Abstract
To understand the differences in the biosynthesis of leaf surface chemicals and their influence on aphid preference for different tobacco cultivars (Nicotiana tabacum), we analyzed the secretory characteristics of glandular trichomes of four commercial cultivars, K326 (flue-cured), Beinhart 1000-1 (cigar), Basma YNOTBS1 (oriental), and Dabai 1 (burley), and their parental species, Nicotiana sylvestris and Nicotiana tomentosiformis. Trichome-type observation showed that K326 and N. sylvestris have three kinds of glandular trichomes (non-glandular, long stalked glandular, and short stalked glandular trichomes), whereas Beinhart 1000-1, Basma YNOTBS1, Dabai 1, and N. tomentosiformis had two kinds of glandular trichomes (long and short stalked glandular trichomes). The gas chromatography–mass spectrometry profiles of leaf exudates indicated that N. tomentosiformis synthesized only labdanoids; N. sylvestris, K326 and Dabai 1 synthesized only cembranoids; and Beinhart 1000-1 and Basma YNOTBS1 synthesized cembranoids and labdanoids. Gene expression pattern analysis revealed that the labdanoid synthesis-related genes NtABS and NtCPS2 were expressed in N. tomentosiformis, Beinhart 1000-1, and Basma YNOTBS1, whereas the cembranoid synthesis-related genes NtCYC and NtCYP71D16 were expressed in N. sylvestris and all four commercial cultivars. Evolutionary analysis indicated that NtCYC and NtCYP71D16 might be phylogenetically originated from N. sylvestris, whereas NtABS and NtCPS2 expressed in Basma YNOTBS1 and Beinhart 1000-1 might be derived from N. tomentosiformis. In addition, aphid attraction (number of aphids) was significantly and positively correlated with the total glandular secretion (r2 = 0.9425, P ≤ 0.05), and it was significantly and positively correlated with amount of CBT-diol (r2 = 0.9224; P ≤ 0.05). These results provide new insights into the biosynthesis of diterpenoids and biotic stress resistance in tobacco.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glandular trichomes on tobacco leaves secret high amounts of important biochemical compounds, such as sucrose esters, waxes, micro-elements, and diterpenoids (Johnson et al. 1985), which represent 10–30% of the leaf dry weight (Wagner 1991). Of these, diterpenoids are related to plant defense, since they have antifungal (Kennedy et al. 1992, 1995) and insecticidal (Lin and Wagner 1994) activity. In addition, diterpenoids, accounting for more than 50% of the foliar chemical composition under optimal conditions, are considered as the precursors of important compounds produced during leaf curing and significantly contribute to tobacco aroma (Wagner 1991; Wagner et al. 2004).
Two types of diterpenes have been found in cultivated tobacco, cembranoids and labdanoids (Wagner 1991). The major labdanoids are Z-abienol (Figs. 1, 2, 3, 4, and 5) and labdenediol (Figs. 1, 2, 3, 4, 5, and 6), whereas the major cembranoids are α- and β-cembratrien-diol (CBT-diols; Figs. 1, 2, 3 and Figs. 1, 2, 3, and 4) along with small amounts of their respective precursors, α- and β-cembratrien-ol (CBT-ols; Figs. 1 and 2) (Severson et al. 1984). The biosynthesis of cembranoids includes two steps: (1) the cyclization of the precursor geranylgeranyl diphosphate (GGPP) that is catalyzed by cembratrien-ol synthase (CBTS) and produces a mixture of α- and β-cembratrien-ols (CBT-ols) (Guo and Wagner 1995), and (2) the oxidation of CBT-ols in the presence of cytochrome P450 oxygenase (CYP450) (Wang et al. 2001). CBTS and CYP450 are encoded by NtCYC and NtCYP71D16, respectively (Ennajdaoui et al. 2010; Wang and Wagner 2003). As proposed in recent studies (Carman et al. 1993; Peters 2010), the biosynthesis of labdanoids also proceeds from GGPP. Accordingly, in the biosynthesis of Z-abienol, copalyl diphosphate synthase (CPS) catalyzes the synthesis of 8-a-hydroxy-copalyl diphosphate (8-OH-CPP), which is then converted to Z-abienol and labdenediol by ABS synthase (Sallaud et al. 2012). In addition, previous studies on NtCPS2 and NtABS have shown that both genes are necessary and sufficient for the biosynthesis of Z-abienol and labdenediol from GGPP in glandular trichomes (Sallaud et al. 2012). The cognition of the biosynthetic pathway of terpenoids can help to elucidate their role in plant defense against insects and fungi, and thus, to improve tobacco resistance by genetic engineering. For instance, the suppression of NtCYP71D16 in plant trichome glands increases the value of CBT-ol/CBT-diol, enhancing aphid resistance in tobacco (Wang and Wagner 2003; Wang et al. 2001).
