of their tolerance, since some abiotic and/or biotic factors facilitate the occurrence of less tolerant species in these environments. In swamp forests, microtopographic changes (Teixeira et al. 2011; Gattringer et al. 2018) and positive interactions between plants (Scarano 2002) can attenuate the stress caused by flooding, favoring the germination process and the survival of species less tolerant or living in flood-prone areas.
Flooding events can last for up to seven months in a year in some swamp forests in the extreme south of Brazil. These forests are located on relief depressions where the ground water is close to the surface or emerging most of the year, with periods of high precipitation rates, configuring long flooding events (Waechter and Jarenkow 1998; Duarte 2012). These forests are characterized by great spatial variations in soil water conditions, containing flooded microenvironments (water line formation) and non-flooded microenvironments (humid soil) (Duarte 2012). The presence of arboreal individuals is noticeably greater in non-flooded sites, being Allophylus edulis (A.St.-Hil et al.) Hieron. ex Niederl one of the species possibly favored by these microenvironments.
Allophylus edulis is a pioneer tree distributed in several countries of South America, including diverse phytogeographic domains throughout Brazil (Abreu et al. 2005). The plantation of this species is recommended to accelerate forest restoration due to its rapid growth and high amount of fruits that attract dispersers of seeds from other species to the site (Turchetto et al. 2017). This species colonizes from open areas to forest formations subject to flooding, and it is more abundant in temporarily flooded forests than in those permanently flooded (Silva et al. 2007).
The species distribution in environments with different degrees of stress can be inferred through the knowledge about their level of tolerance to factors such as soil water conditions. Although important, this information is still scarce for subtropical and tropical tree species in southern South America (Zúñiga-feest et al. 2017), becoming an obstacle to restoration projects. This information is even more relevant if we consider the climate change scenario, which will increase the frequency of extreme weather events such as rainfall and drought, thereby influencing the hydrological regime in areas subject to flooding (Junk 2013; Parolin and Wittmann 2010). In the extreme south of Brazil, the climate change is associated with an increase in precipitations (Copertino et al. 2016), intensifying forest flood events on a spatial and temporal scale. Such changes may impact the structure and distribution of the tree species in flooded forests.
In this sense, we evaluated the tolerance of seeds and young plants of Allophylus edulis to short- and long-term flooding. Our hypothesis was that seeds and young plants of the species would not tolerate a long period of flooding. Thus, short floods would reduce seed germination, growth, content of photosynthetic pigments and photosynthetic indexes (PIs) of young plants. While, long periods of flooding cause plant death and seed unviability.
Materials and methods
Fruits of various trees of Allophylus edulis were collected in a swamp forest located in the municipality of Rio Grande, RS, Brazil (32° 07′ S; 52° 09′ W). This forest remains flooded for approximately seven months per year, with a depth of about 30 cm of water, and the flood periods can be greater or lesser depending on the rainfall regime (Duarte 2012). The seeds for the experiments I and II were collected in November 2016, and in December 2015 for the experiment III. The fruits were pulped and the seeds were used shortly after the collection in the tests to maintain their viability, since the seeds of A. edulis have a recalcitrant behavior.
Experiment I: seed germination in flooded and non-flooded conditions
This experiment consisted of two treatments using eight Gerbox® transparent plastic boxes with 25 seeds each, totaling 200 seeds per treatment: non-flooded (NFL) and flooded (FL), with seed sowing on a sheet of blotting paper moistened with 20 mL and 150 mL of distilled water, respectively, totaling approximately 2 cm of water depth in FL treatment.
The germination tests were carried out for 30 days in a BOD germination chamber under the temperature of 25 °C and constant light, as recommended by Abreu et al. (2005). The criterion considered for germination was the presence of radicles larger than 2 mm in the seeds. The percentage of germination (%G) and germination speed index (GSI) were evaluated according to the equations described by Oliveira et al. (2009). At the end of the experiment, the seedlings that were morphologically perfect had the measured length (cm) and were classified as normal seedlings (NS) and seedlings that had any of their essential structures missing, deformed or severely damaged were classified as abnormal (AS) (Brasil, Ministério da Agricultura, Pecuária e Abastecimento 2009; Oliveira et al. 2009).
Experiment II: germination with relief from flood stress
The experiment II consisted of four tests with eight samples of 25 seeds, totaling 200 seeds per test. All tests were performed in Gerbox® boxes, using a layer of 2 cm of medium-textured, washed and sterilized sand (substrate). The germination tests followed the same criteria of germination, temperature and photoperiod established in experiment I. The seeds were submitted to four temporal levels of flood-related stress. In the control (time 0), the seeds were planted directly on the non-flooded sand, moistened with 20 mL of distilled water. In the other tests, to ensure submersion, the seeds were initially semi-buried in sand substrate and submitted to different flooding periods (15, 30, 60 days), characterizing different stress levels induced by flood duration. To this end, the Gerbox® boxes were filled with 150 mL of distilled water, maintaining a water depth about of 2 cm. At the end of each flooding period, the seeds were removed from the flood conditions (stress relief) and transferred to Gerbox® boxes with the same conditions of the control treatment, to evaluate the germination parameters.
