Abstract
The effects of sugarcane plantation intercropped with soybean on plant growth, yield, enzyme activity, nitrogen and phosphorus contents, the microbe quantity of rhizosphere soil were investigated. Results showed that dry weight of biomass and yield under sugarcane/soybean intercropping were increased by 35.44 and 30.57 % for sugarcane, and decreased by 16.12 and 9.53 % (100-grain weight) for soybean, respectively. The nitrogenase activity of intercropping soybean nodule was significantly increased by 57.4 % as compared with that in monoculture models. The urease activities of intercrops sugarcane and soybean were promoted by 89 and 81 % as compared to that of the monoculture models, respectively. The effective nitrogen and phosphorus contents of rhizospheric soil of intercrops sugarcane and soybean were increased by 66 and 311.7 %, respectively, as compared to those in the monoculture system. Microbe number of rhizosphere soil in the intercropping pattern increased significantly as compared to those in the monoculture models. The quantities of bacteria, fungi, and actinomyces increased by 42.62, 14.5 and 78.5 % in the intercropping sugarcane, while the intercropping soybean increased by 188, 183 and 73 %, respectively. Therefore, growing sugarcanes in combination with soybean can be considered a good agriculture management practice, helping to promote plant growth, yield and increase soil nutrients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sugarcane is a major crop in tropical and subtropical regions of the South China, occupying about 1.4 million hectares. In general, sugarcane has a juvenile period of 100–110 days, so that the intercrops of soybean and sugarcane are widely practiced. Sugarcane is generally planted in 80–100 cm rows, and soybean could be intercropped between two rows of sugarcane at the same time. Recently, the wide-rowed sugarcane plantations with the preferred space of 120–140 cm are tested, which are conducive to conduct intercropping and benefit both crops in intercropping system (Huang 2002). Intercropping has been recognized as a potential system for the augments of productivity over space and time in subsistence farming situations. There is generally a trend toward high yield under intercropping. Even in areas where yield of the companion crop was substantially reduced, total yield was greater (Evan 1960; Aggarwal et al. 1992). Imam et al. (1990) and Bokhtiar et al. (1995) reported that sugarcane intercropping with potato helped to increase both the yields of potato and cane.
The sugarcane crop depletes a considerable amount of nutrients from soil, but soybean in intercropping pattern increases productivity per unit of land and enables the crops more effectively utilize nutrients and improve soil fertility and field ecological conditions (He et al. 2006; Tang et al. 2005). An intercropping partly meets the N requirement of the companion crop due to the transfer of the symbiotically fixed N from the legume to the non-legume (Ledgard et al. 1985). Nitrogen, phosphorus and potassium utilization efficiency in maize/mungbean intercropping was significantly higher than that of the monoculture system (Chowdhury and Rosario 1994), and the organic matter content of sugarcane soil increased due to companion cropping of pulses (Yadav et al. 1987).
Intercropping usually benefits from increased microbial number, and hence improved soil enzyme activity (Chai et al. 2005). Microorganisms abound in the soil are critical to decomposing organic residues and recycling soil nutrients. Soil enzymes involved intimately in the cycling of nutrients, effect fertilizer use efficiency, reflect the microbiological activity in soil and act as indicators of soil change (Dick 1994; Ruggiero and Bollag 1996; Gianfreda and Bollag 1996). Li et al. (2000) studied that the quantity of microbes was positively correlated with the urease activity in wheat/soybean intercropping.
In the present study, our objective was to investigate the growth, yield, rhizosphere soil microbes and enzyme activity related to nutrient availability under sugarcane and soybean intercropping conditions.
Materials and methods
Plant materials
The sugarcane variety ROC22 (Saccharum officinarum) and soybean HuaChun5 (Glycine max L.) were used as the plant materials for the experiments.
Treatments
The treatments include: intercropped sugarcane and soybean, monoculture sugarcane, and monoculture soybean. Each treatment was replicated three times. A plastic potted method was used (150 cm length, 50 cm in width, 50 cm in depth) (Fig. 1).
Plants were grown in a greenhouse under natural light and temperature conditions. The experiment was carried out three times from June 2010 to August 2012. In this paper, the most recent results are shown. Every growing pot contained 30 kg dry (soil belongs to red soil type with pH 5.0, 65.60 mg/kg N, 10.06 mg/kg P), sieved soil and two sugarcane plants or three soybean plants were planted under the monoculture system, or two sugarcane plants, with three soybean plants under the intercropping culture system (Fig. 1). All the treatments were designed in three replications. Pots were rotated periodically. Plants were supplied only tap water until foliage leaves were emerged and then supplied Arnon–Hoagland solution for every 7 days. Plants were harvested periodically until the soybean flowering period and measured the fresh weight and length. Then they were dried in a ventilated oven at 65 °C for 5 days and measured the dry weight. The data obtained were statistically analyzed by student’s t test (Stat View 5.0, SAS Institute Inc.) and SPSS 13.0 software.