Major diterpenoid biosynthesis pathway in glandular trichomes of tobacco. Four key genes controlling diterpenoid synthesis were divided into two groups: NtCYC and NtCYP71D16 that control the synthesis of cembranoids, and NtABS and NtCPS2 that control the synthesis of labdanoids. (1–6): α-CBT-ol, β-CBT-ol, α-CBT-diol, β-CBT-diol, cis-abienol, and labdenediol
a Gas chromatography–mass spectrometry (GC–MS) profiles (34–55 min) of leaf exudates in (a–d) four Nicotiana tabacum cultivars (K326, Beinhart 1000-1, Dabai 1, and Basma YNOTBS1), (e) Nicotiana sylvestris, and (f) Nicotiana tomentosiformis. (1–6) α-CBT-ol, β-CBT-ol, α-CBT-diol, β-CBT-diol, cis-abienol, and labdenediol. Note: the peak 3 and peak 4 in (f) are not α-CBT-diol, β-CBT-diol after mass spectrum analysis (figure S3). b Cembranoid components quantified by GC–MS and expressed in microgram per square centimeter (μg cm−2) of fresh leaf. The data was shown as mean ± SD (n = 3). c Labdanoid components quantified by GC–MS and expressed in microgram per square centimeter (μg cm−2) of fresh leaf. The data were shown as mean ± SD (n = 3)
Leaf trichome morphology of four Nicotiana tabacum cultivars (K326, Beinhart 1000-1, Dabai 1, and Basma YNOTBS1), Nicotiana sylvestris, and Nicotiana tomentosiformis at the five-leaf stage. a Non-glandular trichome (i.e., type III-like); b tall glandular trichome (i.e., types I-like, IV-like, and VI-like); c short glandular trichome (i.e., type VII-like)
Expression patterns of key genes involved in diterpenoid biosynthesis in trichomes of four Nicotiana tabacum cultivars (K326, Beinhart 1000-1, Dabai 1, and Basma YNOTBS1), Nicotiana sylvestris, and Nicotiana tomentosiformis. a General PCR for genetic analysis. b Expression level of NtCYC, NtCYP71D16, NtABS and NtCPS2 was identified with both qRT-PCR (histograms) and semi-quantitative RT-PCR (gels). Top gels, semi-quantitative of NtCYC, NtCYP71D16, NtABS and NtCPS2; bottom gels, L25 was used as an internal control. The data of qRT-PCR were shown as mean ± SD (n = 3)
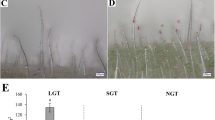
Aphid infestation detection and aphid numbers. a Lower side of aphid-infected leaf blade; b Numbers of aphids collected on the leaves; and c correlation analysis of aphid number and content of total secretion, and content of CBT-diol. The data were shown as mean ± SD (n = 3). Different letters indicate statistically significant differences at P ≤ 0.05
The key genes that involved in diterpenoid biosynthesis are specifically expressed in the gland cells of tobacco trichome tissue (Cui et al. 2011; Brückner et al. 2014; Wang et al. 2002). Trichomes are specialized outgrowths of epidermal cells that exist on most leaves and stems of terrestrial vascular plants (Peng and Hu 2007) and cover approximately 30% of the aerial parts of vascular plants (the surface of stems or leaves) (Fahn 2000). Luckwill (1943) first examined the trichomes of the genus Lycopersicon and categorized them as types I–VII. Depending on their morphology, trichomes can be divided into non-glandular trichomes (i.e., types II, III, and V) and glandular trichomes (i.e., types I, IV, VI, and VII). Non-glandular trichomes that consist of a basal cell and stalk cells do not participate in the synthesis and accumulation of secondary metabolites and are present in most angiosperms, some gymnosperms, and bryophytes (Wagner et al. 2004); for example, the model plant Arabidopsis possesses only non-glandular trichomes (Mathur and Chua 2000; Schwab et al. 2000), which have been widely studied with respect to their development (Larkin et al. 2003). Previous studies showed that more than 30 genes are involved in non-glandular trichomes development (i.e., type III-like) in Arabidopsis, including genes that control the occurrence of trichomes and epidermal hair clusters. Of them, three transcription factors (GL1, GL3, and TTG1) regulate the occurrence and development of trichomes (An et al. 2011). Glandular trichomes consist of basal cells, stalk cells, and apical cells. The latter are responsible for the production of diterpenoids and other important foliar chemical substances. Glandular trichomes can be used as a bioreactor in the production of medical (Weathers et al. 2011) and chemical products (Lange et al. 2011; Schilmiller et al. 2008), as well as in the prevention and control of agricultural diseases and insect pests (Dayan and Duke 2003; Mellon et al. 2012). Tobacco glandular trichomes are divided into short trichomes with a unicellular stalk, possessing a multicellular head (i.e., type VII-like), and tall trichomes with a multicellular stalk, possessing uni- or multicellular heads (i.e., types I-like, IV-like, and VI-like); each kind secretes different components (Meyberg et al. 1991).
Cultivated tobacco (Nicotiana tabacum), which is natural allopolyploid that phylogenetically originated from the hybridization of two species, N. sylvestris Speg. & Comes and N. tomentosiformis Goodsp. (Glas et al. 2012). Here, we studied the differences of leaf trichomes, including their morphology and density, kinds of secreted substances, and expression of genes related to the synthesis of diterpenoids of four cultivars belonging to different types of tobacco and the parental species.