Experiment III: development of young plants under flood conditions
The seedlings for this experiment were obtained from the germination of A. edulis seeds in 290 cm3 tubes. After 60 days, the seedlings were transplanted to 5-L perforated plastic pots containing organic soil obtained from the Federal University of Rio Grande. After seven months, the plants were submitted to two treatments: (I) non-flooded plants (NFL), irrigated daily to keep the substrate moist; (II) flooded (FL) plants, where the pots were placed into a second, to 10-L non-perforated plastic vessel to maintain a water slide of about 3 cm above the substrate. The experiment lasted for 90 days and was conducted in experimental area covered with shade screen that partially diminished the solar radiation incident on the plants (50% shading). Global solar radiation daily (mean ± SD), during 30, 60 and 90 days of flooding was, respectively, 953.30 ± 510.29 kJ/m2, 1451.2 ± 569.35 kJ/m2, and 1626 ± 681.39 kJ/m2. The air temperature for the same periods was 14.9 ± 2.1 °C, 16.1 ± 2.45 °C and 19 ± 2.66 °C, respectively. Daily values of global solar radiation and air temperature were obtained by Automatic Meteorological Station of Rio Grande, RS, Brazil (32° 01′ S 52° 06′ W), distant about 2 km from area of flooding experiment and provided by National Institute of Meteorology (INMET). Climate of the region is classified as Cfa, being characterized as humid subtropical (Alvares et al. 2013).
To evaluate survival and growth, 35 plants of each treatment (NFL and FL) were selected and evaluated after seven different periods: 1, 10, 20, 30, 45, 60 and 90 days after the flooding started. The growth parameters analyzed were: plant height (cm), from the base of the plant until the meristematic apex insertion; number of leaves; and stem diameter (cm), measured at 2 cm above the ground with a pachymeter. Possible visual morphological alterations induced by the treatments, such as hypertrophied lenticels and adventitious roots, were weekly monitored. At the end of the experiment, ten plants of each treatment were randomly selected to estimate leaf area and dry mass. The leaf area was obtained through the foliar contour method, scanning ten mature leaves of each plant (Benincasa 2003). To estimate the dry mass, the plants were separated into leaves, stem and root, individually stored in paper bags, oven-dried at 70 °C for 48 h and weighed.
The concentrations of chlorophyll a (Chl a), chlorophyll b (Chl b), total chlorophyll (Chla+b) and carotenoids (Cx+c) were determined from mature leaves of four plants of each treatment (NFL and FL) at 30, 60 and 90 days after the onset of flooding. The leaves were wrapped in aluminum foil and transported on ice to the laboratory, where a sample of each leaf was withdrawn and weighed. Subsequently, the samples were macerated with 80% (v/v) acetone (Arnon 1949) and filtered using Whatmann n1 filter paper, obtaining the ketone extract from chloroplast pigments. The absorbance readings were obtained from the ketone extracts at the wavelengths of 470, 646 and 663 nm. From the absorbance and dilution factors, the pigment contents were quantified and the results expressed in microgram per gram of fresh mass of leaf tissue (μg pigment g−1 MF), according to the equations described by Wellburn (1994).
The fluorescence of chlorophyll a was measured 1, 3, 10, 20, 30, 45 and 60 days after flooding, using 18 plants of each treatment. In each collection, the central leaflets of two mature leaves of each plant were measured, totaling 36 measurements in each water regime (NFL and FL). The emissions of chlorophyll a fluorescence emission were measured using a portable fluorometer Handy PEA (Hansatech Intruments). The leaves were adapted to the dark for 90 min and exposed to a saturation pulse of 3000 μmol m−2 s−1of photons for 1 s after that period. The fluorescence intensity of chlorophyll a was measured for 50 μs (initial fluorescence, F0), 2 ms (FJ), 30 ms (FI) and maximum fluorescence (FM). The parameters for the JIP test (Strasser and Strasser 1995; Strasser et al. 2004) were then calculated and are described in Table 1. The values of the JIP test parameters were normalized in relation to the control plants (NFL) levels in each period of data collection.
Statistical analyses
The effects of the water conditions (NFL and FL) on the germination parameters (%G, GSI, AS) of experiment I were evaluated with the t test. The effect of the different flood periods on the germination parameters (%G, GSI, AS) of experiment II was evaluated with an analysis of variance (ANOVA) followed by the Tukey’s test. The effects of water conditions (NFL and FL) in different evaluation periods on plant growth (stem diameter, number of leaves and height) were tested with two-way ANOVA with repetitive measures. The differences in the pigment content of the plants were obtained with a two-way ANOVA. Leaf area, dry mass, PIabs and PItotal in different flood regimes (NFL and FL) were evaluated using a t test. The percentage data (%G and AS) and dry mass were transformed using, respectively, arcsine and square root of x, to achieve normality and homoscedasticity. All other data presented normal and homoscedastic distribution and all the statistical tests followed Zar (1999) with a level of significance of 0.05.
