Abstract
The aim of this study was to investigate the effects of NaCl-salinity on the physiological attributes in common reed, Phragmites australis (Cav.) Trin. ex Steudel. Plants grew optimally under salinity treatment with standard nutrient solution without added salt and at NaCl concentrations up to 100 mM. Applied for 21 days, NaCl-salinity (300 and 500 mM) caused a significant reduction in growth allocation of all different tissues of P. australis. Shoot growth of reed plants displayed a highly significant correlation with plant–water relations and photosynthetic parameters. The net photosynthetic rate and stomatal conductance of reed plants treated with NaCl-salinity at varying osmotic potential (ψπ) of nutrient solutions were positively correlated, and the former variable also had a strong positive relationship with transpiration rate. Leaf water potential and ψπ followed similar trends and declined significantly as ψπ of watering solutions was lowered. The increase in total inorganic nutrients resulting from increased Na+ and Cl− in all tissues and K+, Ca2+ and Mg2+ concentrations were maintained even at the most extreme salt concentration. Common reed exhibited high K+/Na+ and Ca2+/Na+ selectivity ratios over a wide range of salinities under NaCl-salinity. These findings suggest that reed plants were able to adapt well to high salinities by lowering their leaf ψπ and the adjustment of osmotically active solutes in the leaves.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The deleterious effects of salinity on plant growth are associated with low osmotic potential (ψπ) of soil solution, nutritional imbalance, specific ion effect, hormonal imbalance and induction of oxidative stress, or a combination of these factors (Greenway and Munns 1980; Marschner 1995; Parida and Das 2005). Responses of species to salt stress depend on several interacting variables, including the magnitude (salt concentration and time of exposure) of the stress, plant genotype, plant developmental stage and cultural environment (Sultana et al. 1999; Jaleel et al. 2007). As indicated by Munns (1993), the effect of salinity on plant growth occurs in two-phase model. The first phase of the growth reduction is due to the osmotic stress of the salt outside the roots, the second phase being due to toxic effects, that is to the accumulation of salt inside the plant to toxic levels. However, by comparing two maize lines with different salt-tolerance, Zhao et al. (2010) showed that the growth response was simultaneously inhibited by ionic and osmotic effects for the two-phase model. Salt tolerance is not exclusively correlated with adaptation to Na+ or Cl− toxicity per se but also reflects adaptations to secondary effects of salinity (Munns 2002). Potassium uptake is particularly important due to the chemical similarities between Na+ and K+, which makes difficult the discrimination between the two ions by transport proteins (Blumwald et al. 2000). Thus, high levels of Na+, or high Na+:K+ ratios can disrupt various enzymatic processes in the cytoplasm. Therefore, one of the key elements in salinity tolerance is the ability to maintain a high cytosolic K+/Na+ selectivity (S K/Na) (Yeo 1998; Maathuis and Amtmann 1999; Blumwald et al. 2000).
Plant biomass production depends on the accumulation of carbon products through photosynthesis, but elevated salinity can adversely affect photosynthesis (Zhu 2001; Ashraf 2003). A decline in photosynthetic capacity of many plant species in response to salt stress has been ascribed to either stomatal, restricting CO2 entry into leaves (Steduto et al. 2000; Meloni et al. 2003), or non-stomatal limitation that results in inhibition or down-regulation of photosynthesis (Dunn and Neales 1993; Sultana et al. 1999). The presence of salt decreases the ψπ of the medium, so plants have problems with respect to absorption of water. In order to compensate for the negative values of the nutrient solution, plants have to decrease their water potential (ψw); this involves a decrease of the ψπ, to maintain turgor and achieve osmotic adjustment (Blum et al. 1996). Salinity reduces the ability of plants to take up water, causing a reduction in growth along with a suite of metabolic changes (Munns 2002). A metabolic response to salt stress is the synthesis of compatible osmolytes (Ashraf and Foolad 2007). These mediate osmotic adjustment and therefore protect sub-cellular structures and reduce oxidative damage caused by free radicals, produced in response to high salinity (Zhu 2001).