Materials and methods
Plant material
In this study, we used four tobacco cultivars, K326 (flue-cured), Basma YNOTBS1 (oriental), Dabai 1 (burley), and Beinhart 1000-1 (cigar), with different aromas during curing (Table S1) and two wild species N. sylvestris and N. tomentosiformis. Seeds were sown in mixed soil (1:1 vermiculite: humus) under greenhouse conditions (temperature of 22 °C, relative humidity of 70%, natural diurnal rhythm, and natural illumination), and the seedlings were transplanted to the experimental fields of Henan Agricultural University, Xuchang, Henan Province, China. Fertilization and irrigation were applied as required.
Microscopic observation of trichome morphology and density
For trichome morphology analysis, five plants were randomly selected for each cultivar. Leaves at different growing stage were sampled. At five-leaf stage the third leaf from the stem base were used, while at mature stage, the 16th–18th (upper) and 10th–12th (middle) leaves from the stem base were chosen. The bottom four leaves of each tobacco plant were removed at the beginning of the mature stage.
Leaves at the five-leaf stage were also used for trichome density analysis. Leaves between the main vein and the left leaf margin located between the third and fourth branch vein from the leaf apex to leaf base were sampled. Twenty squares of the sampled leaves were cut out (area, 1 × 1 cm). Samples were observed under a real-time and 3D digital microscope (VHX-5000; Keyence Corporation, Osaka, Japan). The mean number of trichomes per mm2 of leaf area was calculated.
RNA extraction and RT-PCR
Total RNA was extracted from the roots, stems, and leaves without trichomes, as well as from trichomes using RNeasy kit (Qiagen, Hilden, Germany), according to the manufacturer’s instructions. Trichomes were harvested by freezing three or four mature leaves (20 cm in length) on an aluminum sheet over a liquid nitrogen bath. Once frozen, each leaf was brushed with a paintbrush over a liquid nitrogen-cooled mortar to collect trichomes, whereas the rest of the tissue was collected as leaf without trichomes. Trichomes and leaves were frozen immediately with liquid nitrogen and stored at − 80 °C for RNA extraction. To remove traces of genomic DNA, RNA samples were treated with DNase (Invitrogen, Carlsbad, CA, USA), according to the manufacturer’s instructions. RNA quantity was determined using a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA), and its quality was analyzed using 1% agarose gel electrophoresis stained with ethidium bromide. For reverse transcription (RT), each RT reaction consisted of 0.5 μg RNA, 2 μl of PrimerScript Buffer, 0.5 μl of oligo dT, 0.5 μl of random 6-mers, and 0.5 μl of PrimerScript RT Enzyme Mix I (TaKaRa, Tokyo, Japan) in a total volume of 10 μl and performed with a GeneAmp® PCR System 9700 (Applied Biosystems, Foster City, CA, USA) for 15 min at 37 °C, followed by heat inactivation for 5 s at 85 °C. The RT reaction mix was then diluted tenfold in nuclease-free water and stored at − 20 °C.
Gene expression pattern analysis
For gene expression pattern analysis, we designed primers based on mRNA sequences obtained from the SOL Genomics Network (SGN; https://www.sgn.cornell,edu) database and synthesized by Generay Biotech (Shanghai, China) (the primer sequences are presented in Table 1). Four genes (NtCYC, NtCYP71D16, NtABS and NtCPS2) were amplified in the four tobacco cultivars and two parental species.
Gene expression quantification was performed by semi-quantitative interpretation and real-time quantitative PCR (qPCR). Each semi-quantitative interpretation was same with PCR reaction. Each RT-qPCR reaction consisted of 1 μl of cDNA, 5 μl of 2 × LightCycler® 480 SYBR Green I Master (Roche, Basel, Switzerland), 0.2 μl of forward primer (Table 1), 0.2 μl of reverse primer, and 3.6 μl of nuclease-free water in a total volume of 10 μl and performed with a LightCycler® 480 II Real-time PCR (Roche). PCR conditions were as follows: 10 min at 95 °C, followed by 40 cycles of 10 s at 95 °C and 30 s at 60 °C. Each sample was tested in triplicate. Melting curve analysis was performed to validate the specific amplification of the expected PCR product. The L25 gene was used as an internal control for data normalization, and the expression levels were calculated using the 2−ΔΔCt method (Livak and Schmittgen 2001).
Gas chromatography–mass spectrometry (GC–MS) analysis
Five healthy plants with strong growth, no pests, and similar height were selected from each tobacco cultivar in the field, and their middle leaves (10th to 12th leaf from the stem base) were collected at maturity stage for extraction and determination of leaf exudates. Leaf sample preparation and pretreatment were performed using the leaf disk method as described by Han (1995). Briefly, leaf disks of 10 cm in diameter were cut out along both sides of the midrib, and 60 disks were obtained and divided into three repeats. The leaf disks were immersed in dichloromethane successively for extracting leaf exudates. Each immersion was repeated eight times of 2 s each. A total of 1 ml of internal standard solution (mixture of 2.020 mg ml−1 sucrose octaacetate and 2.542 mg ml−1 n-heptadecane alcohol) was added to each extract, mixed, concentrated in a rotary evaporator, and subjected to silylation treatment. Sample solutions were analyzed by GC–MS using TRACE GC ULTRA-DSQ II (Thermo Fisher Scientific, Waltham, MA, USA) as described by Wang et al. (2013); the profiles were compared qualitatively using the NIST12 spectral library.