Sampling: plants along with soil were taken out from the pots, the adhered soil near the roots was stripped gently, maintained in sterilized paper bags for analysis.
Soil analysis
The number of bacteria, fungi and actinomyces in rhizosphere soil was investigated with a diluting flat method and counting technique. The media used for these counts comprised (1) tryptone yeast extract for bacteria; (2) caseinate-asparagine agar for fungi; and (3) potato dextrose agar for actinomycetes. The analysis of urease and acid phosphatase measurements was carried out by the method of Hoffman, Johnson and Hoffman, respectively (Guan 1986). Inorganic nitrogen in soil was measured by the semi-micro Kjeldahl procedure; inorganic phosphate and potassium in soil were by flame photometry; organic matter was carried out by the potassium dichromate oxidation method of Walkley and Black (Liu et al. 1996).
Measurement of nitrogen-fixing activity
Nitrogen-fixing activity of nodulated roots was measured by the acetylene reduction method of Sasakawa (Sasakawa et al. 1986).
Results
Plant dry matter weight and yield
Sugarcane showed the increasing effect in intercropping sugarcane and soybean, but soybean showed a certain degree of plant dry matter loss during intercropping. Results showed that dry weight of biomass and yield under sugarcane/soybean intercropping was increased by 35.44 and 30.57 % for sugarcane, and decreased by 16.12 and 9.53 % (100-grain weight) for soybean, respectively (Fig. 2; Table 1). The total biomass and yield were increased by 21.5 and 10.37 % than monoculture models, respectively (Fig. 2; Table 1). Thus, mixed grown sugarcane and soybean have some degree of advantages in terms of growth and yield.
Rhizosphere soil nitrogen, phosphorus and potassium
Intercropping improved the soil inorganic nitrogen and phosphorus in the intercropping sugarcane and soybean. The inorganic nitrogen content of soybean rhizospheric soil in the intercropping system was 217.02 mg/kg, approximately 66 % higher than those in the monoculture system (Fig. 3). A slight increase in the inorganic nitrogen of intercropping sugarcane was also observed. Inorganic phosphorus content in intercropping sugarcane and soybean was 93.52 and 123.58 mg/kg, approximately 199 and 311.7 % higher than those in the sole plantation, respectively (Fig. 4). Potassium and organic matter contents were also increased slightly in the intercropping system, respectively (Figs. 5, 6).
Soil microorganisms
Photographs of the microbial culture are shown in Figs. 7, 8, and 9. The microbial decomposition of the crop residues could provide nutrients to the plant. Soil microbe numbers varied greatly under different treatments in this study (Table 2). Total soil microbe (bacteria, fungi and actinomyces) number was significantly higher in sugarcane-soybean combinations as compared with both monoculture plantations. The increase of bacteria, fungi, and actinomyces in the intercropping soybean was occurred more remarkably than in the monoculture sugarcane. The quantities of bacteria, fungi, and actinomyces in the intercropping sugarcane were 5.8 × 106, 3.62 × 104 and 6.39 × 105, increased by 42.62, 14.5 and 78.5 %, respectively, while in the intercropping soybean were 9.13 × 106, 3.1 × 104 and 6.78 × 105, increased by 188, 183 and 73 % as compared to that of the monoculture soybean, respectively (Table 2).
Enzyme activities of rhizosphere soil
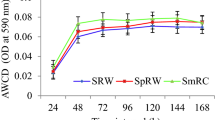
The acetylene reduction activities (ARAs) of the intercropping models were 0.71 μmol C2H −14 plant−1 higher than those in the monoculture soybean (1.02 μmol C2H −14 plant−1) (Fig. 10). The urease in both intercropping models of sugarcane (0.7421 mg/g) and soybean (0.8076 mg/g) were significantly promoted by 89 and 81 % as compared to that of the monoculture models, respectively (Table 3). Intercropping models also showed slightly greater acid phosphatase activity than monoculture models, but not significantly (Table 3).
