Abstract
An efficient and reproducible Agrobacterium-mediated transformation system via repetitive secondary somatic embryogenesis was developed for Rosa rugosa ‘Bao white’. Somatic embryogenesis was induced from in vitro-derived unexpanded leaflet explants on MS medium supplemented with 4.0 mg/L 2,4-dichlorophenoxyacetic acid (2,4-D), 0.05 mg/L Kinetin and 30 g/L glucose. Secondary somatic embryos were successfully proliferated via cyclic secondary somatic embryogenesis on MS medium containing 1.0 mg/L 2,4-D, 0.01 mg/L 6-benzyladenine and 45 g/L glucose under light intensity of 500–1,000 lux. The highest germination rate (86.33 %) of somatic embryos was observed on 1/2-strength MS medium containing 1.0 mg/L BA. Relying on the repetitive secondary somatic embryogenesis and A. tumefaciens strain EHA105 harboring the binary vector pBI121, a stable and effective Agrobacterium-mediated transformation pattern was developed. The presented transformation protocol, in which somatic embryo clumps at globular stage (0.02–0.04 g) were infected by Agrobacterium for 60 min and co-cultivated for 2 days, and then selected under a procedure of 3 steps, were confirmed to be optional by GUS histochemical assay and Southern blot analysis. The procedure described here will be very useful for the introgression of desired genes into R. rugosa ‘Bao white’ and the molecular analysis of gene function.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rosa rugosa Thunb, distinct significantly from common Rosa hybrida L., is an important commercial crop due to its fragrant blossoms and medicinal functions. Essential oil from the petals of R . rugosa, contains a lot of alcohols, esters, talkanes, terpenes, aldehydes, ketones and ethers, is one of the most important natural raw materials used in perfume, cosmetics, aromatherapy, spices, and nutrition industry (Feng et al. 2010). R. rugosa also contains a number of medicinally important metabolites, such as flavonoids, tormentic acid, gallic acid derivative, polysaccharides and Rosamultin (Ng et al. 2004; Park et al. 2005; An et al. 2011). A large number of medicinal properties such as antidiabetic, hypolipidemic, anti-inflammatory, pain releasing and anti-cancer have been attributed to the pharmacologically active metabolites in R. rugosa (Park et al. 2005; Lee et al. 2008). However, R. rugosa is non-recurrent species, and is always attacked and affected by many biotic stresses, i.e. fungal diseases, which cause the decreases in biomass and essential oil yield. Genetic engineering appears to be an effective approach to develop disease resistant and recurrent flowering R. rugosa plants, and furthermore, offers opportunities to enhance secondary metabolite contents and to understand the molecular basics and regulation mechanism of secondary metabolism.
A highly efficient plant regeneration system is the prerequisite of genetic transformation. Somatic embryogenesis, which allows the production of plants from somatic cells, is an important approach for plant regeneration in woody species (Bao et al. 2012). The efficiency of regeneration via somatic embryogenesis usually depends on a high multiplication rate and increased level of uniformity of secondary somatic embryogenesis system. Secondary somatic embryogenesis, independent of the original explants quantity, permits to yield a large number of reproductive units in a prolonged period and offers increased level of synchronization with less variability. Until now, successful establishment of plant regeneration via primary and secondary somatic embryogenesis has been reported in a number of woody plants including Rosa species (Estabrooks et al. 2007; Shi et al. 2010; Dai et al. 2011; Bao et al. 2012), which indicated that primary and repetitive secondary somatic embryogenesis is a particularly powerful system for regeneration of woody plants.
Repetitive secondary somatic embryogenesis procedure provides a sustainable source of embryogenic material and thus is also important in many genetic transformation programs. (Raemakers et al. 1995). By this way, Agrobactierum-mediated transformation has been reported in several wood species (Katsumoto et al. 2007; Ribas et al. 2011). However, transformation protocol using secondary somatic embryogenesis system was observed in only a few of in rose cultivars (Li et al. 2002b; Kim et al. 2004; Vergne et al. 2010). Up to date, to our best acknowledge, systems for high-frequency plant regeneration and transformation through cyclic leave-originated secondary embryogenesis have not yet been reported for R. rugosa.
In this study, we developed a high-frequency regeneration procedure for R. rugosa ‘Bao White’ using repetitive secondary somatic embryogenesis system. Based on the established efficient regeneration system, for the first time, a successful new Arobactierum-mediated transformation protocol for R. rugosa ‘Bao White’ was developed and this new confirmed transgenic strategy has long-term implications in genetic engineering of R. rugosa ‘Bao White’ for desired traits.