Aphid preference to different leaves
One young leaf (10–15 cm in length) from each cultivar was placed in a closed container at room temperature; they were arranged evenly in a circle. Repeat 3 times. Horizontal clapboard with small holes was placed in the container; water was added under the clapboard, and gauze and filter papers were placed on the clapboard. To prevent wilting and keep the surfaces dry, a small plastic disk was placed on the filter paper. The blades of the leaves were placed on the plastic film, whereas the leaf base was coated with absorbent cotton outside the filter paper. A few wingless aphids were released in the center of the circle to migrate to the leaves of preference. The number of aphids on each leaf was counted 2 d after the release.
Statistical analysis
One-way analysis of variance (ANOVA) in conjunction with the least significant difference multiple comparison test was performed to identify differences among the cultivars. Pearson’s correlation coefficients between aphid numbers and content of total secretion, content of CBT-diol were also calculated. All analyses were performed using SPSS 19 software (IBM, New York, NY, USA).
Results
Diversity of foliar chemical profiles
The GC–MS profiles showed that the two parental species differed in the composition and content of trichome secretions, especially for diterpenoids (Fig. 2a). The glandular trichomes of N. sylvestris could synthesized and secreted cembranoids, but not labdanoids (Fig. 2b), whereas the glandular trichomes of N. tomentosiformis could synthesized and secreted small amount of labdanoids, but not cembranoids (Fig. 2c). The trichomes of all four tobacco cultivars could synthesize and secrete cembranoids (Fig. 2b); however, only the trichomes of Beinhart 1000-1 and Basma YNOTBS1, but not that of K326 and Dabai 1, could synthesize and secrete labdanoids (Fig. 2c). The quantity of diterpenoids for each cultivar is presented in Table 2. The results showed that the content of diterpenoids was significantly different among cultivars. The total diterpenoids content in N. sylvestris was the highest, followed by Beinhart 1000-1, Basma YNOTBS1, K326, and N. tomentosiformis. The cembranoids content in N. sylvestris was the highest, followed by Beinhart 1000-1, Basma YNOTBS1, and K326; while the labdanoids content in Basma YNOTBS1 was the highest, followed by Beinhart 1000-1, and N. tomentosiformis.
Trichome morphology and density
The morphology of the glandular trichomes varies greatly between the two parental species (Fig. 3). The leaf surface of N. sylvestris contained three kinds of trichomes: long-stalked glandular trichomes (i.e., types I-like, IV-like, and VI-like), short-stalked glandular trichomes (i.e., type VII-like), and non-glandular trichomes (i.e., type III-like), whereas the leaf surface of N. tomentosiformis did not contain any non-glandular trichomes (i.e., type III-like). Additionally, the leaf surface of K326 contained three kinds of trichomes (i.e., types I-like, III-like, IV-like, VI-like, and VII-like), whereas the leaf surface of Beinhart 1000-1, Basma YNOTBS1, and Dabai 1 did not contain any non-glandular trichomes (i.e., type III-like). In addition, according to the observations of the morphology of glandular trichomes on different leaves in same adult plants of four Nicotiana tabacum cultivars and two parental species,the morphology of trichomes did not show any changes with plant growth and leaf position (Fig. S1).
The total trichome density per mm2 in N. sylvestris (31.30) was significantly higher than that in N. tomentosiformis (19.33) (P ≤ 0.05). The total trichome density in the four cultivars differed greatly, with the highest in Beinhart 1000-1 (37.86), followed by K326 (22.27), and relatively low in Dabai 1 (4.54) and Basma YNOTBS1 (2.91). All of them were significantly different with the two parental species (P ≤ 0.05) (Fig. 4).
Expression of key genes involved in diterpenoid biosynthesis
Two key genes responsible for cembratrien biosynthesis, NtCYC and NtCYP71D16, were expressed in N. sylvestris, but not in N. tomentosiformis. However, two key genes for labdanoid biosynthesis, NtABS and NtCPS2, were expressed in N. tomentosiformis, but not in N. sylvestris (Fig. 5a). The four genes were highly expressed in Basma YNOTBS1 and a bit less in Beinhart 1000-1. For K326 and Dabai 1, NtABS, NtCYC and NtCYP71D16 had high expression patterns, while NtCPS2 was expressed at a markedly low level (Fig. 5b).