Phragmites australis (Cav.) Trin. ex Steudel (synonymous to P. communis Trin.), common reed, is a widespread species in the temperate regions of the world (Den Hartog et al. 1989). Its typical habitats are fresh and brackish water areas of swamps, riversides, and lakesides. It is often the key-species in wetland ecosystems and propagates both through rhizome growth and seed germination in sparsely populated patches (Gorai et al. 2006). However, reed plants have adapted to terrestrial habitats, and various ecotypes have evolved with resistance to drought, salinity, and low temperature (Matoh et al. 1988; Wang et al. 1998; Pagter et al. 2005; Gorai et al. 2007; Engloner 2009; Gorai 2009). Among these, salinity is a well-known stressor of P. australis, leading to reduced vigour and success in brackish and salt marshes (Burdick et al. 2001). The aim of the present study was to investigate, for P. australis plants grown under greenhouse conditions, whether NaCl-salinity at varying ψπ of nutrient solutions was related to growth, leaf gas exchange, water (and ion) relations and osmotic adjustment. Additionally, the correlation between growth and different physiological attributes was evaluated.
Materials and methods
Plant material and culture conditions
Seeds of Phragmites australis were collected in November 2003 from a saline location in Zerkine, Gabès (33°43′N, 10°16′E; southeast Tunisia). One thousand seeds weighed, on average, 100 mg (Gorai et al. 2007). This area is arid to semi-arid with a typical Mediterranean climate, characterized by irregular rainfall events and a harsh dry summer period. Annual rainfall is around 187 mm and annual mean evapo-transpiration 996 mm. Mean annual temperature is 19.3°C with a minimum temperature 5.9°C in January and 32.7°C maximum in August.
Seeds were surface sterilized with sodium hypochlorite solution for 1 min and germinated on filter paper in 90 mm Petri dishes at controlled conditions (Gorai et al. 2006). Seedlings were transferred to 3 l-plastic tanks for hydroponic growth, using aerated Hewitt nutrient solution (Hewitt 1966), containing macronutrients (mM): MgSO4 (1.5), KH2PO4 (1.6), K2HPO4 (0.4), KNO3 (3), NH4NO3 (2), Ca(NO3)2 (3.5). The medium contained also iron as complex EDTA–K–Fe (Jacobson 1951) and micronutrients as a mixture of salts: MnCl2; CuSO4, 5H2O; ZnSO4, 7H2O; Mo7O24(NH4)6, 4H2O and H3BO3 (Arnon and Hoagland 1940). Plants were grown in a growth chamber under the following conditions: 25 ± 1°C temperature, 50% day and 75% night relative humidity and 16 h light/8 h dark regime with 250 μmol m−2 s−1 photosynthetic active radiations (PAR). The nutrient solutions were replaced after every 3rd day. The solution pH was adjusted to 6.5 ± 0.1 every day with NaOH or HCl, as required.
The experiment was arranged in a growth chamber in a completely randomized design with four NaCl-salinity treatments × six replicates. Plants were supplied with a control nutrient solution (0 mM NaCl, ψπ = −0.04 MPa,) or saline nutrient solutions (100, 300 and 500 mM NaCl) at varying ψπ of −0.53, −1.51 and −2.49 MPa, respectively. The osmolality of the solutions was analysed using a vapour pressure osmometer (Wescor 5520, Logan, UT, USA). To avoid osmotic shock, the final salt concentration (100, 300 or 500 mM) was progressively adjusted by adding NaCl of 50 mM twice daily. Two harvests were made, at the beginning of treatment (2-month-old plants) and 21 days later. At the harvests, leaves, stems and roots separated from rhizomes were successively rinsed three times in cold water and blotted between two layers of filter-paper. The fresh mass (FM) was measured immediately, and the dry mass (DM) after 48 h of desiccation in an oven at 60°C. Plant relative growth rate (RGR) was determined as RGR = ΔM/M Δt, where Δ is the difference between values at the final and initial harvests, t is the time (days) and M is the whole plant DM (g). M is the logarithmic mean of M calculated over the Δt period (Hunt 1990): M = ΔM/Δ ln(M).
Leaf water relations
The water content (WC) of different tissues was determined as WC (g H2O g−1 DM) = (FM − DM)/DM. Leaf ψw was measured using a pressure chamber (PMS Instruments Co., Corvallis, OR, USA) after 21 days of salt treatment, according to Scholander et al. (1965). After measuring of ψw, the samples were frozen in liquid nitrogen and stored at −20°C. Leaf tissues were thawed and centrifuged at 1,200×g for 25 min at 4°C to extract the cell sap. A vapour pressure osmometer (Wescor 5520, Logan, UT, USA) was used to determine osmolality of the sap expressed from leaves, which was converted to ψπ, by the van’t Hoff equation: ψπ = −ciRT, where ci is the value reading from the instrument, R is the ideal gas constant and T is the absolute temperature (Nobel 1991). Turgor potential (ψp) was determined using the relationship: ψp = ψw − ψπ.