Materials and methods
Plant material and culture condition
Explants of R. rugosa ‘Bao white’ were obtained from the in vitro culture sterile shoots. Shoots culture procedures and conditions were performed as previously described (Xing et al. 2010). The pH of all media, was adjusted to 5.8–6.0 with 1 mM NaOH prior to autoclaving at 121 °C for 20 min. Stock solutions of AS and antibiotics were filter-sterilized and mixed with autoclaved media cooled to 50–55 °C. All the cultures were incubated at 24 ± 2 °C, either in continuous darkness or under a 14 h photoperiod with a light intensity of 2,000–2,500 lux or 500–1,000 lux provided by cool white fluorescent lights.
Initiation of somatic embryogenesis
Unexpanded leaves were excised from 2 weeks proliferated shoots, and then these were divided into the three or five separate leaflets with the respective petioles present. For induction of somatic embryogenesis, the leaflets explants were incubated on basal medium containing full-strength MS salts, various concentration of 2,4-D (0, 1, 2, 3, 4, 5, 6 mg/L) and Kinetin (KT) (0, 0.05, 0.1 mg/L), plus 30 g/L glucose, and solidified using 3.0 g/L Phytagel. The cultures were kept in darkness for 60 days.
Optimizations of proliferation and germination on somatic embryo
Three individual experiments were carried out to optimize the condition of secondary somatic embryos proliferation:
-
1.
to investigate the effect of basal medium and PGRs, somatic embryos were cultured under a light intensity of 500–1,000 lux on full-strength MS or 1/2-strength MS basal medium supplemented different concentration of 2,4-D (0.5,1.0 mg/L) and BA (0, 0.01 mg/L), all of which contained 45 g/L glucose and 3.0 g/L Phytagel for 30 days;
-
2.
to investigate the effect of glucose concentration, somatic embryos were cultured under a light intensity of 500–1,000 lux on full-strength MS basal medium supplemented different concentration of 1.0 mg/L 2,4-D and 0.01 mg/L BA, plus varied concentration of glucose (30, 45, 60 g/L) and solidified using 3.0 g/L Phytagel for 30 days;
-
3.
in order to investigate the effect of light conditions on somatic embryo proliferation, somatic embryos were cultured on MS basal medium supplemented with 1.0 mg/L 2,4-D, 0.01 mg/L BA, 3.0 g/L Phytagel and 45 g/L glucose and then were treated under a intensity of 2,000–2,500 lux and 500–1,000 lux respectively, for 30 days.
At last, to further optimize the condition of secondary somatic embryos germination, secondary somatic embryos were cultured under a light intensity of 2,000–2,500 lux on full-strength MS or half-strength MS basal medium supplemented with BA (0.5, 1.0 mg/L) or 0.5 mg/L ABA or 0.5 mg/L TDZ respectively, plus 30 g/L glucose and solidified using 3.0 g/L Phytagel for 60 days.
Phytotoxic levels of antibiotics, bacterial strains and plasmid
Varied concentration of Kan (0, 25, 50, 75, 100 mg/L), combining 150 mg/L Cef respectively, were used to determine the somatic embryo sensitivity to kanamycin. Data were recorded by calculating the percentage of somatic embryos forming new secondary embryos after continuous 60 days culture. The disarmed Agrobacterium tumefaciens strains EHA105 (Hood et al. 1993) harboring the binary vector pBI121 (Jefferson 1987) were employed in transformation experiments. The T-DNA region of the plasmid contains the nptII gene drivened by nos promoter and GUS reporter gene driven by CaMV35S promoter (Fig. 1).
T-DNA constructs of binary vector pBI121 containning gus gene with intron used for transformation of Rosa rugosa (Jefferson 1987). RB right border, nos-P nopaline synthase promoter, nptII neomycin phosphotransferase gene, nos-T nopaline sythase terminator, 35S-P cauliflower mosaic virus (CaMV) 35S promoter, gus b-glucuronidase gene, LB left border
Transformation procedure
Standard procedures for bacterial preparation were according to Ning et al. (2012). The OD600 of bacterial was 0.4–0.6. Bacterial cells were harvested in liquid MS media supplemented with 200 Μm AS, and then incubated in shaker incubator at 28 °C and 200 rpm for about 2 h. 50 somatic embryo clumps were infected with 50 ml bacterial suspension in each experiment. In order to find the optimal procedure for transformation, three individual experiments were performed subsequently:
-
1.
In order to investigate the effect of secondary somatic embryos type on transformation, fully developed somatic embryos were first separated into small clumps at different weight and type (0–0.01 g, 0.02–0.04 g, cotyledonary somatic embryos), and then were immersed in the bacterial suspension for 60 min. Subsequently after dried on a sterilized filter paper, they were co-cultivated with Agrobacterium for 2 days in the co-cultivation medium (Table 1).
Table 1 Constituents of somatic embryo and plant culture medium in transformation -
2.
In order to investigate the effect of period of infection and cocultivation on transformation, somatic embryos (0.02–0.04 g) were immersed in the bacterial suspension for 40–60 min. Subsequently after dried on a sterilized filter paper, they were co-cultivated with Agrobacterium for 2–3 days in the co-cultivation medium (Table 1).