The correlation of microorganisms quantity and enzyme activity
The results showed that the bacteria quantity in the soil, ranked first, accounts for 91.5 % of total microorganisms during the whole growing period of the seedlings, and then were actinomyces and fungi (Table 2). Further, analysis showed that the total quantity of bacteria, fungi and actinomycete in the intercropping was positively correlated with enzyme activity. The correlation coefficient of bacteria, fungi and actinomycete was 0.8843, 0.5289, 0.958 with urease, and 0.5843, 0.8780, 0.8665 with phosphatase, respectively (Table 4).
Discussion
Previous reports showed that intercropping of cereal and legumes has many advantages in terms of growth and some other agronomical properties (Singh et al. 1986; Putnam et al. 1986). There are also significant handicaps of mixed grown component crops such as root competition for water and nutrients and competition for light (Ofori and Stern 1987). Sathyavely et al. (1991) studied on intercropping sugarcane with pluses and oilseeds, all of which were not significantly different for cane grown either in mixtures or in pure stands. However, our study indicated that the biomass and yield of intercropped sugarcane were significantly higher than that in the monoculture sugarcane, suggesting sugarcane-soybean combinations can promote the growth and yield of sugarcane (Fig. 2; Table 1). By contrast, the biomass and yield of soybean in sugarcane-soybean combinations were lower than that of the monoculture soybean (Fig. 2; Table 1). This reduction may reasonably be attributed to competition for nutrients or water, or a combined effect of these factors. The total biomass and yield of sugarcane-soybean combinations were higher than those of the monoculture system (Fig. 2; Table 1). These suggested that mixed grown sugarcane and soybean have some degree of advantages in terms of growth. The ARAs of the intercropping soybean was significantly higher than those in the monoculture (Fig. 10). This may be due to the higher demand for nitrogen in sugarcane, as soybean itself fixes nitrogen and has a lower demand for nitrogen, and sugarcane roots absorbed more nitrogen, thus stimulating the soybean biological nitrogen fixation.
In this study, soil microbe numbers varied greatly under both intercropping and monoculture treatments (Table 2). Compared with sole plantation of sugarcane or soybean, the numbers of bacteria, fungi and actinomyces were increased significantly. The differences in soil microorganisms might be attributed to a combined factor, such as root biomass, root exudates, root and microclimatic environment of community. The microbial decomposition of the crop residues could provide nutrients to the plant, thus a positive relationship between the abundance of microorganism and nutrient and soil organic matter, which has been confirmed by some studies (Berg et al. 1998; Marschner et al. 2002).
The urease activities in the intercropping system were significantly higher than those in the monoculture system (Table 3). The quantity of microbes was positively correlated with urease and phosphatase activities (Table 4), these results were in accordance with Li et al. (2000). Enzyme activities in soil are important to support those biochemical processes which are essential for the maintenance of soil quality (Moscatelli et al. 2001). Our data showed the inorganic nitrogen in the intercropping system was significantly higher than that of the monoculture system (Fig. 3). This is in agreement with a previous report of Singh et al. (1986). In addition, the phosphorus content had also a significant increase and a slight increase in potassium and organic matter content was also obtained in our experiment. These may be due to the more soil microbes that are present, the more unavailable nitrogen can be more converted into inorganic nitrogen compounds which will be absorbed by plants, accelerating nitrogen transformation, and improving nitrogen utilization. Unavailable nitrogen can be more converted into inorganic nitrogen as the urease activities increase.
In conclusion, intercropped sugarcane with legumes are far more effective than monoculture sugarcane to produce higher shoot dry weight, increase the quantity of microorganism which was positively correlated with enzyme activity, involve in organic matter decomposition and nutrient cycling in intercropping system.
Author contribution
XP Li and H Nian conceived and designed the study; XP Li, YH Mu, YB Cheng and XG Liu performed the experiments; XP Li analyzed data and wrote the paper; H Nian revised the paper. All authors read and approved the final manuscript.