CO2 and H2O gas-exchange
After 21 days at 0, 100, 300 and 500 mM NaCl, photosynthetic gas exchange parameters were measured between 10:00 and 12:00 h using an LCpro + portable photosynthesis system (ADC, BioScientific Ltd, UK). The CO2 concentration in the leaf chamber was set at 360 μmol mol−1. The leaf was irradiated with PAR of 1,500 μmol m−2 s−1 of internal light source. The third youngest fully expanded leaf was used for these measurements. Readings were logged every 30 s until stable values for net photosynthetic rate (P N), stomatal conductance (g s), transpiration rate (E), and internal CO2 concentration (C i) were reached. The water use efficiency (WUE) and intrinsic WUE (WUEi) were calculated as P N/E and P N/g s, respectively.
Organic and inorganic solute determination
The proline was quantified spectrophotometrically by the ninhydrin method according to Bates et al. (1973). The plant material was homogenized in 3% aqueous sulfosalicylic acid and the homogenate was centrifuged at 14,000 rpm. The supernatant was used for the estimation of the proline concentration. The reaction mixture consisted of 2 ml of acid ninhydrin and 2 ml of glacial acetic acid, which was boiled at 100°C for 1 h. After termination of reaction in ice bath, the reaction mixture was extracted with 4 ml of toluene, and absorbance was read at 520 nm. The leaf proline concentration was expressed on dry weight basis.
Ions were extracted from dried, milled plant material with nitric acid (HNO3, 0.5%). Concentrations of Na+, K+, Ca2+ and Mg2+ were determined using an atomic absorption spectrophotometer (Schimazu AA 6800, Schimazu Crop, Kyoto, Japan), while Cl− concentration was determined on the same extract with a chloride meter (Jenway PC LM3, London, UK).
K+/Na+ and Ca2+/Na+ selectivity ratios
The selectivity ratios of K+ and Ca2+ over Na+ (S K/Na, S Ca/Na) for accumulation, uptake and transport were estimated as: [K or Ca/Na]whole plant/[K or Ca/Na]medium, [K or Ca/Na]root/[K or Ca/Na]medium and [K or Ca/Na]shoot/[K or Ca/Na]root, respectively (Gorai et al. 2010). K, Ca and Na represent, respectively, the quantity found in the whole plant (accumulation), roots (uptake) and shoots (transport). In the medium and roots, rather it is concentration.
Statistical analysis
Data were analysed using SPSS statistical package (SPSS 2002). Data were tested for normal distribution using the Shapiro–Wilk Test, and heterogeneities of variance within treatments were tested using Levene’s Test. When necessary, log transformations were used to normalize distributions. Student–Newman–Keuls test was used to estimate least significant range between means. Correlation coefficients between data were examined using Pearson’s correlation coefficient at or below the 5% significance level.
Results
Growth analysis
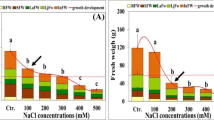
A one-way ANOVA on the biomass production of P. australis indicated that salinity significantly affected DM (P < 0.001). Root dry mass increased significantly in salinity of 100 mM NaCl, but yield of roots progressively declined with further increases in salinity (Fig. 1a). The RGR followed a similar trend that dry matter allocation and decreased significantly in all tissues (P < 0.001) at the highest NaCl concentration. At all levels of salinity, leaves had the lowest growth activity. Root growth was less sensitive to NaCl than was shoot growth, translating a decrease in dry matter allocation to the shoots (Fig. 1b). At 500 mM NaCl, the fresh:dry weight ratio for all tissues was significantly decreased compared with lower salinity and we recorded 60, 59 and 65% of controls, respectively, for leaves, stems and roots (Fig. 1c).