Selection and regeneration procedure
After cocultivation, somatic embryos were transferred into selection and proliferation medium (Table 1). Following 75 days of selection of proliferation, the kanamycin-resistant somatic embryos were separated and transferred to selection and germination medium (Table 1) for 2 months to promote the germination and shoot regeneration. Kanamycin-resistant shoots were subcultured onto selection and shoot elongation medium (Table 1) for 1 month. During selection process, explants were subcultured to a fresh selection medium once every 2 weeks. Vigorously growing kanamycin-resistant shoots were transferred to selection rooting medium (Table 1) for 20 days for rooting. Rooted plantlets were transferred the mixture soil consisting of turf soil + garden soil + sand (2:2:1; v/v/v).
GUS Histochemical assay and molecular analysis of transformation
Standard procedures for GUS histochemical assay were performed according to the methods reported by Jefferson et al. (1987) and Ning et al. (2012). Genomic DNA isolation from leaves of transgenic and non-transgenic R. rugosa ‘Bao White’ plants were based on the method described by Wang et al. (2011), and the PCR amplification analysis was carried out through Ning et al. (2012). The specific primers for the nptII gene were used:
-
Forward primer: 5′-CCATCGGCTGCTCTGATGCCGCCGT-3′
-
Reverse primer: 5′-AAGCGATAGAAGGCGATGGCTGC-3′.
For southern blot analysis, North2South® Chemiluminescent Hybridization and Detection Kit (Thermo 17,097) was used. 25 μg genomic DNA from each PCR-positive and control plant was digested overnight with EcoRI, The digested genomic DNA were separated by 0.8 % agarose gel electrophoresis and transferred onto hybond-N + nylon membrane. A fragment of 431 bp GUS reporter gene was labeled with biotin according to manual of North2South® Biotin Random Prime Labeling Kit (Thermo 17,075), and used as a probe. After hybridization, a combination of enhanced luminol and horseradish peroxidase (HRP) was used as Chemiluminescence substrate for detection of hybridization signals using X-ray film autography.
Statistical analysis
In this study, each treatment was repeated thrice, and each replicate consisted of a Petri plate with 10–15 explants, and totally contained 30–45 explants. Except 15 cm Petri plate used in the maturation and germination experiments, 10 cm Petri plates were used in all the other experiments. Each Petri plate was closed by 5 cm PE preservative film.
Data were evaluated by analysis of variance (ANOVA) and means were compared using LSD (least significant difference) tests. All computations were made using the generalized linear model procedures of SAS. The proliferation of somatic embryos was calculated as previous described method (Bao et al. 2012). The proliferation coefficient of somatic embryos was calculated as the ratio of weight of somatic embryos after proliferation to the weight of somatic embryos before proliferation. Percentage data were transformed via arcsine before analysis.
Results
Somatic embryogenesis initiated from unexpanded leaflets
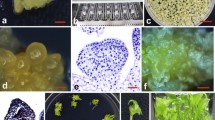
When unexpanded leaflets with attached petioles were incubated on MS medium supplemented with 1–2 mg/L or 5–6 mg/L 2,4-D and 0–0.1 mg/L KT, callus and rhizoid was observed at the cut surface on all the media within 3 weeks. Initially, these calluses were white and water-soaked, but turned brown gradually and became moribund eventually, without forming somatic embryo. Whereas, on the medium containing 3–4 mg/L 2,4-D and 0–0.1 mg/L KT, after 6–8 weeks, somatic embryos were directly induced at the proximal cut surface of the petiole. The frequency of somatic embryo formation differed significantly after the various treatments (Table 2). High concentration of KT (0.1 mg/L) promotes the expanded and thin embryos, which could not proliferate. The highest frequency (8.15 %) of primary somatic embryo induction was observed on the medium containing 4 mg/L 2,4-D and 0.05 mg/L KT, where predominately thickened and tufted embryos were obtained (Fig. 2a).
Plant regeneration via direct somatic embryogenesis for Rosa rugosa. a Primary somatic embryo on the medium with 4.0 mg/L 2,4-D and 0.01 mg/L KT (bar 1.0 mm), b proliferation of secondary somatic embryo under light intensity of 50–100 lx (bar 1.0 mm), c proliferation of secondary somatic embryo under light intensity of 50–100 lx (bar 1.0 mm), d proliferation of secondary somatic embryo under light intensity of 1,000–1,500 lx (bar 5.0 mm), e secondary somatic embryo turned green on germination medium (bar 3.0 mm), f germinated plantlets with both shoots and radicles (bar 5.0 mm)
Proliferation and maintenance of somatic embryos
Effect of light conditions on somatic embryo proliferation
When somatic embryos were cultured on a given medium composition (MS containing 1.0 mg/L 2,4-D, 0.01 mg/L BA, 3.0 g/L Phytagel and 45 g/L glucose), the conditions of continuous dark or light intensity of 500–1,000 lux and 2,000–2,500 lux resulted in the development of somatic embryos with notably different color and texture characteristics. Either normal light intensity of 2,000–2,500 lux or darkness inhibited the proliferation of somatic embryos. Under the normal light intensity, the primary somatic embryos turned green and exhibited an expansion (Fig. 2d); in the dark, the somatic embryo appeared to be brownish, withered and accompanied usually with the formation of waterlogged callus. In contrast, somatic embryos which developed under low light intensity of 500–1,000 lux were the vigorous and golden-yellow (Fig. 2b).