Results
Seed germination in flood conditions and after relief of flood stress
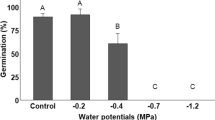
The flood reduced the percentage of germinated seeds and the GSI (Table 2). In addition, seeds germinated in the FL treatment produced smaller NS and a high number of abnormal seedlings (Fig. 1a, Table 2), with short hypocotyls and atrophied radicles (Fig. 1b). The germination began on the sixth day after sowing and the germination rate was high in the control test (NFL). The stress relief after 15 days of flooding resulted in germination on the third day, with high germination rate, high GSI and larger normal seedlings, indicating greater germinative vigor. The potential to germinate continued after 30 days of flooding, with seeds germinating on the fifth day after removed from the flood and transferred to a humid substrate. In spite of this, the germination rate and GSI decreased and the number of abnormal seedlings increased. After 60 days of flooding, the seeds became non-viable (Table 2).
Morphological aspects of seedlings and plants of Allophylus edulis. Normal seedlings originated from non-flooded treatment (a); abnormal seedlings originated from flooded treatment (b); young plants after 90 days of flooding presenting symptoms such as wilted leaves and chlorotic spots (c); non-flooded and flooded plants at 90 days (d)
Survival and growth of young plants
Plants of A. edulis from the NFL treatment had an increase of approximately 67% in stem diameter at 90 days (Fig. 2a). In contrast, the stem growth of plants in the FL treatment was inhibited, lacking morphoanatomical adaptations such as adventitious roots, hypertrophied lenticels, cortical cracks and thickening of the stem due to the formation of aerenchymal tissue, which are normally observed in species tolerant to flooded environments. NFL plants had a progressive increase in height, 48.16% larger at the end of the experiment, differing from the FL plants (Fig. 2b). The flooding induced a stoppage in plant growth, and the plant height measurements on the first day did not differ from the measure after 90 days of flooding. In the NFL plants, after 90 days of flooding, the number of leaves doubled in relation to the first day (Fig. 2c). There was a leaf fall in the FL treatment after 20 days of flooding, followed by a small increase in the number of leaves at 30 days and another fall after 90 days of flood, when we verified a leaf number 26.3% lower than at the first day of flood.
After 90 days of experiment, all NFL plants were still alive, unlike plants undergoing FL treatment, which showed a mortality of 28.57%. The surviving plants of the FL treatment had severe stress symptoms such as leaf chlorosis and withered leaves (Fig. 1c) as well as lower dry mass for both shoot and root (Fig. 1d). The flood caused a reduction in leaf area, so that FL plants had leaves about six times smaller than the NFL plants (Table 3). The values of Chl a, Chl b and Chla+b were lower in FL plants at 60 days of flooding and remained stable (Fig. 3).
Chlorophyll a fluorescence transient
After 30 and 60 days, there was an increase in the flux of light energy absorption (ABS/RC) and captured energy flow (TR0/RC) per reaction center in the plants submitted to FL treatment. Additionally, there was an increase in the dissipated energy flow (DI0/RC) reaching values of 76% (30 days) and 61% (60 days) in relation to the plants submitted to the NFL treatment (control) (Fig. 4). For the ET0/RC and RE0/RC parameters, there was no discrepancy between FL and NFL (control) treatments. We verified a tendency of reduced ET0/TR0 (Fig. 4) levels in conditions of flood, indicating a lower probability that the captured exciton moves electrons for the CTE besides the QA−. This can be confirmed by the decline in the values of the parameters associated with the quantum electron transport yield of QA−. (1) For the electron acceptors of the intersystem (ET0/ABS); and (2) for the final electron acceptor of photosystem I (FSI) (RE0/ABS). The maximum photochemical quantum yield (TR0/ABS or FV/FM) did not suffer influence of the stress duration, with values close to control levels throughout all periods evaluated.
After the first day of flooding, there was a reduction in the performance indexes PIabs (33%) and PItotal (35%) in relation to the control treatment. After three days, the NFL (control) and FL plants resembled in relation to PIabs and PItotal. However, on the tenth day of evaluation, FL treatment plants showed a significant drop in the value of the PIabs that remained in decline until 60 days of flooding. In the same way, there was a progressive decrease in the PItotal as the flooding time increased. After 30 and 60 days of flooding, the plants had the values of PIabs and PItotal reduced in more than 50% in relation to the control (Figs. 4 and 5).
Discussion
Allophylus edulis plants tolerate flooding for a short period, maintaining the seed germination capacity and inhibiting the growth of young plants, though both stages are sensitive to prolonged flooding. These characteristics, associated with the efficient dispersion mechanism, justify the wide distribution of the species in temporarily flooded areas ().
The success in establishing a species in flooded areas depends initially on the strategy of fruit dispersal associated with factors that allow seed germination and seedling survival during flooding (Marques and Joly 2000). The dispersal of A. edulis fruits can occur by zoochory, hydrochory and autochory. Zoochoric dispersal is promoted especially by birds (Abreu et al. 2005; Gagetti et al. 2016) and hydrochoric dispersion occurs due to the frequent location of the specie near watercourses (Silva et al. 2007), contributing to that seeds are dispersed in places which experience a wide variety of hydrological conditions.