Aphid preference assay and correlation with glandular secretions
Aphid preference assay revealed that aphid attraction and host preference were different between tobacco species and cultivars (Fig. 6a). The number of aphids on the leaves of N. sylvestris (126) was significantly higher than that of N. tomentosiformis (25) (P ≤ 0.05). Additionally, the number of aphids on the leaves of Beinhart 1000-1 (140) was significantly higher than that of Basma YNOTBS1 (73), K326 (66), and Dabai 1 (60) (P ≤ 0.05) (Fig. 6b). The number of aphids on the leaves of Beinhart 1000-1 was not different from that on the leaves of N. sylvestris, but significantly higher than that on the leaves of N. tomentosiformis (P ≤ 0.05); the number of aphids on the leaves of Basma YNOTBS1 and K326 was significantly lower than that on the leaves of N. sylvestris, but significantly higher than that on the leaves of N. tomentosiformis (P ≤ 0.05); and the number of aphids on the leaves of Dabai 1 was significantly higher than that on the leaves of N. tomentosiformis, but significantly lower than that on the leaves of N. sylvestris.
Correlation analysis showed that insect attraction and host preference were significantly and positively correlated with the total amount of glandular secretions (r2 = 0.9425; P ≤ 0.05) (Fig. 6c) and the CBT-diol amount (r2 = 0.9224; P ≤ 0.05) (Fig. 6c).
Discussion
In this study, the surface of K326 leaves contained three kinds of trichomes (i.e., types I-like, III-like, IV-like, VI-like, and VII-like), whereas that of Beinhart 1000-1, Basma YNOTBS1, and Dabai 1 leaves did not contain any non-glandular trichomes (i.e., type III-like). The parental species N. sylvestris and N. tomentosiformis also showed different glandular morphology: the surface of N. sylvestris leaves contained both glandular and non-glandular trichomes (i.e., types I-like, III-like, IV-like, VI-like, and VII-like), whereas that of N. tomentosiformis leaves contained only glandular trichomes (i.e., types I-like, IV-like, VI-like, and VII-like). Therefore, the non-glandular trichomes (i.e., type III-like) of K326 probably originated from N. sylvestris.
In tobacco, the trichome density is affected by environmental conditions such as illumination, temperature, fertilization, and irrigation (Liang et al. 2009; Shi et al. 1999; Weng et al. 2009; Wilkens et al. 1996; Zhang et al. 2012), therefore, the trichome density of mature leaves might be biased by agronomic and environmental factors (Zhang et al. 2015). That is why we used young leaves for trichome density analysis, and to minimize the influence of external environment, the growing young plants were kept at a certain environment that was controlled by human.
Our results were in agreement with those reported by Simmons and Gurr (2006), which found that N. sylvestris produces only cembranoids, whereas N. tomentosiformis produces labdanoids only; however, they were in disagreement with those reported by Glas et al. (2012), which found that N. tomentosiformis produces both labdanoids and cembranoids, whereas N. sylvestris produces only the latter type of diterpenoids.
According to previous research on the tobacco trichome function, the tall glandular trichomes are the main synthesis and secretion site of diterpenoids (Meyberg et al. 1991), and the short glandular trichomes are the main synthesis and secretion site of the surface-localized defensive protein phylloplanins controlled by the gene T-phylloplanin (Shepherd et al. 2005); thus, the secretory function of trichomes differs in relation to their morphology (Yong et al. 2012). Wahlberg and Enzell (1987) reported that the biochemical profile of diterpenoids differs among the tobacco types. Oriental tobacco normally contains both types of diterpenoids, whereas burley tobacco contains only cembranoids. Additionally, Leffingwell (1999) revealed that cigar tobacco also contains two kinds of diterpenoids, whereas flue-cured tobacco contains only cembranoids. Our results are in line with the above findings, the gas chromatography–mass spectrometry profiles of leaf exudates indicated that Beinhart 1000-1 (cigar) and Basma YNOTBS1 (oriental) synthesized cembranoids and labdanoids; and K326 (flue-cured) and Dabai 1 (burley) synthesized only cembranoids.
The expression analysis of genes related to diterpenoid biosynthesis in the two parental species showed that the two key genes responsible for cembratrien biosynthesis, NtCYC and NtCYP71D16, were expressed in N. sylvestris, but not in N. tomentosiformis; however, two key genes involved in labdanoid biosynthesis, NtABS and NtCPS2, were expressed in N. tomentosiformis, but not in N. sylvestris. These results might explain the reason why N. sylvestris synthesizes only cembranoids, whereas N. tomentosiformis synthesizes only labdanoids (Simmons and Gurr 2006). In addition, the four key genes mentioned above, NtCYC, NtCYP71D16, NtABS, and NtCPS2, were highly expressed in Basma YNOTBS1 and Beinhart 1000-1. The genes responsible for cembratrien biosynthesis, NtCYC and NtCYP71D16, and the gene involved in labdanoid biosynthesis, NtABS, were highly expressed in K326 and Dabai 1, whereas NtCPS2 was expressed at a markedly low level. These results might explain why Basma YNOTBS1 and Beinhart 1000-1 synthesize both types of diterpenoids, whereas K326 and Dabai 1 synthesize only cembranoids. Thus, NtCYC and NtCYP71D16 expressed in four tobacco cultivars might be phylogenetically originate from N. sylvestris, whereas NtABS and NtCPS2 expressed in Basma YNOTBS1 and Beinhart 1000-1 might be derived from N. tomentosiformis.