Effect of basal medium and PGRs on somatic embryo proliferation
The effects of basal media and PGRs on secondary somatic embryos proliferation are described in Table 3. No significant differences in secondary somatic embryos proliferation coefficient was observed when different basal media and varied concentration of BA were used. However, both basal medium and concentration of BA had significant influences on the frequency of abnormal somatic embryo (not proliferate again) formation. 1/2-strength MS media and high concentration of BA promoted the formation of abnormal somatic embryo. The concentration of 2,4-D had significant differences in the proliferation coefficient of secondary somatic embryos and the frequency of abnormal somatic embryo formation. When cultured on the MS basal medium containing 0.5 mg/L 2,4-D with or without BA, although the proliferation frequency of secondary somatic embryos (>4.0) were higher, the newly proliferated somatic embryo appeared compact and white in color, which cannot proliferate repetitively. The best results were obtained on MS basal medium containing 1.0 mg/L 2,4-D combined with 0.01 mg/L BA, most of the somatic embryos appeared healthy and vigorous (Fig. 2c), the proliferation coefficient was 4.56, and less abnormal somatic embryo formed.
Effect of glucose concentration on somatic embryo proliferation
The effects of concentration of glucose on somatic embryo proliferation are described in Table 4. Concentration of glucose had significant influences on somatic embryo proliferation. Medium containing 45 g/L glucose was proved to be more effective than 30 and 60 g/L glucose, where highest proliferation coefficient was achieved and secondary somatic embryos appeared healthy and golden-yellow.
High frequency germination of secondary somatic embryos
The effects of hormones and basal medium on the germination frequency of secondary somatic embryos are described in Table 5. Significant differences in the germination of somatic embryos were observed among varied hormones treatments. When cultured on the MS medium containing 0.5 mg/L BA, the higher germination frequency (73.67 %) of somatic embryos was obtained. The statistic results also indicated that both basal medium and concentration of BA also had significant influences on the germination of somatic embryos. The frequency of germination on 1/2-strength MS media was higher than that on full-strength MS media. The highest germination rate (86.3 %) of somatic embryos was observed on 1/2-strength MS medium containing 1.0 mg/L BA. Furthermore, all most germinated somatic embryos, swelled in size and turned green (Fig. 2e), finally developed into bipolar plantlets on this medium (Fig. 2f).
Optimization of transformation parameters
Various concentrations of Kan (25–100 mg/L) were used to determine the threshold for selective putative transformed secondary somatic embryos. The results showed that 100 mg/L Kan was the most optional level.
Effect of secondary somatic embryos type on transformation
Embryos state (different types of somatic embryo) significantly affected the frequency of GUS transient expression. The best results, 69.8 % GUS expression, were obtained in somatic embryo clumps (0.02–0.04 g) at globular stages. While the smaller clumps (0–0.01 g) and cotyledonary somatic embryos exhibited a decrease in the percentage of GUS expression explants (33.3 and 51.1 %, respectively) (Fig. 3). Moreover no secondary somatic embryo was induced from the smaller somatic embryos clumps (0–0.01 g) and cotyledonary somatic embryos on the selection and proliferation medium. When the somatic embryo clumps (0.02–0.04 g), after co-cultivation, are cultured on selection on selection and proliferation medium for 3 months, kanamycin-resistant secondary somatic embryos were produced.
Transient GUS expression in the three types of secondary somatic embryos from Rosa rugosa. Frequency of transient GUS expression represent the percentage of inoculated explants displaying GUS + spots 7 days after infection and co-cultivation. Values shown are mean ± standard errors. Values that are significantly different within columns at the 5 % level of significance are indicated with different letters
Effect of co-cultivation and inoculation period on transformation
Significant differences in the percentage of GUS responding explants were observed when varied inoculation period were performed. The optimum time for getting the highest transient transformation efficiency was 60 min during infectious periods (Table 6). The optimizing co-cultivation period tests showed that although no significant differences in transient GUS expression were observed after 2–3 days of co-cultivation, longer co-cultivation duration led to overgrowth of bacteria which consequently decreased proliferations of secondary somatic embryos.