References
Aggarwal PK, Garrity DP, Liboon SP, Morris RA (1992) Resource use and plant interactions in a rice-mungbean intercrop. Agron J 84:71–78
Berg MP, Kniese JP, Verhoef HA (1998) Dynamics and stratification of bacteria and fungi in the organic layers of a Scots pine forest soil. Biol Fertil Soils 26:313–322
Bokhtiar SM, Majid MA, Islam MJ (1995) Fertilizer management for sugarcane-potato intercropping in the Old Himalayan Piedmont Plain soils of Bangladesh. Bangladesh J Sugarcane 17:107–112
Chai Q, Huang P, Huang GB (2005) Effect of intercropping on soil microbial and enzyme activity in the rhizosphere. Acta Pratacul Turae Sin 14(5):105–110
Chowdhury MK, Rosario EL (1994) Comparison of nitrogen, phosphorus and potassium utilization efficiency in maize/mungbean intercropping. J Agric Sci 122:193–199
Dick RP (1994) Soil enzyme activities as indicators of soil quality. In: Doran JW, Coleman DC, Bezdicek DF, Stewart BA (eds) Defining soil quality for a sustainable environment, SSSA Special Publication Number 35. Soil Science Society of America, Madison, pp 107–124
Evan AC (1960) Studies in intercropping. I. Maize or sorghum with groundnuts. East Afr Agric J 26:1–10
Gianfreda L, Bollag JM (1996) Influence of natural and anthropogenic factors on enzyme activity in soil. In: Stotzky G, Bollag JM (eds) Soil biochemistry, vol 9, pp 123–193
Guan SY (1986) Soil enzyme and its analysis method. Agriculture Press, Beijing
He JF, Huang GQ, Liao P, Liu XY, Su YH (2006) Effects on disaster reduction of maize/soybean intercropping ecological system on upland red soil. Meteorol Disaster Reduct Res 29(4):31–35
Huang RH (2002) The primary report of comparative experiments of wide-rows cultivation for sugarcane. Guangxi Sugarcane Canesugar 4:16–17
Imam SA, Hossain AHMD, Sikka LC, Midmore DJ (1990) Agronomic management of potato/sugarcane intercropping and its implication. Field Crop Res 25:111–122
Ledgard SF, Frency JR, Simpson JR (1985) Assessing nitrogen transfer from legumes to associated grasses soil. Boil Biochem 17:575–577
Li L, Li XL, Zhang FS, Yang SC, Lu MJ (2000) Uptake and utilization of nutrition as related to yield advantage in wheat/soybean intercropping. Plant Nutr Fertil Sci 6(2):140–146
Liu GS, Jiang NH, Zhang LD, Liu ZL (1996) Soil physical and chemical analysis and description of soil profiles. In: Sun HL, Liu GS (eds) Standard methods for observation and analysis in Chinese Ecosystem Research Network. Standards Press of China, Beijing, pp 5–40
Marschner P, Marino W, Lieberei R (2002) Seasonal effects on microorganisms in the rhizosphere of two tropical plants in a polyculture agroforestry system in Central Amazonia, Brazil. Biol Fertil Soils 35:68–71
Moscatelli MC, Fonck M, Angelis PD, Larbi H, Macuz A, Rambelli A, Grego S (2001) Mediterranean natural forest living at elevated carbon dioxide: soil biological properties and plant biomass growth. Soil Use Manag 17:195–202
Ofori F, Stern WR (1987) Cereal–legume intercropping systems. Adv Agron 41:41–90
Putnam DH, Herbert SJ, Vargas A (1986) Intercropped corn-soyabean density studies, II. Yield composition and protein. Exp Agric 22:373–381
Ruggiero PDJ, Bollag JM (1996) Soil as a catalytic system. In: Stotzky G, Bollag JM (eds) Soil biochemistry, vol 9, pp 79–122
Sasakawa H, Trung BC, Yoshida S (1986) Stem nodulation on Aeschynomene indica plants by isolated rhizobia. Soil Sci Plant Nutr 32:145–149
Sathyavely A, Chinnasamy K, Rujubekarum S (1991) Studies on intercropping in sugarcane with pulses and oilseeds. SISSTA Sugar J 17:35
Singh NB, Singh PP, Nair KPP (1986) Effect of legume intercropping on enrichment of soil nitrogen, bacterial activity and productivity of associated maize crops. Exp Agric 22:339–344
Tang JC, Mboreha IA, She L, Liao H, Chen HZ, Sun ZD, Yan XL (2005) Nutritional effects of soybean root architecture in a maize/soybean intercropping system. Sci Agric Sin 38(6):1196–1203
Yadav RL, Prasad SR, Singh K (1987) Fertilizer requirement and row arrangement of pulses in sugarcane based cropping systems. Indian J Agron 32:80–84
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 31171508), Special Fund for Agro-scientific Research in the Public Interest (No. 200903002), and Modern Agro-industry Technology Research System (No. CARS-04-PS09).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by O. Ferrarese-Filho.
Rights and permissions
About this article
Cite this article
Li, X., Mu, Y., Cheng, Y. et al. Effects of intercropping sugarcane and soybean on growth, rhizosphere soil microbes, nitrogen and phosphorus availability. Acta Physiol Plant 35, 1113–1119 (2013). https://doi.org/10.1007/s11738-012-1148-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-012-1148-y