Our results showed that the relationship between the leaf trichome density and secretion amount was quite complex. For example, our results show that N. sylvestris synthesized and excreted more diterpenoids, although its trichome density was lower than that of Beinhart 1000-1. Our results were in agreement with those reported by Peng-Fei (2008) that showed a positive correlation between the trichome density and secretion accumulation. Therefore, at the molecular level, the content of trichome exudates is determined by both the density of glandular trichomes and the intensity of trichome metabolism. The expression level of NtCYC and NtCYP71D16 in Basma YNOTBS1 was significantly higher than that in Beinhart 1000-1, whereas the trichome density followed the opposite trend. These results showed that, at the transcriptional level, Basma YNOTBS1 had a relatively higher ability to synthesize cembranoids than Beinhart 1000-1. Therefore, the high expression level of key genes might explain the high secretory volume of trichomes.
Labdanoids were detected in Beinhart 1000-1 and Basma YNOTBS1; labdenediol was detected only in Basma YNOTBS1, whereas Z-abienol was detected in both cultivars. Furthermore, the content of Z-abienol was the highest in Basma YNOTBS1. To further analyze the results, we examined the expression level of key genes involved in labdanoid biosynthesis. The results showed that, although the expression levels of NtABS were all high among the four cultivars, NtCPS2, the upstream regulation gene of NtABS in the labdanoid synthesis pathway was highly expressed only in Beinhart 1000-1 and Basma YNOTBS1. These results might explain the molecular mechanism of the foliar chemical characteristics at the transcriptional level in Basma YNOTBS1. The relationship between the expression of NtCPS2 and the content of secreted labdanoids confirmed that the role of NtCPS2 in labdanoid biosynthesis is critical and that it is possible to improve the aroma quality of K326 by restoring the labdanoid synthesis pathway.
Many studies have confirmed the role of diterpenoids in insect resistance (Ennajdaoui et al. 2010; Kennedy et al. 1995; Lin and Wagner 1994; Wang et al. 2001). Our results indicated that the aphid resistance of Beinhart 1000-1 and N. sylvestris was lower than that of the other studied materials. We also identified a significantly positive correlation between aphid preference and total glandular secretion as well as CBT-diol components. No relationship was found between aphid preference and other diterpene components (Fig. S2). Thus, further studies are needed to the better understand the contribution of diterpenoids to insect resistance.
Diterpenoids are important precursors of tobacco aroma substances and also influence insect resistance; thus, identifying the factors involved in the diterpenoid synthesis and secretion of glandular trichomes might help to further improve the aroma of tobacco and the biotic stress resistance of hairy plants. Our results showed that glandular trichome secretion was affected by the density of glandular hairs and the expression of key genes, and thus, they might be useful for improving abiotic and biotic stress resistance in crop plants.
Author contribution statement
Mingyue Huang and Hongying Zhang performed the experiments. Zhaojun Wang participated in data analysis. Dexin Niu performed the qRT-PCR experiments, and Yanhua Li projected design and supervision.
References
An L, Zhou Z, Yan A, Gan Y (2011) Progress on trichome development regulated by phytohormone signaling. Plant Signal Behav 6(12):1959–1962. https://doi.org/10.4161/psb.6.12.18120
Brückner K, Božić D, Manzano D, Papaefthimiou D, Pateraki I, Scheler U, Ferrer A, de Vos RC, Kanellis AK, Tissier A (2014) Characterization of two genes for the biosynthesis of abietane-type diterpenes in rosemary (Rosmarinus officinalis) glandular trichomes. Phytochemistry 101:52–64. https://doi.org/10.1016/j.phytochem.2014.01.021
Carman RM, Duffield AR (1993) The biosynthesis of labdanoids. The optical purity of naturally occurring manool and abienol. Aust J Chem 46(7):1105–1114. https://doi.org/10.1071/ch9931105
Cui H, Zhang ST, Yang HJ, Ji H, Wang XJ (2011) Gene expression profile analysis of tobacco leaf trichomes. BMC Plant Biol 11(1):76–77. https://doi.org/10.1186/1471-2229-11-76
Dayan FE, Duke SO (2003) Trichomes and root hairs: natural pesticide factories. Pestic Outlook 14(4):175–178. https://doi.org/10.1039/B308491B
Ennajdaoui H, Vachon G, Giacalone C, Besse I, Sallaud C, Herzog M, Tissier A (2010) Trichome specific expression of the tobacco (Nicotiana sylvestris) cembratrien-ol synthase genes is controlled by both activating and repressing cis-regions. Plant Mol Biol 73(6):673–685. https://doi.org/10.1007/s11103-010-9648-x