High-frequency transgenic plants production via secondary somatic embryogenesis
Depending on effective transformation pattern via cyclic secondary somatic embryogenesis, the transgenic somatic embryos (Fig. 4a) were obtained after 2 months culture on the selection and proliferation medium, the transgenic shoots (Fig. 4b) were obtained from transgenic embryos after 4–6 months culture on germination selective medium, finally, the transgenic shoots of 2–3 cm were successfully rooting in rooting selective medium (Fig. 4c). In our study, a total of 500 explants were transformed with A. tumefaciens strain EHA105:p35SGUSINT, which resulted in 248 somatic embryo clumps after the first step of kanamycin selection and 175 somatic embryo clumps showed varying levels of GUS activity (Fig. 4a). After the germination selection step, these transgenic somatic embryos formed 102 putative transgenic shoots. Among these, 57 rooted plantlets showed GUS activity in both leaves and roots (Fig. 4d), and the transformation efficiency was 11.4 %. Rooted transgenic plantlets were transferred to soil (Fig. 4e). The GUS-positive R. rugosa ‘Bao White’ lines were verified for the presence of nptII gene by PCR analysis (Fig. 5). Southern blot analysis of 5 random selected plants that are positive in both PCR and GUS activity test suggested that most transgenic plants harboring 1–2 copies of the Gus reporter gene (Fig. 6).
Stable transformation of Rosa rugosa. a GUS positive somatic embryos (bar 1 mm), b GUS expression in germinated shoots, CK was the untransformed shoots (bar 1 cm), c GUS expression in germinated plantlets before transfer to soil (bar 1 cm), d GUS expression in whole germinated plantlets (bar 1 cm), e transgenic plants after transfer to soil (bar 5 cm)
Discussion
The presented paper typically describes a stable and high-efficiency Agrobacterium-mediated transformation protocol using secondary somatic embryos as explants in wood species, and it is also the first report of effective transformation via secondary somatic embryogenesis and in R. rugosa ‘Bao White’.
Although the method for plant regeneration via somatic embryogenesis in R. rugosa has already been reported by Kunitake et al. (1993) and Kim et al. (2009), the explants used in induction of somatic embryogenesis were immature seeds and zygotic embryo respectively. However, these explants cannot inherit all the agronomical traits of mother plant. In our study, somatic embryos were initiated from in vitro-derived leaflets, which can well inherit all the agronomical traits of maternal ramet as the major vegetative organ.
2,4-D is considered to be the most routinely used hormone in somatic embryos induction in vitro culture within most species. (Kim et al. 2009; Prange et al. 2010; Bao et al. 2012). In our study, it was found that the addition of certain concentration KT together with 2,4-D would affect the induction and the morphology of somatic embryos. 0.05 mg/L KT could promote the induction of normal somatic embryos, while 0.1 mg/L KT encourage the expanded and thin petal-shaped embryo. One explanation would be that the somatic embryo of R. rugosa ‘Bao White’ was sensitive to KT, which promotes the maturation of somatic embryo untimely.
Secondary somatic embryogenesis has been occasionally reported in trees (Pinto et al. 2008; Shi et al. 2010), and also has been observed in a few of R. hybrida and R. chinese cultivars (Li et al. 2002a; Vergne et al. 2010; Bao et al. 2012). In our study, cyclic secondary somatic embryogenesis also can be successfully induced in R. rugosa ‘Bao White’. Furthermore it was observed that light condition was the key factor in the proliferation of secondary somatic embryos. The proliferation also was significantly affected by the type of basal medium, the concentration of carbohydrates and PGRs. In addition, the repetitive secondary somatic embryogenesis system, evaluated by our study, can maintain the embryogenic potential for more than 3 years. The optimal light intensity of somatic embryo proliferation was 500–1,000 lux, while the normal light intensity stimulated the accumulation of some endogenous chlorophylls, anthocyanins and hormones in somatic embryos, which inhibited the proliferation of secondary somatic embryos. This makes R. rugosa ‘Bao White’ special and distinct significantly from other reported Rosa cultivars (Li et al. 2002a; Vergne et al. 2010; Bao et al. 2012). The effect of basal medium, PGRs and carbohydrates (glucose) on the proliferation of somatic embryos was reported in different species previously (Pinto et al. 2008; Bao et al. 2012). However, in Rosa species, previous work on the suitable basal medium, PGRs and carbohydrates for proliferation of somatic embryos were few. Bao et al. (2012) first evaluated the effects of various types and concentrations of carbohydrates and PGRs on the proliferation of secondary somatic embryos of R hybrida ‘samantha’ in detail. In this study, we found that in addition to concentrations of carbohydrates and PGRs, basal medium affect the proliferation of somatic embryos as well in R. rugosa ‘Bao White’.