Some seeds show great success in germination after flooding (Ferreira et al. 2006; Wittmann et al. 2007) and others maintain viability after a long period of submersion (Scarano and Crawford 1992; Parolin and Wittmann 2010). However, for most species, stress has a negative effect causing loss of viability (Kozlowski 1997; Okamoto and Joly 2000). In our study, we verified that some seeds started germination under flooding conditions, forming abnormal seedlings or small-size normal seedlings which decreases the chances of establishment in the soil. The fluctuation of seed may have exercised a positive effect on germination under flood conditions. There is variation in seed size of A. edulis (Abreu et al. 2005) which can have an effect on both seed fluctuating capacity and on the content of reserves accumulated. In swamp forests, submerged seeds are exposed to an environment with less availability of oxygen and light due to turbid waters. In experiment II, when the seeds were half-buried and, therefore, prevented from floating, the start of germination occurred only when the seeds were transferred to the non-flooded treatment. Submersion possibly induced the physiological dormancy in seed, postponing germination until conditions were favorable. The seed dormancy is an ecologically advantageous response in flood-prone habitats (Scarano et al. 2003).
The stress relief test showed that the germination of A. edulis seeds can be favored after a short flood period, since seeds flooded for 15 days and subsequently transferred to the non-flooded treatment had high germination rate, higher germination speed producing also more vigorous seedlings. Other studies have shown that short-term soaking in water increases the germination of forest species seeds (Parolin et al. 2003; Lucas et al. 2012; Conserva et al. 2018). Soaking seeds in water probably breaks the physical barriers imposed on germination by softening the coating and favoring the imbibition process (Lucas et al. 2012). The soaking treatment for 15 days may have promoted the imbibition of A. edulis seeds. Thus, when the seeds were transferred to the non-flooded substrate, they germinated faster.
The seeds of A. edulis have a thin integument (Abreu et al. 2005) and imbibition is generally rapid in seeds with permeable coating (Baskin and Baskin 2014). After the germination process has started, as maintenance of seeds in hypoxic conditions possibly caused gradual damage to embryonic tissue and consequent loss of germination capacity. In addition, other characteristics of A. edulis seed such as relatively small size, absence of endosperm and foliar cotyledons (Abreu et al. 2005) indicate that the seeds store low energy reserves. Thus, the decline in germination rate may also be linked to the depletion of energy resources to maintain fermentative metabolism. Under low-humidity conditions, A. edulis seeds also lose viability as they are recalcitrant (José et al. 2007). This type of seed has a strong tendency to accumulate soluble sugars, especially sucrose (Berjak and Pammenter 2007) which is an immediate use reserve for energy production (Buckeridge et. al. 2004). Thus, the rapid germination and formation of seedlings when conditions become favorable may be associated with the presence of these sugars. In addition, soluble sugars may have exerted an osmoprotective effect, while the seeds were flooded, maintaining cell turgidity, membrane stability and preventing protein degradation (Tewari and Mishra 2018).
In southern Brazil, the ripening of A. edulis fruits occurs at the end of the flooded phase, when the water level is falling. Thereby, many seeds must go through a brief period of flooding and begin germination while floating or as soon as conditions of normoxia are reestablished. The germination in the hydrological transition period is beneficial as it prevents the seed from being exposed to extreme flood or dry conditions. The dry phase is characterized by high temperatures and high evaporation (Reboita and Kruche 2018) rates that can be as detrimental to seed germination as a long period of flooding.
Given that plants with recalcitrant seeds do not form seed banks in the soil (Barbedo and Marcos Filho 1998), it is important to invest in seeds with rapid germination to form a seedling bank (Berjak and Pammenter 2007). This investment becomes even more important in floodable forests that offer a narrow window for seedling regeneration (Parolin et al. 2003; Wittmann et al. 2007). Furthermore, there is an association between functional type of cotyledon and seed germination speed, being that seedlings with foliar cotyledon develop faster because they have limited energy reserves (Parolin et al. 2003). The rapid germination and emergence of foliar cotyledons when flooding ceases probably maximize photosynthetic activity and growth seedling in the non-flooded period (Parolin et al. 2003; Conserva et al. 2018).
According to Joly and Crawford (1982), a plant can be considered flood tolerant when it is able to grow and increase its biomass under flood conditions. In our study, we verified that the young plants of A. edulis respond to flood conditions with changes in the energy flow of the photosynthetic electron transport chain (ETC), reduction in plant size and biomass, and leaf fall, which indicate reduced tolerance to prolonged flood. The hypoxic and/or anoxic conditions imposed by the flood affect the growth of sensitive plants, as the water and nutrient uptake is compromised and the stomatal conductance of the leaves and photosynthetic capacity are reduced (Batista et al. 2008; Yu et al. 2015; Bidalia et al. 2018). A deviation from aerobic to anaerobic root system metabolism also occurs under these conditions, resulting in a reduced energy efficiency (Drew 1997). To compensate low energy yields, root cells increase their demand for carbohydrates, reducing their availability to other plant functions (Bailey-Serres and Voesenek 2008). Thus, it is common for plants to respond to flooding by inhibiting their growth or reducing their biomass (Andrade et al. 1999; Batista et al. 2008; Peng et al. 2017; Bidalia et al. 2018).