Changes in tissue a dry mass, b relative growth rate, c fresh:dry weight ratio and d water content of Phragmites australis when 2-month old plants were subjected for 21 days to salinity at varying NaCl concentrations (0, 100, 300 or 500 mM) in nutrient solution. Data represent means ± SE (n = 6). Different letters indicate significant differences between treatments at P < 0.05 according to the Student–Newman–Keuls test
Water relations
Salinity significantly affected tissue WC of P. australis tissues (P < 0.001). In all treatments, WC was lower in stems and leaves than in roots. Root WC increased slightly at 100 mM NaCl, but declined at higher salinities (Fig. 1d). All leaf water relation parameters decreased with increasing NaCl concentration (Fig. 2a–c). A one-way ANOVA of the water status of P. australis revealed that salinity significantly affected ψw, ψπ and ψp (P < 0.001) of salinized plant leaves relative to control ones. Linear regression analysis was used to determine the relationships between leaf water relations and ψπ of the watering solutions. There was a strong positive relationship between ψπ of solutions and ψw or ψπ of leaves (Fig. 2a, b), with R 2 values 0.96 and 0.95, respectively, while a negative correlation was identified between ψπ of solutions and leaf ψp, with R 2 = 0.66 (Fig. 2c). This decrease in water relations of P. australis plants under salt stress indicated dehydration and turgor loss.
Regression plots for a water potential, ψw; b osmotic potential, ψπ; and c turgor potential, ψp in leaves of Phragmites australis when 2-month-old plants were subjected for 21 days to salinity with a standard nutrient solution (ψπ = −0.04 MPa) or NaCl solutions of various osmotic potentials (−0.53, −1.51 or −2.49 MPa). Lines describing the evolution of each parameter were obtained using a linear regression. Values are from the four treatments with six replicates (n = 24)
CO2 exchange, transpiration and stomatal conductance
The P N, g s, E and C i were significantly affected by NaCl treatment (Fig. 3a–d). There was a significant negative relationship between water potential of solutions and P N, g s, E and C i, with coefficient of correlation (R 2) values of 0.75, 0.79, 0.78 and 0.46, respectively. The P N significantly decreased with increase in NaCl concentration (P < 0.001). It was reduced from 10.9 μmol m−2 s−1 in control plants to an approximately fivefold lower rate in the most stressed plants (Fig. 3d). The C i was slightly reduced in salinized plants, as compared with controls. This concentration did not differ statistically between control plants and those at 100 mM NaCl (Fig. 3a). The E was severely decreased with increase in NaCl concentration in the nutrient solution. The highest rate was recorded in control plants (3.46 mmol m−2 s−1), and approximately sevenfold lower rate in plants stressed at 500 mM NaCl (Fig. 3b). The decrease in g s was more than those in P N and E with increasing NaCl concentration (Fig. 3c). As illustrated in Fig. 3e and f, salinity treatment with decreasing ψπ of solutions had a significant effect on WUEi (P < 0.05, R 2 = 0.36) as compared with controls; however, the WUE values did not differ significantly (P > 0.05, R 2 = 0.10).
Changes in a internal CO2 concentration, C i; b transpiration rate, E; c stomatal conductance, g s; d net photosynthetic rate, P N; e water use efficiency, WUE; and f intrinsic WUE, WUEi of Phragmites australis when 2-month-old plants were subjected for 21 days to salinity with a standard nutrient solution (ψπ = −0.04 MPa) or NaCl solutions of various osmotic potentials (−0.53, −1.51 or −2.49 MPa). Lines describing the dependencies were obtained using a linear regression. Values are from four treatments with six replicates (n = 24)
Changes in Na+ (a), Cl− (b), K+ (c), Ca2+ (d) and Mg2+ (e) concentrations in leaves, stems and roots and proline (f) concentration in leaves of Phragmites australis grown on nutrient solution supplied with different NaCl concentrations (0, 100, 300 or 500 mM). Concentration was calculated on a tissue water basis. Data represent means ± SE (n = 6). Different letters indicate significant differences between treatments at P < 0.05 according to the Student–Newman–Keuls test
Correlations between growth and physiological attributes
Table 1 shows the correlation coefficients between growth and different physiological attributes. As expected, high correlation coefficients were found between growth (shoot DM and shoot RGR) and all photosynthetic parameters of P. australis plants except WUE, when exposed to salinity at varying ψπ of watering solutions (Table 1). Additionally, the correlation coefficients between shoot DM and leaf water relations were significantly high, suggesting strong relationships (shoot DM × ψw, R 2 = 0.92; shoot DM × ψπ, R 2 = 0.92; shoot DM × ψp, R 2 = 0.72).