In some Rosa cultivars, two developmental types of somatic embryos were observed in the germination process, namely those with shoots only, and those with both shoots and roots (kaur et al. 2006; Bao et al. 2012). However, in R. rugosa ‘Bao White’, most of the germinated somatic embryos had normal appearance with both shoots and radicles (Fig. 2f). This phenomenon indicated that the presented culture condition was suitable for germination of somatic embryo and plant regeneration, and thus the secondary somatic embryos of R. rugosa ‘Bao White’ have a normal bipolar morphology. A positive effect of ABA has been observed during maturation and germination of somatic embryos in many species (Li et al. 2002a; Mauri and Manzanera 2004; Sharma et al. 2004; Shi et al. 2010), and the presence of TDZ also was proved to promote the maturation of somatic embryos in some species (Khan et al. 2006; Bao et al. 2012). Whereas in R. rugosa ‘Bao White’, it was found that both ABA and TDZ did not have positive effects on maturation and germination of somatic embryos, while, BA was the most effective hormone treatment. This fact is possibly due to the sensitivity of R. rugosa’s somatic embryos to BA in the maturation and germination progress. In some way, exogenous application of BA may change the endogenous hormone levels, nucleic acid and protein synthesis, as well as nitrogen metabolism. The actual role played by BA in stimulating maturation of R. rugosa somatic embryos remains unclear, and the further research on this phenomenon is needed. The medium strength will affect the development of somatic embryo via directly changing osmotic pressure (Komatsuda et al. 1992). Bao et al. (2012) reported that the frequency of somatic embryo germination on standard MS medium was higher than that on 1/2-strength MS medium in R. hybrida ‘Samantha’. However, in our study, the highest frequency of maturation and germination of secondary somatic embryos was achieved on1/2-strength MS. This result is concordant with the finding in R. hybrida ‘arizona’ (Murali et al. 1996).
Agrobacterium-mediated transformation via somatic embryogenesis have reported in many Rosa species (Dohm et al. 2001; Li et al. 2002b; Kim et al. 2004; Katsumoto et al. 2007; Vergne et al. 2010). In contrast to most previous reports on rose transformation which using embryogenic callus as target of transformation (Li et al. 2002b; Kim et al. 2004; Katsumoto et al. 2007), secondary somatic embryos was used in our study. Though it is confirmed to resist stubbornly generating embryogenic callus in R. rugosa ‘Bao White’, a stable and efficient Agrobacterium-mediated transformation protocol using secondary somatic embryos explants was developed in the first time by us. Same inoculation material was chosen by Dohm et al. (2001). However, in our study, the transgenic plants were regenerated via secondary somatic embryo proliferation and germination instead of adventitious shoots formation in their study.
Several factors play major roles in Agrobacterium-mediated transformation. 1 h of inoculation accomplished with 2 days of co-cultivation was the optimum transformation pattern, which is consistent with the report on transformation of Rosa chinensis ‘Old Blush’ (Vergne et al. 2010). However, in contrast to Vergne et al. (2010), km-resistance buds directly produced by transformed cotyledonary embryos can grow to shoots, and died in the selection procedure. The best results were achieved with 0.02–0.04 g somatic embryo clumps. As active cellular division was thought to be important for Agrobacterium transformation (Peña et al. 2004), this fact may be explained by that 0.02–0.04 g somatic embryo clumps contains a greater number of active embryogenic cells, compared with smaller clumples, and cotyledonary embryos. In our study, three selection procedures, including cyclic secondary somatic embryogenesis, germination and rooting respectively, were extremely necessary to transformation of R. rugosa ‘Bao White’. After all these procedures, untransformed escapes were almost eliminated. GUS activity and southern blot analysis not only confirmed stable integration of GUS reporter gene into the plant genome, but also verified that a high percentage of positive transgenic plants (11.4 %) were generated. In the previous reports of transformation in rose, the highest transformation efficiency (15.6 %) was obtained by Marchand et al. (1998) via biolistic methods. While for Agrobacterium-mediated transformation, the transformation efficiency was among 2–9 %, the highest of which (9 %) was Li et al. (2002a, b) in a Agrobacterium-mediated transformation of embryogenic callus by taking advantage of induced secondary somatic embryogenesis. In this study, based on secondary somatic embryogenesis and three selection procedures, a higher transformation efficiency of 11.4 % was achieved using somatic embryos as explants.
In conclusion, primary and cyclic secondary somatic embryogenesis procedure was established in a commercial woody species-R. rugosa ‘Bao White’. Based on this efficient regeneration system, successful Arobactierum-mediated transformation protocol was founded and improved via repetitive secondary somatic embryogenesis system, which was robustly confirmed by GUS activity and Southern blot analysis The transformation system described here opens up an avenue for future genetic improvement programs, including disease resistance and secondary metabolism engineering, for this important industrial fragrance oil bearing crop.