Some plants are able to grow vigorously during long periods of flooding (Kolb and Joly 2009). In general, species that present high flood tolerance tend to inhibit growth when stress begins and return to growth after a period of acclimatization (Larré et al. 2013; Zúñiga-Feest et al. 2017). During this period, the plant performs metabolic (Joly and Crawford 1982; Alves et al. 2013; Larré et al. 2016) and photosynthetic (Medina et al. 2009; Larré et al. 2013) adjustments, developing morphoanatomical structures that allow the plant to survive and thrive in conditions of water saturation (Medina et al. 2009; Oliveira and Joly 2010; Larré et al. 2013). In this context, the formation of hypertrophied lenticels, cortical cracks and adventitious roots are common morphological responses that confer advantage to the species (Zhang et al. 2017). Although individuals withstand a long period of flooding, the plants of A. edulis did not develop any morphological changes that could help the plant survive the anoxia conditions. Stress conditions were signaled by injury symptoms, such as chlorosis, loss of leaf mass and reduced growth.
The flood may compromise photosynthesis through stomatal and non-stomatal limitations, decreasing the photosynthetic capacity due to a reduction in chlorophyll concentrations, carboxylation efficiency or electron transport rates (Gravatt and Kirby 1998; Herrera et al. 2008; Larré et al. 2013; Polacik and Maricle 2013; Junior et al. 2015). The distinction between stomatal and non-stomatal limitations is important, since the stomatal closure is a transient regulatory response that can be reverted when stressful conditions cease; whereas, the other responses reflect permanent changes (Herrera et al. 2008).
The plants of the FL treatment showed a reduction in chlorophyll content (Fig. 3) and changes in JIP-test parameters (Fig. 4), which reflect the energy flux in the photosynthetic ETC, signaling that long-term flooding promotes a decrease in photosynthetic capacity. The reduction in pigment contents is a typical symptom of stress due to oxidative processes in the chloroplast, resulting in slow synthesis or rapid breakdown of pigments (Smirnoff 1993). Some species with a greater tolerance to flooding do not present a reduction in their content of photosynthetic pigments (Junior et al. 2015), even increasing their pigment content in response to flooding (Parolin 2001). However, many plants, classified as tolerant or sensitive to flooding, show a reduced chlorophyll content in water saturation conditions (Junior et al. 2015; Kozlowski and Pallardy 2002). During flooding, metabolic adjustments can occur, reducing the chlorophyll content in the leaves, resulting in a lower absorption of light energy by leaf area to protect PSII from photo-oxidation (Du et al. 2012).
In our study, the flood increased the absorption flux (ABS/RC) by the antenna pigments, as well as the captured energy flow (TR0/RC), mainly after the first day, 30 and 60 days of flooding. The decrease in the ET0/TR0, ET0/ABS and RE0/ABS parameters shows a lower electron transfer to the ETC (Fig. 4). This can lead to the inactivation of a fraction of the PSII reaction centers, converting them into non-reducing centers of QA− resulting in an increase in the mean antenna size by active RC (Junior et al. 2015). Consequently, an increase in the energy flux dissipated by PSII reaction center (DI0/RC) was triggered. The mechanism of dissipating energy in the form of heat or fluorescence may have been employed by A. edulis plants as a measure to avoid photoinhibition and consequent damage to the photosystems.
Substantial changes in the electron transport per active reaction center were noted with flooding. In general, the performance indexes (PIabs and PItotal), energy flow parameters per reaction center (ABS/RC, TR0/RC and DI0/RC) and parameters related to quantum yields (ET0/TR0, ET0/ABS and RE0/ABS) show increased stress on the first day of flood. However, the stress was followed by an acclimatization mechanism after the third day, when FL treatment plants showed values similar to the control (Figs. 4 and 5). After this short period, the discrepancies between the NFL and flooded treatments increased as the flood time elapsed, culminating in low PI values at 30 and 60 days, which indicate damage to PSII. The maximum quantum yield of primary PSII photochemistry (TR0/ABS or FV/FM), widely used to monitor environmental stress in plants (Oxborough and Baker 1997), was not an effective indicator in our study, which was also observed for other tree species under flood conditions (Larré et al. 2013; Martinazzo et al. 2013).
The distribution of species in flooded forests is regulated by the duration of flooding, their inherent mechanisms to tolerate flood-related stress (Bailey-Serres and Voesenek 2008), and the existence of microenvironments where stress is attenuated and the conditions become favorable for the plant development. In this way, species with different levels of flood tolerance may coexist in a flooded environment seasonally or permanently.
We verified positive germinative response in A. edulis seeds after a short period of flooding (15 days) and low mortality of young plants after a long period of flooding (90 days). However, the period of 30 days of flooding was sufficient to cause a sharp drop in seed germination and plant photosynthetic performance indexes (PIs). After 60 days of flooding, the seeds lost viability and the PIs remained low, signaling damage to the photosynthetic apparatus. At 90 days of flooding, the surviving plants presented serious evidence of injury caused by the flood. Thus, we conclude that the initial development of A. edulis is associated with environments with short periods of flooding, which explains the greater abundance of individuals of the species in temporarily flooded forests. The occurrence of the species in flooded forests for long periods is conditioned by the occurrence of microenvironments protected from long floods. This species is recommended for the restoration of flooded areas if planted where they are exposed to short periods of flooding.