Fahn A (2000) Structure and function of secretory cells. Adv Bot Res 31:37–75. https://doi.org/10.1016/S0065-2296(00)31006-0
Glas JJ, Schimmel BCJ, Alba JM, Escobar-Bravo R, Schuurink RC, Kant MR (2012) Plant glandular trichomes as targets for breeding or engineering of resistance to herbivores. Int J Mol Sci 13(12):17077–17103. https://doi.org/10.3390/ijms131217077
Guo Z, Wagner GJ (1995) Biosynthesis of cembratrienols in cell-free extracts from trichomes of Nicotiana tabacum. Plant Sci 110(1):1–10. https://doi.org/10.1016/0168-9452(95)04174-S
Han J, Wang G, Yuan T, Liu W, Wang Y (1995) Preliminary study on trichome exudate of leaf surface of flue-cured tobacco. Chin Tob Sci 2:10–13
Johnson AW, Severson RF, Hudson J (1985) Tobacco leaf trichomes and their exudates. Tobacco Control 27:67–72
Kennedy BS, Nielsen MT, Severson RF, Sisson VA, Stephenson MK, Jackson DM (1992) Leaf surface chemicals from Nicotiana affecting germination of Peronospora tabacina (adam) sporangia. J Chem Ecol 18(9):1467–1479. https://doi.org/10.1007/bf00993221
Kennedy BS, Nielsen MT, Severson RF (1995) Biorationals from Nicotiana protect cucumbers against Colletotrichum lagenarium (Pass.) ell. & halst disease development. J Chem Ecol 21(2):221–231. https://doi.org/10.1007/bf02036653
Lange BM, Mahmoud SS, Wildung MR, Turner GW, Davis EM, Lange I, Baker RC, Boydston RA, Croteau RB (2011) Improving peppermint essential oil yield and composition by metabolic engineering. Proc Natl Acad Sci USA 108(41):16944–16949. https://doi.org/10.1073/pnas.1111558108
Larkin JC, Brown ML, Schiefelbein J (2003) How do cells know what they want to be when they grow up? Lessons from epidermal patterning in Arabidopsis. Annu Rev Plant Biol 54(1):403–430. https://doi.org/10.1146/annurev.arplant.54.031902.134823
Leffingwell J (1999) Leaf chemistry—basic chemical constituents of tobacco leaf and differences among tobacco types. Blackwell, London, pp 265–284. https://doi.org/10.13140/2.1.5173.6645
Li H, Chen ZF, Xie ZF, Li GY, Yang XY, Jin DM, Xiang J, Yang WL (2016) Breeding and Application of Dabai 1, a New Burley Tobacco Variety with Good Quality and High Yield. Guizhou Agricu Sci 44(5):1–4. https://doi.org/10.3969/j.issn.1001-3601.2016.05.001
Liang ZM, Ji H, Zhang H, Weng ML, Cui H (2009) Morphology and structure of chloroplast in flue-cured tobacco trichomes after applying fertilizer. Acta Bot Boreali-Occid Sinica 29(2):291–295
Lin Y, Wagner GJ (1994) Surface disposition and stability of pest-interactive, trichome-exuded diterpenes and sucrose esters of tobacco. J Chem Ecol 20(8):1907–1921. https://doi.org/10.1007/bf02066232
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 25(4):402–408. https://doi.org/10.1006/meth.2001.1262
Luckwill LC (1943) The genus Lycopersicon: a historical, biological and taxonomic survey of the wild and cultivated tomato. Aberd Univ Stud 120:1–44
Mathur J, Chua NH (2000) Microtubule stabilization leads to growth reorientation in Arabidopsis trichomes. Plant Cell 12(4):465–478. https://doi.org/10.1105/tpc.12.4.465
Mellon JE, Zelaya CA, Dowd MK, Beltz SB, Klich MA (2012) Inhibitory effects of gossypol, gossypolone, and apogossypolone on a collection of economically important filamentous fungi. J Agric Food Chem 60(10):2740–2745. https://doi.org/10.1021/jf2044394
Meyberg M, Krohn S, Brümmer B, Kristen U (1991) Ultrastructure and secretion of glandular trichomes of tobacco leaves. Flora 185(5):357–363. https://doi.org/10.1016/S0367-2530(17)30495-4
Peng L, Hu ZH (2007) Morphogenesis and histochemical investigation of peltate glandular trichomes on Glycyrrhiza uralensis Fisch. Leaves. J Mol Cell Biol 40(6):395–402
Peng-Fei LI, Zhou JH, Zhang JP, Yang HQ, Xiao ZX (2008) Changes of trichome density and content of leaf surface secretion on different flue-cured tobacco leaves during aging. J Hunan Agric Univ 34(3):293–297
Peters RJ (2010) Two rings in them all: the labdane-related diterpenoids. Nat Prod Rep 27(11):1521–1630. https://doi.org/10.1039/c0np00019a
Sallaud C, Giacalone C, Topfer R, Goepfert S, Bakaher N, Rosti S, Tissier A (2012) Characterization of two genes for the biosynthesis of the labdane diterpene Z-abienol in tobacco (Nicotiana tabacum) glandular trichomes. Plant J 72(1):1–17. https://doi.org/10.1111/j.1365-313X.2012.05068.x
Schilmiller AL, Last RL, Pichersky E (2008) Harnessing plant trichome biochemistry for the production of useful compounds. Plant J 54(4):702–711. https://doi.org/10.1111/j.1365-313X.2008.03432.x