Author contribution
Xing wen performed the experiments and drafted the manuscript. Bao ying and Luo ping help to perform the experiment. Bao manzhu and Ning guogui finalized the paper. Ning guogui supervised the project. All authors read and approved the final manuscript.
Abbreviations
- MS:
-
Murashige and Skoog (1976)
- 2,4-D:
-
2,4-Dichlorophenoxyacetic acid
- TDZ:
-
Thidiazuron
- BA:
-
6-Benzyladenine
- NAA:
-
a-Naphthalene acetic acid
- KT:
-
Kinetin
- ABA:
-
Gibberellin
- PGR:
-
Plant growth regulator
- nptII :
-
Neomycin phosphotransferase
- GUS:
-
β-Glucuronidase
- Kan:
-
Kanamycin
- Cef:
-
Cefotaxime
- AS:
-
Acetosyringone
- X-Gluc:
-
5-Bromo-4-chloro-3-indolyl-β-d-glucuronide
References
An JH, Kim TI, Park HJ, Kim HM, Choi HJ, Lee TK (2011) Tormentic acid, a triterpenoid saponin, isolated from Rosa rugosa, inhibited LPS-induced iNOS, COX-2, and TNF-α expression through inactivation of the nuclear factor-κb pathway in RAW 264.7 macrophages. Int Immunopharmacol 11:504–510
Bao Y, Liu GF, Shi XP, Xing W, Ning GG, Liu J, Bao MZ (2012) Primary and repetitive secondary somatic embryogenesis in Rosa hybrida ‘Samantha’. Plant Cell Tissue Organ Cult 109:411–418
Dai JL, Tan X, Zhan YG, Zhang YQ, Xiao S, Gao Y, Dong WX, Wang T, Wang XC, You XL (2011) Rapid and repetitive plant regeneration of Aralia elata Seem. via somatic embryogenesis. Plant Cell Tissue Organ Cult 104:125–130
Dohm A, Ludwig C, Schilling D, Debener T (2001) Transformation of roses with genes for antifungal protein. Acta Hortic 547:27–31
Estabrooks T, Browne T, Dong ZM (2007) 2, 4, 5-Trichlorophenoxy-acetic acid promotes somatic embryogenesis in the rose cultivar‘Livin’ Easy’ (Rosa sp.). Plant Cell Rep 26:153–160
Feng LG, Chen C, Sheng LX, Liu P, Tao J, Su JL, Zhao LY (2010) Comparative analysis of headspace volatiles of Chinese Rosa rugosa. Molecules 15:8390–8399
Hood EE, Gelvin SB, Melchers LS, Hoekema A (1993) New Agrobacterium vectors for plant transformation. Transgenic Res 2:208–218
Jefferson RA (1987) Assaying chimeric genes in plants: the GUS gene fusion system. Plant Mol Biol Rep 5:387–405
Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusions: B-glucuronidase as a sensitive and versatile gene marker in higher plants. EMBO J 6:3901–3907
Katsumoto Y, Fukuchi-Mizutani M, Fukui Y et al (2007) Engineering of the rose flavonoid biosynthetic pathway successfully generated blue-hued flowers accumulating delphinidin. Plant Cell Physiol 48:1589–1600
Kaur N, Pratap PK, Sharma M, Ahuja PS (2006) Somatic embryogenesis from immature zygotic embryos of Rosa bourboniana Desp. In Vitro Cell Dev Biol Plant 42:124–127
Khan H, Siddique I, Anis M (2006) Thidiazuron induced somatic embryogenesis and plant regeneration in Capsicum annuum. Biol Plant 50:789–792
Kim CK, Chung JD, Park SH, Burrell AM, Kamo KK, Byrne DH (2004) Agrobacterium tumefaciens-mediated transformation of Rosa hybrida using the green fluorescent protein (GFP) gene. Plant Cell Tissue Organ Cult 78:107–111
Kim SW, Oh JM, Liu JR (2009) Somatic embryogenesis and plant regeneration in zygotic embryo explant cultures of Rugosa rose. Plant Biotechnol Rep 3:199–203
Komatsuda T, Lee W, Oka S (1992) Maturation and germination of somatic embryos as affected by sucrose and plant growth regulators in soybeans Glycine gracilis Skvortz and Glycine max (L.) Merr. Plant Cell Tissue Organ Cult 28:103–113
Kunitake H, Imamizo H, Mii M (1993) Somatic embryogenesis and plant regeneration from immature seed-derived calli of rugosa rose (Rosa rugosa Thunb.). Plant Sci 90:187–194
Lee YH, Jung MG, Kang HB, Choi KC, Haam S, Jun W, Kim YJ, Cho HY, Yooh HG (2008) Effect of anti-histone acetyltransferase activity from Rosa rugosa Thunb. (Rosaceae) extracts on androgen receptor-mediated transcriptional regulation. J Ethnopharmacol 118:412–417
Li XQ, Krasnyanski FS, Korban SS (2002a) Somatic embryogenesis, secondary somatic embryogenesis, and shoot organogenesis in Rosa. J Plant Physiol 159:313–319