Author contribution statement
CI Duarte—designed the experiment, data collection, data interpretation, literature searches and writing; EG Martinazzo—designed the experiment, data analysis and fluorescence parameter analyses; MA Bacarin—data analysis and fluorescence parameter analyses; IG Colares—designed the experiment and writing.
References
Abreu DCA, Kuniyoshi YS, Nogueira AC, Medeiros A (2005) Caracterização morfológica de frutos, sementes e germinação de Allophylus edulis (St.-Hil.) Radlk. (Sapindaceae). Rev bras Sementes 27:59–66
Alvares CA, Stape JL, Sentelhas PC, Moraes G, Leonardo J, Sparovek G (2013) Köppen's climate classification map for Brazil. Meteorol Z 22:711–728
Alves JD, Zanandrea I, Deuner S, Goulart PFP, Souza KRD, Santos MO (2013) Antioxidative responses and morpho-anatomical adaptations to waterlogging in Sesbania virgata. Trees 27:717–728
Andrade ACSD, Ramos FN, Souza AFD, Loureiro MB, Bastos R (1999) Flooding effects in seedlings of Cytharexyllum myrianthum Cham. and Genipa americana L.: responses of two neotropical lowland tree species. Rev Bras Bot 22:281–285
Arnon DI (1949) Copper enzymes in isolated chloroplasts. Polyphrenol oxidase in Beta vulgaris. Plant Physiol 24:1–15
Bailey-Serres J, Voesenek LACJ (2008) Flooding stress: acclimations and genetic diversity. Annu Rev Plant Biol 59:313–339
Barbedo CJ, Marcos Filho J (1998) Tolerância à dessecação em sementes. Acta Bot Bras 12:145–164
Baskin CC, Baskin JM (2014) Seeds: ecology, biogeography and evolution of dormancy and germination. Academic Press, San Diego
Batista CUN, Medri ME, Bianchini E, Medri C, Pimenta JA (2008) Flood tolerance in Cecropia pachystachya Trec. (Cecropiaceae): ecophysiological and morpho-anatomical aspects. Acta Bot Bras 22:91–98
Benincasa MMP (2003) Análise de crescimento de plantas: noções básicas. Funep, Jaboticabal
Berjak P, Pammenter NW (2007) From Avicennia to Zizania: seed recalcitrance in perspective. Ann Bot 101:213–228
Bidalia A, Okram Z, Hanief M, Rao KS (2018) Assessment of tolerances in Mitragyna parvifolia (Roxb.) Korth and Syzygium cumini Keels. seedlings to waterlogging. Photosynthetica 56:707–717
Brasil, Ministério da Agricultura, Pecuária e Abastecimento (2009) Regras para análise de sementes. MAPA, Brasília
Buckeridge MS, Aidar MPM, Santos HP, Tiné MAS (2004) Acúmulo de reservas. In: Ferreira AG, Borghetti F (eds) Germinação: do básico ao aplicado. Artmed, Porto Alegre, pp 31–50
Colmer T, Voesenek L (2009) Flooding tolerance: suites of plant traits in variable environments. Funct Plant Biol 36:665–681
Conserva A, Camargo JLC, Santana DG, Piedade MTF (2018) Germinative behaviour of ten tree species in white-water floodplain forests in central Amazonia. Folia Geobot 53:89–101
Copertino MS, Creed JC, Lanari MO, Magalhães K, Barros K, Lana PC, Sordo L, Horta PA (2016) Seagrass and submerged aquatic vegetation (VAS) habitats off the coast of Brazil: state of knowledge, conservation and main threats. Braz J Oceanogr 64:53–80
da Paz AA, Ribeiro C, Azevedo AA, Lima ERD, Carmo FMDS (2017) Induced flooding as environmental filter for riparian tree species. Environ Exp Bot 139:31–38
Drew MC (1997) Oxygen deficiency and root metabolism: injury and acclimation under hypoxia and anoxia. Annu Rev Plant Physiol Plant Mol Biol 48:223–250
Duarte CI (2012) Influência da variação hidrológica e da luminosidade na composição e estrutura do componente herbáceo em uma floresta paludosa no extremo sul do Brasil. Dissertation, Universidade Federal do Rio Grande
Du K, Xu L, Wu H, Tu B, Zheng B (2012) Ecophysiological and morphological adaption to soil flooding of two poplar clones differing in flood-tolerance. Flora 207:96–106
Ferreira CS, Piedade MTF, Bonates LC (2006) Germinação de sementes e sobrevivência de plântulas de Himatanthus sucuuba (Spruce) Wood em resposta ao alagamento nas várzeas da Amazônia Central. Acta Amaz 36:413–418
Ferreira CS, Piedade MTF, Tiné MAS, Rossatto DR, Parolin P, Buckeridge MS (2009) The role of carbohydrates in seed germination and seedling establishment of Himatanthus sucuuba, an Amazonian tree with populations adapted to flooded and non-flooded conditions. Ann Bot 104:1111–1119
Gagetti BL, Piratelli AJ, Piña-Rodrigues FCM (2016) Fruit color preference by birds and applications to ecological restoration. Braz J Biol 76:955–966
Gattringer JP, Ludewig K, Harvolk-Schöning S, Donath TW, Otte A (2018) Interaction between depth and duration matters: flooding tolerance of 12 floodplain meadow species. Plant Ecol 219:973–984