Inorganic and organic solute concentrations
A two-way ANOVA showed significant individual effects of plant tissue, salinity treatment and their interactions on ion concentrations (Table 2). Reed plants cultivated under varying NaCl concentrations showed significantly higher Na+ accumulation in the stems (P < 0.001), as compared with that in the leaves (P < 0.001) and the roots (P < 0.001) (Fig. 4a). Stem Na+ concentration ranged from 0.018 to 0.814 mol l−1 at 100 and 500 mM NaCl, respectively, whereas those of leaves and roots varied from 0.017 to 0.518 mol l−1 and 0.011 to 0.378 mol l−1, respectively (Fig. 4a). Chloride concentration showed similar changes, but at lower levels than sodium (P values < 0.001; Fig. 4b). Potassium concentration in leaves significantly increased at 500 mM NaCl, but there were no differences in both stems and roots with increasing NaCl supply (Fig. 4c). As to Ca2+ and Mg2+ accumulation, NaCl treatment had no effect on concentrations of these ions in leaves at salinity levels of 0–300 mM NaCl, and differences were significantly increased at the highest concentration. Increasing NaCl-salinity had no adverse effect on Ca2+ and Mg2+ concentrations in stems and roots and high amounts were reached at a NaCl concentration of 500 mM (Fig. 4d–e).
As shown in Fig. 4f, proline concentration in leaves of P. australis was significantly increased by solutions of increased osmolality (P < 0.001). The leaves of plants subjected to 500 mM NaCl accumulated sevenfold more proline than the controls.
Figure 5 shows the selective accumulation of K+ and Ca2+ over Na+ estimated by the K+/Na+ and Ca2+/Na+ ratios of these ions in the culture medium and P. australis organs. The S K/Na and S Ca/Na for accumulation ranged from 27.78 to 34.70 and from 6.01 to 9.52 at 100 and 500 mM NaCl, respectively, whereas that for uptake varied from 16.2 to 20.6 and from 2.36 to 5.62, respectively. However, the S K/Na and S Ca/Na for transport in shoots were lower than those of uptake and accumulation (Fig. 5).
Changes in the K+/Na+ and Ca2+/Na+ selectivity ratios for accumulation, uptake and transport in Phragmites australis when 2-month-old plants were subjected for 21 days to salinity at varying NaCl concentrations (0, 100, 300 or 500 mM) in nutrient solution. Data represent means ± SE (n = 6). Different letters indicate significant differences between treatments at P < 0.05 according to the Student–Newman–Keuls test
Discussion
Common reed’s growth decreased progressively with increase in NaCl concentration in the nutrient solution. This was in agreement with previous reports (Gorai et al. 2007, 2010; Pagter et al. 2009). The depressive effect of salinity on plant growth is commonly due to both osmotic and ion-specific effects (Greenway and Munns 1980; Munns 2002). It has been reported that reductions in growth depend on the period of time over which the plants have grown in saline conditions, leading to the Munns’ two-phase hypothesis in response to salt stress. During the period of treatment, reed plants showed a two-phase growth response to salinity.
Several research reports that plants grown under salt stress manifest acclimation to success their establishment, by lowering both leaf ψw and ψπ (Sultana et al. 1999; Koyro 2006). Osmotic adjustment by net accumulation of solutes in cells in response to a fall in the ψw of their environment can in part offset this deterioration of growth conditions. As a consequence of this net accumulation, the cell ψπ is lowered, and turgor pressure tends to be maintained (Blum et al. 1996). In the present experiment, increasing salinity was accompanied by a decrease in WC, leaf ψw and ψπ of reed plants. Thus, turgor could be maintained, and obviously the osmotic adjustment was sufficient to compensate the reduction in leaf ψw in these reed plants growing under salinity conditions. According to Gorai et al. (2010), leaf ψw and ψπ of reed plants exposed to hypoxia at varying NaCl-salinity concentrations declined significantly as ψπ of watering solutions was lowered.