Li XQ, Krasnyanski FS, Korban SS (2002b) Optimization of the uidA gene transfer into somatic embryo of rose via Agrobacterium tumefaciens. Plant Physiol Biochem 40:453–459
Marchant R, Power JB, Lucas JA, Davey MR (1998) Biolistic transformation of rose (Rosa hybrida L.). Ann Bot 81:109–114
Mauri PV, Manzanera JA (2004) Effect of abscisic acid and stratification on somatic embryo maturation and germination of holm oak (Quercus ilex L.). In Vitro Cell Dev Biol Plant 40:495–498
Murali S, Sreedhar D, Lokeswari TS (1996) Regeneration through somatic embryogenesis from petal-derived calli of Rosa hybrida L. cv arizona (hybrid tea). Euphytica 91:271–275
Murashige T, Skoog F (1976) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497
Ng TB, He JS, Niu SM, Zhao L, Pi ZF, Shao W, Liu F (2004) A gallic acid derivative and polysaccharides with antioxidative activity from rose (Rosa rugosa) flowers. J Pharm Pharmacol 56:537–545
Ning GG, Xiao X, Lv HY, Li X, Zuo Y, Bao MZ (2012) Shortening tobacco life cycle accelerates functional gene identification in genomic research. Plant Biol 14:934–943
Park JC, Kim SC, Choi MR, Song SH, Yoo EJ, Kim SH, Miyashro H, Hattori M (2005) Anti-HIV protease activity from rosa family plant extracts and rosamultin from Rosa rugosa. J Med Food 8:107–109
Peña L, Pérez RM, Cervera M, Juárez JA, Navarro L (2004) Early events in Agrobacterium-mediated genetic transformation of Citrus explants. Ann Bot 94:67–74
Pinto G, Silva S, Park YS, Neves L, Araujo C, Santos C (2008) Factors influencing somatic embryogenesis induction in Eucalyptus globulus Labill.: basal medium and anti-browning agents. Plant Cell Tissue Organ Cult 95:69–78
Prange ANS, Serek M, Bartsch M, Winkelmann T (2010) Efficient and stable regeneration from protoplasts of Cyclamen coum Miller via somatic embryogenesis. Plant Cell Tiss Organ Cult 101:171–182
Raemakers CJJM, Jacobsen E, Visser RGF (1995) Secondary somatic embryogenesis and applications in plant breeding. Euphytica 81:93–107
Ribas AF, Dechamp E, Champion A, Bertrand B, Combes MC, Verdeil JL, Lapeyre F, Lashermes P, Etienne H (2011) Agrobacterium-mediated genetic transformation of Coffea arabica (L.) is greatly enhanced by using established embryogenic callus cultures. BMC Plant Bio 11:92. doi:10.1186/1471-2229-11-92
Sharma P, Pandey S, Bhattacharya A, Nagar PK, Ahuja PS (2004) ABA associated bio-chemical changes during somatic embryo development in Camellia sinensis (L.) O Kuntze. J Plant Physiol 161:1269–1276
Shi XP, Dai XG, Liu GF, Zhang JW, Ning GG, Bao MZ (2010) Cyclic secondary somatic embryogenesis and efficient plant regeneration in camphor tree (Cinnamomum camphora L.). In Vitro Cell Dev Biol Plant 46:117–125
Vergne P, Maene M, Guillaume G, Aurelie C, Debener T, Mohammed B (2010) Somatic embryogenesis and transformation of the diploid Rosa chinensis cv Old Blush. Plant Cell Tissue Organ Cult 100:73–81
Wang Z, Ye SF, Li JJ, Zheng B, Bao MZ, Ning GG (2011) Fusion primer and nested integrated PCR (FPNI-PCR): a new high-efficiency strategy for rapid chromosome walking or flanking sequence cloning. BMC Biotech 11:109
Xing W, Bao MZ, Qin HD, Ning GG (2010) Micropropagation of Rosa rugosa through axillary shoot proliferation. Acta Biol Cracov Bot 52:69–75
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (No. 31171985) and the National Science and Technology Ministry of China (No. 2011AA100208). We thank all the colleagues in our lab for constructive discussion and technical support. We are also grateful to Dr. Alex C. McCormac for critical editing of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by S. Werbrouck.
Rights and permissions
About this article
Cite this article
Xing, W., Bao, Y., Luo, P. et al. An efficient system to produce transgenic plants via cyclic leave-originated secondary somatic embryogenesis in Rosa rugosa . Acta Physiol Plant 36, 2013–2023 (2014). https://doi.org/10.1007/s11738-014-1578-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-014-1578-9