Gravatt DA, Kirby CJ (1998) Patterns of photosynthesis and starch allocation in seedlings of four bottomland hardwood tree species subjected to flooding. Tree Physiol 18:411–417
Herrera A, Tezara W, Marín O, Rengifo E (2008) Stomatal and non-stomatal limitations of photosynthesis in trees of a tropical seasonally flooded forest. Physiol Plant 134:41–48
Joly CA, Crawford RMM (1982) Variation in tolerance and metabolic responses to flooding in some tropical trees. J Exp Bot 33:799–809
José AC, Silva EA, Davide AC (2007) Classificação fisiológica de sementes de cinco espécies arbóreas de mata ciliar quanto a tolerância à dessecação e ao armazenamento. Rev Bras Sementes 29:171–178. https://doi.org/10.1590/S0101-31222007000200023
Junior UMS, Gonçalves JFC, Strasser RJ, Fearnside PM (2015) Flooding of tropical forests in central Amazonia: what do the effects on the photosynthetic apparatus of trees tell us about species suitability for reforestation in extreme environments created by hydroelectric dams? Acta Physiol Plant 37:166
Junk WJ (2013) Current state of knowledge regarding South America wetlands and their future under global climate change. Aquat Sci 75:113–131
Kolb RM, Joly CA (2009) Flooding tolerance of Tabebuia cassinoides: metabolic, morphological and growth responses. Flora 204:528–535
Kozlowski TT (1997) Responses of woody plants to flooding and salinity. Tree Physiol. https://doi.org/10.1093/treephys/17.7.490
Kozlowski TT, Pallardy SG (2002) Acclimation and adaptive responses of woody plants to environmental stresses. Bot Rev 68:270–334
Larré CF, Fernando JA, Marini P, Bacarin MA, Peters JA (2013) Growth and chlorophyll a fluorescence in Erythrina crista-galli L. plants under flooding conditions. Acta Physiol Plant 35:1463–1471
Larré C, Leivas-Moraes C, Borella J, Amarante L, Deune S, Peters JA (2016) Antioxidant activity and fermentative metabolism in the plant Erythrina crista-galli L. under flood conditions. Semina Ciênc Agrár 37:567–580
Liu B, Rennenberg H, Kreuzwieser J (2015) Hypoxia affects nitrogen uptake and distribution in young poplar (Populus × canescens) trees. PLoS ONE 10(8):e0136579
Lucas CM, Mekdeçe F, Nascimento CM, Holanda ASS, Braga J, Dias S, Souza S, Rosa P, Suemitsu C (2012) Effects of short-term and prolonged saturation on seed germination of Amazonian floodplain forest species. Aquat Bot 99:49–55
Marques M, Joly CA (2000) Germinação e crescimento de Calophyllum brasiliense (Clusiaceae), uma espécie típica de florestas inundadas. Acta Bot Bras 14:113–120
Martinazzo EG, Perboni AT, Oliveira PV, Bianchi VJ, Bacarin MA (2013) Atividade fotossintética em plantas de ameixeira submetidas ao déficit hídrico e ao alagamento. Cienc Rural 43:35–41
Medina CL, Sanches MC, Tucci MLS, Sousa CA, Cuzzuol GRF, Joly CA (2009) Erythrina speciosa (Leguminosae-Papilionoideae) under soil water saturation: morphophysiological and growth responses. Ann Bot 104:671–680
Okamoto J, Joly CA (2000) Ecophysiology and respiratory metabolism during the germination of Inga sessilis (Vell.) Mart. (Mimosaceae) seeds subjected to hypoxia and anoxia. Braz J Bot 23:51–57
Oliveira VC, Joly CA (2010) Flooding tolerance of Calophyllum brasiliense Camb. (Clusiaceae): morphological, physiological and growth responses. Trees 24:185–193
Oliveira ACS, Martins GN, Silva RF, Vieira HD (2009) Testes de vigor em sementes baseados no desempenho de plântulas. InterSciencePlace 1:1–21
Oxborough K, Baker NR (1997) Resolving chlorophyll a fluorescence images of photosynthetic efficiency into photochemical and non-photochemical components-calculation of qP and Fv−/Fm−; without measuring Fo. Photosynth Res 54:135–142
Parolin P (2001) Senna reticulata, a pioneer tree from Amazonian várzea floodplains. Bot Rev 67:239–254
Parolin P, Wittman F (2010) Struggle in the flood: tree response to flooding stress in four tropical floodplain systems. AoB Plants. https://doi.org/10.1093/aobpla/plq003
Parolin P, Ferreira LV, Junk WJ (2003) Germination characteristics and establishment of trees from central Amazonian flood plains. Trop Ecol 44:157–169
Parolin P, De Simone O, Haase K, Waldhoff D, Rottenberger S, Kuhn U, Kesselmeier J, Kleiss B, Schmidt W, Piedade M et al (2004) Central Amazonian floodplain forests: tree adaptations in a pulsing system. Bot Rev 70:357–380
Peng Y, Zhou Z, Tong R, Hu X, Du K (2017) Anatomy and ultrastructure adaptations to soil flooding of two full-sib poplar clones differing in flood-tolerance. Flora 233:90–98