Photosynthetic activity is one of the major factors controlling growth (Tezara et al. 2002; Ashraf 2004). The P N presented here clearly shows a correlation with shoot growth of P. australis treated with increased osmolarity of solutions. The present study depicts that P N and g s of P. australis under salt stress were positively associated and the former variable also had a strong positive relationship with E. A similar result was obtained by Pagter et al. (2009) showing that stomatal conductance in P. australis was negatively influenced by decreasing ψπ of nutrient solutions caused by NaCl or Na2SO4. Choi et al. (2005) showed that carbon isotope discrimination in P. australis grown in a constructed saline wetland decreased as salinity increased, indicating a decline in stomatal and/or mesophyll conductance. Working on the same species, Wang et al. (1998), Lissner et al. (1999), Pagter et al. (2009) and Gorai et al. (2010) showed that increasing osmolarity of growth medium affects P N not only due to its effects on stomatal regulation but also due to other non-stomatal responses. In reed plants subjected to hypoxia at various ψπ, Gorai et al. (2010) showed no clear relationships between growth and photosynthetic parameters except for gs, whereas growth displayed a highly significant correlation with plant-water relations.
Sodium contribution to the total amount of cations (Na+, K+, Ca2+ and Mg2+) became important with an increase in NaCl-salinity level and more marked in the shoots, as compared with the roots. There are several reports on considerable inhibition of nutrient uptake, notably K+, Ca2+ and Mg2+ uptake under salinity (Marschner 1995; Munns 2002; Netondo et al. 2004). The common reed, in contrast, showed increased accumulation of shoot K+ and root and shoot Ca2+ and Mg2+ concentrations, and therefore able to adapt well to high salinities. The present data agree with findings reported on Cynodon dactylon (Hameed and Ashraf 2008) in which concentrations of K+ and Ca2+ in the roots as well as shoots increased with an increase of salt level. Phragmites australis appears, thus, able to maintain a high S K/Na for accumulation in the whole plant and the uptake by roots; however, the transport in shoots reached lower values when salinity increases in the medium. Many studies on halophytes and some tolerant glycophytes plants showed that a high S K/Na for transport is a salt tolerance criterion (Gorham et al. 1990; Shachtman et al. 1991; Wolf et al. 1991; Yeo 1998). Selectivity ratio for transport of K+ over Na+ in Cakile maritima, a halophytic species occurring on dunes along the Tunisian seashore (Debez et al. 2004) was 3.5-fold higher than in common reed (present study) at a NaCl concentration of 500 mM. Yeo (1998) suggested that dry matter production is proportional to leaf K+ nutrition. The capacity of plants to counteract salinity stress strongly depends on the status of their potassium nutrition (Zhu 2001). The ability of reed species to maintain a substantial growth rate under saline conditions is directly related to an efficient S K/Na and S Ca/Na. Moreover, P. australis tolerance was due to its capacity to limit Na+ transport and to enhance K+ supply to the shoots. Between different ecotypes of reed plants differences in NaCl tolerance have been ascribed to differences in ion selectivity of a high-affinity plasma membrane K+ transporter resulting in higher K/Na ratios in salt-tolerant than in salt-sensitive ecotypes (Takahashi et al. 2007). Common reed has an efficient mechanism of Na+ exclusion from the leaves and exhibited a high leaf S K/Na over a wide range of salinities under hypoxia treatment (Gorai et al. 2010).
Proline is a compatible solute that accumulates in response to osmotic stress, and the accumulation of this osmolyte represents an important adaptive response to salt and drought stress (Ashraf and Harris 2004; Parida and Das 2005). Reed plants subjected to salt stress accumulated sevenfold more proline than controls. There was a strong positive correlation between proline concentration and osmolality in the leaves of P. australis in response to NaCl concentrations in the culture medium.
Overall, common reed’s growth declined significantly as water potential of watering solutions was lowered. Shoot growth was positively associated with a decrease in leaf gas exchange characteristics and water potential components. The g s was reduced linearly as ψπ of solutions was decreased and influenced accordingly P N and E. Plants were able to adapt well to salinity at varying ψπ of nutrient solutions by lowering their leaf ψπ. Reduction in the leaf ψw was achieved by the adjustment of osmotically active solutes in the leaves.
