Abstract
Biosynthesis of six saponins (ginsenosides) in suspension culture of P. quinquefolium Z5 was investigated. Ginsenoside content in biomass reached the highest level, nearly 30 mg g−1 d.w., between 25 and 30 days of the culture. Saponins were synthesized simultaneously with cell growth but their synthesis rate was not proportional to the growth rate. During the phase of rapid biomass multiplication, after which biomass reached 90% of its maximum yield, only half examined ginsenosides was produced. The second half of the final saponins yield was produced during the slow growth phase, in which only 10% of biomass was grown. During the intensive growth phase the productivity of six saponins examined per biomass (dry weight) unit was 3.4 μg mg−1 d.w. day−1, however, this parameter calculated for slow growth phase reached nearly 30 μg mg−1 d.w. day−1. There were differences in increase of the contents of six saponins determined in biomass, and it was the highest for saponins Re (20(S)-protopanaxatriol-6-[O-α-l-rhamnopyranosyl(1 → 2)-β-d-glucopyranoside]-20-O-β-d-glucopyranoside) and Rg1 (20(S)-protopanaxatriol-6,20-di-O-β-d-glucoside).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
A North American Panax—P. quinquefolium (Araliaceae), similarly to the most famous known representative of the Panax genus—P. ginseng, contains ginsenosides—triterpene saponins. These metabolites are responsible for medical activity of Panax, i.e., adaptogenic effect and antistress effect (Rai et al. 2003; Lee et al. 2006), neuroprotective activity (Lian et al. 2005; Naval et al. 2007), immunological activity (Belegortsera et al. 2000; Keith and Block 2003; Predy et al. 2005). Ginsenosides (panaxosides) are glycosides in which aglycon part may be: protopanaxadiol, protopanaksatriol or oleanolic acid. A carbohydrate part is built from 3 to 6 residues of sugars, such as glucose, arabinose, rhamnose, xylose or glucuronic acid. In the present article, dynamics of ginsenosides production in the cell suspension culture of P. quinquefolium Z5 is presented.
Materials and methods
Plant material
Plant material used for initiation of in vitro cultures of Panax quinquefolium originated from the plantation of the University of Agriculture in Lublin (Poland). Seeds of P. quinquefolium used for the plantation initiation were supplied by Prof. Bryan F. Zilkey (Dehli Station, Ontario, Canada), where this plant was identified. Suspension culture of the selected cell line Z5 of P. quinquefolium was initiated from callus after IX passage, growing in dark on medium MS with 2,4-D 1 mg/l, NAA 1 mg/l, BAP 0.5 mg/l and 50 g/l sucrose.
Culture conditions
Suspension culture was conducted in Erlenmeyer flasks (300 ml) containing 50 ml of a liquid MS medium with 2,4-D 0.2 mg/l and TDZ 0.002 mg/l, pH 5.6–5.8. The medium was sterilized in temperature of 123°C and 1 atm pressure for 16 min. The flasks were placed on a rotary shaker (100 rpm), 26 ± 2°C temperature, 90% humidity, light 40 μE m−2 s−1 for 40 days. Average fresh biomass of inoculum was 9.56 g/l, and average dry weight was 0.86 g/l.
Biomass from 5, 10, 15, 20, 25, 30, 35 and 40 days of cultivation was separated through filtration (using vacuum pomp) and dried at a room temperature. This material was used for ginsenosides extraction.
Extraction procedure
Samples of 1 g of dry raw material (weighed to 0.1 g tolerance) were placed in the 250 ml flasks. They were extracted three times with 50 ml of 80% methanol for 30 min at the solvent’s boiling temperature under reflux condenser. Combined methanol extracts were evaporated until dryness in vacuum evaporator under lowered pressure at 60°C. The flask with dried residues was placed in a desiccator filled with a drying agent. The dried methanol extract was weighed.
Ginsenoside analysis using HPLC methods
Dried extracts were dissolved in 2 ml of methanol (HPLC grade) and filtered through a 0.2 μm pore diameter Millex®-FG Hydrophobic Fluoropore filters (PTFE). Then aliquots of 20 μl were introduced to liquid chromatography system consisting of LiChroART® 250-4, Waters 600 Controller pump and UV–Vis Waters 996 detector combined with Pentium 60 PC hardware equipped with Millenium software. Two different mixtures of acetonitrile with water were used as eluent. Acetonitrile to water ratio 30:70 was used for determination of the ginsenosides Rb1, Rb2, Rc, Rd (flow rate 2 ml/min, analysis time 45 min), and 18:82 ratio was used for determination of the ginsenosides Rg1 and Re (flow rate 3 ml/min, analysis time 40 min). Ginsenoside detection was made at 203 nm wavelength.