Pezeshki SR (2001) Wetland plant responses to soil flooding. Environ Exp Bot 46:299–312
Polacik KA, Maricle BR (2013) Effects of flooding on photosynthesis and root respiration in salt cedar (Tamarix ramosissima), an invasive riparian shrub. Environ Exp Bot 89:19–27
Reboita MS, Kruche N (2018) Normais Climatológicas Provisórias de 1991 a 2010 para Rio Grande. RS Rev bras meteorol 33:165–179
Rengifo E, Tezara W, Herrera A (2005) Water relations, chlorophyll a fluorescence, and contents of saccharides in tree species of a tropical forest in response to flood. Photosynthetica 43:203–210
Scarano FR (2002) Structure, function and floristic relationships of plant communities in stressful habitats marginal to the Brazilian Atlantic rainforest. Ann Bot 90:517–524
Scarano FR, Crawford RM (1992) Ontogeny and the concept of anoxia-tolerance: the case of the Amazonian leguminous tree Parkia pendula. J Trop Ecol 8:349–352
Scarano FR, Pereira TS, Rôças G (2003) Seed germination in Seed germination during floatation and seedling growth of Carapa guianensis, a tree from flood-prone forests of the Amazon. Plant Ecol 168:291–296
Silva AC, Van den Berg E, Higuchi P, Oliveira Filho AT (2007) Comparação florística de florestas inundáveis das regiões sudeste e sul do Brasil. Rev Bras Bot 30:257–269
Silva AC, Higuchi P, Van den Berg E, Nunes MH, Carvalho DA (2012) Florestas Inundáveis: Ecologia, florística e adaptações das espécies. Editora UFLA, Lavras
Smirnoff N (1993) The role of active oxygen in the response of plants to water deficit and desiccation. New Phytol 125:27–58
Strasser BJ, Strasser RJ (1995) Measuring fast fluorescence transients to address environmental questions: the JIP-test. In: Mathis P (ed) Photosynthesis: from light to biosphere. Kluwer Academic Publishers, Dordrecht, pp 977–980
Strasser RJ, Tsimilli-Michael M, Srivastava A (2004) Analysis of the chlorophyll a fluorescence transient. In: Papageorgiou GC, Govindjee G (eds) Chlorophyll a fluorescence: a signature of photosynthesis. Advances in photosynthesis and respiration series. Springer, Dordrecht, pp 321–362
Teixeira AP, Assis MA, Luize BG (2011) Vegetation and environment relationships in a neotropical swamp forest in southeastern Brazil (Itirapina, SP). Aquat Bot 94:17–23
Tewari S, Mishra A (2018) Flooding stress in plants and approaches to overcome. In: Ahmad P, Ahanger MA, Singh VP, Tripathi DK, Pravej Alam P, Alyemeni MN (eds) Plant metabolites and regulation under environmental stress. Academic Press, London, pp 356–366
Turchetto F, Araujo MM, Callegaro RM, Griebeler AM, Mezzomo JC, Berghetti ÁL, Rorato DG (2017) Phytosociology as a tool for forest restoration: a study case in the extreme South of Atlantic Forest Biome. Biodivers Conserv 26:1463–1480
Waechter JL, Jarenkow JA (1998) Composição e estrutura do componente arbóreo nas matas turfosas do Taim, Rio Grande do Sul. Biotemas 11:45–69
Wellburn AR (1994) The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J Plant Physiol 144:307–313
Wittmann AO, Piedade MTF, Parolin P, Wittmann F (2007) Germination of four low-varzea tree species in central Amazonia. Aquat Bot 86:197–203
Yu B, Zhao CY, Li J, Li JY, Peng G (2015) Morphological, physiological, and biochemical responses of Populus euphratica to soil flooding. Photosynthetica 53:110–117
Zar JH (1999) Biostatistical analysis. Prentice-Hall, Upper Saddle River
Zhang Q, Huber H, Beljaars SJ, Birnbaum D, Best S, Kroon H, Visser EJ (2017) Benefits of flooding-induced aquatic adventitious roots depend on the duration of submergence: linking plant performance to root functioning. Ann Bot 120:171–180
Zúñiga-Feest A, Bustos-Salazar A, Alves F, Martinez V, Smith-Ramírez C (2017) Physiological and morphological responses to permanent and intermittent waterlogging in seedlings of four evergreen trees of temperate swamp forests. Tree Physiol 37:779–789
Acknowledgements
We thank Ana Roschildt, Daniel Villanova, Mateus Negrini and Roger Oliveira their support in data collection. We also thank Ana S. Rolon, Fabiana Barbosa, Marianna Lanari for suggestions in previous version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by P. Wojtaszek.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Duarte, C.I., Martinazzo, E.G., Bacarin, M.A. et al. Seed germination, growth and chlorophyll a fluorescence in young plants of Allophylus edulis in different periods of flooding. Acta Physiol Plant 42, 80 (2020). https://doi.org/10.1007/s11738-020-03063-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-020-03063-7