Abbreviations
- C i :
-
Internal CO2 concentration
- DM:
-
Dry mass
- E :
-
Transpiration rate
- FM:
-
Fresh mass
- g s :
-
Stomatal conductance
- P N :
-
Net photosynthetic rate
- RGR:
-
Relative growth rate
- S K/Na :
-
K+/Na+ selectivity ratio
- S Ca/Na :
-
Ca2+/Na+ selectivity ratio
- ψπ :
-
Osmotic potential
- ψp :
-
Turgor potential
- ψw :
-
Water potential
- WUE:
-
Water use efficiency
- WUEi :
-
Intrinsic WUE
References
Arnon DI, Hoagland DR (1940) Crop production in artificial solutions and in soils with special reference to factors affecting yields and absorption of inorganic nutrient. Soil Sci 50:463–484
Ashraf M (2003) Relationships between leaf gas exchange characteristics and growth of differently adapted populations of Blue panicgrass (Panicum antidotale Retz.) under salinity or waterlogging. Plant Sci 165:69–75
Ashraf M (2004) Some important physiological selection criteria for salt tolerance in plants. Flora 199:361–376
Ashraf M, Foolad MR (2007) Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ Exp Bot 59:206–216
Ashraf M, Harris PJC (2004) Potential biochemical indicators of salinity tolerance in plants. Plant Sci 166:3–16
Bates S, Waldren RP, Teare ID (1973) Rapid determination of the free proline in water stress studies. Plant Soil 39:205–208
Blum A, Munns R, Passioura JB, Turner C (1996) Genetically engineered plants resistant to soil drying and salt stress: how to interpret osmotic relations? Plant Physiol 110:1051–1053
Blumwald E, Aharon GS, Apse MP (2000) Sodium transport in plant cells. Biochim Biophys Acta 1465:140–151
Burdick DN, Buchsbaum R, Holt E (2001) Variation in soil salinity associated with expansion of Phragmites australis in salt marshes. Environ Exp Bot 46:247–261
Choi WJ, Ro H-M, Chang SX (2005) Carbon isotope composition of Phragmites australis in a constructed saline wetland. Aquat Bot 82:27–38
Debez A, Ben Hamed K, Grignon C, Abdelly C (2004) Salinity effects on germination, growth and seed production of the halophyte Cakile maritima. Plant Soil 262:179–189
Den Hartog C, Kvet J, Sukopp H (1989) Reed A common species in decline. Aquat Bot 35:1–4
Dunn GM, Neales TF (1993) Are the effects of salinity on growth and leaf gas-exchange related. Photosynthetica 29:33–42
Engloner AI (2009) Structure, growth dynamics and biomass of reed (Phragmites australis): a review. Flora 204:331–346
Gorai M (2009) Effects of salinity and hypoxia on physiological behavior of the common reed (Phragmites australis (Cav.) Trin. ex Steud.). PhD. Thesis. Tunis El Manar University, Faculty of Sciences of Tunis, Tunisia (in French)
Gorai M, Vadel MA, Neffati M (2006) Seed germination characteristics of Phragmites communis: effects of temperature and salinity. Belg J Bot 139:78–86
Gorai M, Vadel AM, Neffati M, Khemira H (2007) The effect of sodium chloride salinity on the growth, water status, and ion content of Phragmites communis Trin. Pak J Biol Sci 13:2225–2230
Gorai M, Ennajeh M, Khemira H, Neffati M (2010) Combined effect of NaCl-salinity and hypoxia on growth, photosynthesis, water relations and solute accumulation in Phragmites australis plants. Flora 205:462–470
Gorham J, Bristol A, Yopung EM, Wyn Jones RG, Kashour G (1990) Salt tolerance in the Triticeae: K/Na Discrimination in Barley. J Exp Bot 41:1095–1101
Greenway H, Munns R (1980) Mechanisms of salt tolerance in nonhalophytes. Ann Rev Plant Physiol 31:149–190
Hameed M, Ashraf M (2008) Physiological and biochemical adaptations of Cynodon dactylon (L.) Pers. from the salt range (Pakistan) to salinity stress. Flora 203:683–694
Hewitt EJ (1966) Sand and water culture methods used in the study of plant nutrition. Commonw Bur Hortic Tech Com 22:431–446
Hunt R (1990) Basic growth analysis. Plant growth analysis for beginners. Unwin Hyman, London
Jacobson L (1951) Maintenance of iron supply in nutrient solutions by a single addition of ferric-potassium-ethylene-diamine-tetracetate. Plant Physiol 26:411–413
Jaleel CA, Gopi R, Sankar B, Manivannan P, Kishorekumar A, Sridharan R, Panneerselvam R (2007) Alterations in germination, seedling vigour, lipid peroxidation and proline metabolism in Catharanthus roseus seedlings under salt stress. S Afr J Bot 73:190–195