Schwab B, Folkers U, Ilgenfritz H, Hülskamp M (2000) Trichome morphogenesis in Arabidopsis. Philos Trans Royal Soc 355(1399):879–883. https://doi.org/10.1098/rstb.2000.0623
Severson RF, Arrendale RF, Chortyk OT, Johnson AW, Jackson DM, Gwynn GR, Chaplin JF, Stephenson MG (1984) Quantitation of the major cuticular components from green leaf of different tobacco types. J Agric Food Chem 32(3):566–570. https://doi.org/10.1021/jf00123a037
Shepherd RW, Bass WT, Houtz RL, Wagner GJ (2005) Phylloplanins of tobacco are defensive proteins deployed on aerial surfaces by short glandular trichomes. Plant Cell 17(6):1851–1861. https://doi.org/10.1105/tpc.105.031559
Shi HZ, Liu GS (1998) Tobacco aromatics. In: Guo K (ed) Variation in aroma content of tobacco, 4th edn. China Agriculture Press, Beijing, pp 81–82
Shi XD, Liu GS, Han JF, Li GC (1999) Effect of various fertilizers on the type and distribution of trichome in fluecurd tobacco. Acta Tabacaria Sinica 5(2):19–22
Simmons AT, Gurr GM (2006) Trichomes of Lycopersicon species and their hybrids: effects on pests and natural enemies. Agric For Entomol 7(4):265–276. https://doi.org/10.1111/j.1461-9555.2005.00271.x
Wagner GJ (1991) Secreting glandular trichomes: more than just hairs. Plant Physiol 96(3):675–679. https://doi.org/10.1104/pp.96.3.675
Wagner GJ, Wang E, Shepherd RW (2004) New approaches for studying and exploiting an old protuberance, the plant trichome. Ann Bot 93(1):3–11. https://doi.org/10.1093/aob/mch011
Wahlberg L, Enzell CR (1987) Tobacco isoprenoids. Nat Prod Rep 4:237–276. https://doi.org/10.1039/NP9870400237
Wang E, Wagner GJ (2003) Elucidation of the functions of genes central to diterpene metabolism in tobacco trichomes using posttranscriptional gene silencing. Planta 216(4):686–691. https://doi.org/10.1007/s00425-002-0904-4
Wang E, Wang R, Deparasis J, Loughrin JH, Gan S, Wagner GJ (2001) Suppression of a P450 hydroxylase gene in plant trichome glands enhances natural-product-based aphid resistance. Nat Biotechnol 19(4):371–374. https://doi.org/10.1038/86770
Wang E, Gan S, Wagner GJ (2002) Isolation and characterization of the CYP71D16 trichome-specific promoter from Nicotiana tabacum L. J Exp Bot 53(376):1891–1897. https://doi.org/10.1093/jxb/erf054
Wang X, Yang Y, Zhang S, Feng Q, Wang J, Dongling WU (2013) Trichome morphology and secreting feature in neutral flavor type flue-cured tobacco leaf. Acta Tabacaria Sinica 3:45–48. https://doi.org/10.3969/j.issn.1004-5708.2013.03.009
Weathers PJ, Arsenault PR, Covello PS, Mcmickle A, Teoh KH, Reed DW (2011) Artemisinin production in Artemisia annua: studies in planta and results of a novel delivery method for treating malaria and other neglected diseases. Phytochem Rev 10(2):173–183. https://doi.org/10.1007/s11101-010-9166-0
Weng ML, Liang ZM, Cui H (2009) Effects of shaded treatment on density of central trichome and content of glandular secretions in tobacco. J Hen Agric Sci 38(4):52–55. https://doi.org/10.3969/j.issn.1004-3268.2009.04.014
Wilkens RT, Shea GO, Stamp NE (1996) Resource availability and the trichome defenses of tomato plants. Oecologia 106(2):181–191. https://doi.org/10.1007/BF00328597
Yin D, Zhang CD, Du SM, Qu SB, Qiao LZ, Song YC (2008) Breeding and evaluation of new oriental tobacco variety YNOTBS1 and its characteristics. Chin Tob Sci 1:11–15
Yong EC, Lim S, Kim HJ, Han JY, Lee MH, Yang Y, Kim JA, Kim YS (2012) Tobacco NtLTP1, a glandular-specific lipid transfer protein, is required for lipid secretion from glandular trichomes. Plant J 70(3):480–491. https://doi.org/10.1111/j.1365-313X.2011.04886.x
Zhang H, Cui H, Hao JI, Liang ZM, Weng ML (2012) Effects of drought stress on the development and gene expression of tobacco trichomes. J Zhengzhou Coll Anim Husb Eng 32(1):4–8
Zhang Y, Xu QH, Mao ZC, Pu WD, Li JY, Chen YP (2015) Comparison of density and morphology of glandular trichome of tobacco leaves of four species at different collection periods. J Yunnan Agric Univ 30(1):35–43. https://doi.org/10.16211/j.issn.1004-390X(n).2015.01.007
Acknowledgements
This study was supported by the State Tobacco Monopoly Administration of China [Grant No. 110201401003 (JY-03)], and the Technology Center, China Tobacco Henan Industrial Co., Ltd. [Grant No. ZW2014004].
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by H. Peng.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Huang, M., Zhang, H., Wang, Z. et al. Comparative studies of leaf surface chemical biosynthesis in different tobacco cultivars. Acta Physiol Plant 40, 67 (2018). https://doi.org/10.1007/s11738-018-2642-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-018-2642-7