Koyro HW (2006) Effect of salinity on growth, photosynthesis water relations and solute composition of the potential cash crop halophyte Plantago coronopus (L.). Environ Exp Bot 56:136–146
Lissner J, Schierup HH, Comín FA, Astorga V (1999) Effect of climate on salt tolerance of two Phragmites australis population. II. Diurnal CO2 exchange and transpiration. Aquat Bot 64:335–350
Maathuis FJM, Amtmann A (1999) K+ nutrition toxicity: the basis of cellular K+/Na+ ratios. Ann Bot 84:112–133
Marschner H (1995) Mineral nutrition of higher plants. Academic Press, London
Matoh T, Matsushita N, Takahashi E (1988) Salt tolerance of the reed plant Phragmites communis. Physiol Plant 72:8–14
Meloni DA, Oliva MA, Martinez CA, Cambraia J (2003) Photosynthesis and activity of superoxide dismutase, peroxidase and glutathione reductase in cotton under salt stress. Environ Exp Bot 49:69–76
Munns R (1993) Physiological processes limiting plant growth in saline soil: some dogmas and hypotheses. Plant Cell Environ 16:15–24
Munns R (2002) Comparative physiology of salt and water stress. Plant Cell Environ 25:239–250
Netondo GW, Onyangoa JC, Beck E (2004) Sorghum and salinity, I: response of growth, water relations, and ion accumulation to NaCl salinity. Crop Sci 44:797–805
Nobel PS (1991) Physicochemical and environmental plant physiology. Academic Press, San Diego
Pagter M, Bragato C, Brix H (2005) Tolerance and physiological responses of Phragmites australis to water deficit. Aquat Bot 81:285–299
Pagter M, Bragato C, Malagoli M, Brix H (2009) Osmotic and ionic effects of NaCl and Na2SO4 salinity on Phragmites australis. Aquat Bot 90:43–51
Parida AK, Das AB (2005) Salt tolerance and salinity effects on plants: a review. Ecotoxicol Environ Saf 60:324–349
Scholander PF, Hammel HT, Bradstreet ED, Henningsen EA (1965) Sap pressure in vascular plants. Science 148:339–346
Shachtman D, Munns R, White Cross MI (1991) Variation in sodium exclusion and salt tolerance in Triticum tauschii. Crop Sci 31:992–997
SPSS (2002) SPSS 11.5 for windows update. SPSS Inc, Chicago
Steduto P, Albrizio R, Giorio P, Sorrentino G (2000) Gas-exchange response and stomatal and non-stomatal limitations to carbon assimilation of sunflower under salinity. Environ Exp Bot 44:243–255
Sultana N, Ikeda T, Itoh R (1999) Effect of NaCl salinity on photosynthesis and dry matter accumulation in developing rice grains. Environ Exp Bot 42:211–220
Takahashi R, Nishio T, Ichizen N, Takano T (2007) Cloning and functional analysis of the K+ transporter, PhaHAK2, from salt-sensitive and salt-tolerant reed plants. Biotechnol Lett 29:501–506
Tezara W, Mitchell V, Driscoll SP, Lawlor DW (2002) Effects of water deficit and its interaction with CO2 supply on the biochemistry and physiology of photosynthesis in sunflower. J Exp Bot 53:1781–1791
Wang HL, Hao LM, Wen JQ, Zhang CL, Liang HG (1998) Differential expression of photosynthesis-related genes of reed ecotypes in response to drought and saline habitats. Photosynthetica 35:61–69
Wolf O, Munns R, Tounet ML, Jeschke WD (1991) The role of the stem in the partitioning of Na+ and K+ in salt-treated barley. J Exp Bot 42:697–704
Yeo AR (1998) Molecular biology of salt tolerance in the context of whole-plant physiology. J Exp Bot 49:915–929
Zhao KF, Song J, Fan H, Zhou S, Zhao M (2010) Growth response to ionic and osmotic stress of NaCl in salt-tolerant and salt-sensitive Maize. J Integr Plant Biol 52:468–475
Zhu JK (2001) Plant salt tolerance. Trends Plant Sci 6:66–71
Acknowledgments
We are thankful to the journal associate editor Prof. Sylvie Renault and three anonymous referees for their constructive comments on earlier versions of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by S. Renault.
Rights and permissions
About this article
Cite this article
Gorai, M., Ennajeh, M., Khemira, H. et al. Influence of NaCl-salinity on growth, photosynthesis, water relations and solute accumulation in Phragmites australis . Acta Physiol Plant 33, 963–971 (2011). https://doi.org/10.1007/s11738-010-0628-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-010-0628-1