Results
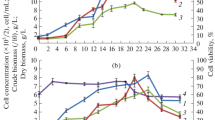
The presence of six ginsenosides, i.e., Rb1, Rb2, Rc, Rd, Re and Rg1 in biomass of growing cell culture of P. quinquefolium Z5 was determined. Content of ginsenosides in 1 g of dry biomass increased reaching the highest level after 25–30 days culture. The content of two metabolites Rg1 and Re (both belong to the Rg group) showed the highest increase from about 2.8 to 7.6 mg g−1 d.w. and 3.4 to 8 mg g−1 d.w., respectively. Contents of the other saponins had minor increase dynamics—from 0.8–1.6 mg g−1 d.m. to 2.4–4.8 mg g−1 d.w. (Table 1). The total content of examined ginsenosides increased nearly 3-fold from over 11 mg g−1 d.m. to over 29 mg g−1 d.m. Prolongation of the P. quinquefolium culture beyond the growth phase caused great decrease of the saponin level (Figs. 1, 2).
Dynamics for both the cell growth and saponins biosynthesis was determined (Fig. 1). Ginsenosides production accompanied the biomass increase, however, during the most intensive biomass growth between 10 and 20 days of culture (linear growth phase G1, ∆X = 5.2 mg/ml, over 76% final biomass yield) only about 43% of the final saponin yield was produced (∆P = 85.7 μg/ml) (Table 2). After that, during the slow growth phase, the biomass increase was 0.65 mg/ml only, but ginsenoside production was very efficient (∆P = 97.4 μg/ml) what constituted nearly 50% of its final yield. After the biomass growth was stopped the saponin contents dropped (Table 2).
Discussion
Ginsenosides content in biomass reached the greatest level in 25 days of cultivation. The level of all examined metabolites in biomass increased almost 3-fold, and was the highest for saponins Re and Rg1 (Table 1; Fig. 2). Saponin biosynthesis and growth of biomass during the process proceeded simultaneously but their dynamics was different. For the rapid growth phase (G1) between 10 and 20 days, 90% of total biomass yield and only 50% of product was accumulated with low synthesis rate of about 3.4 μg mg−1 d.w. day−1. The saponin content reached about 17 μg mg−1 d.w. in this phase. The rest of the product (50%) was accumulated with high rate nearly 30 μg mg−1 d.w. day−1 in the slow growth phase (G2) in which biomass increased by only 10%. The saponin content reached maximum nearly 150 μg mg−1 d.w. in this phase (Table 2). These relationships clearly show, that although saponins are synthesized already simultaneously with the cell growth, their synthesis rate is not proportional to the growth rate (Fig. 3).
Graphical interpretation of the relationship between ginsenoside production per 1 ml of culture and biomass growth per 1 ml of culture in the suspension culture of P. quinquefolium Z5. Group Rb—sum of protopanaxadiol derivatives: Rb1, Rb2, Rc, Rd; Group Rg—sum of protopanaxatriol derivatives: Rg1 and Re
Some decrease of ginsenoside contents was observed after biomass growth has been stopped. It can be assumed that it is a result of the activities of enzymes involved in saponin metabolism. Moreover, it is probably that lower saponins content in biomass is due to a cell lysis facilitating ginsenoside extraction into medium. Mathur et al. (1994) examined that amount of various fractions of ginsenosides decrease in biomass, but increased in the medium after 25 days cultivation of P. quinquefolium suspension.
For a culture of P. ginseng dynamics of saponin production was dependent on mineral nutrients. Liu and Zhong (1996, 1997) had presented the similar course of the process as in our study, and they also showed saponins production (20–50 mg g−1 d.w.) after cell multiplication; in stationary phase was stopped, what was depended on both N-source and potassium concentration. Akalezi et al. (1999) have shown that saponin content initially decreases and afterwards keeps constant level independent on inoculum size with 30 g/l saccharose in medium. When sugar content was increased to 60 and 80 g/l and inoculum to 3 g/l intensive ginsenosides biosynthesis (maximum 0.275 g/l) was observed at the beginning (10–15 days of cultivation time) of a phase of biomass growth (5–20 days of cultivation time). Khodakovsaya et al. (1998) showed that maximum saponin content in different P. ginseng lines growing in dark or light or with addition of ginsenoside precursors into medium or transformed lines, was oscillating between 7 and 17 mg g−1 d.w. According to Zhong and Wang (1998) during the growth phase (between 10 and 35 days of cultivation) of P. quinquefolium suspension culture ginsenoside synthesis had a slow rate, which clearly increased to 1.5 g/l (measured by colorimetric method), after 35 days of the culture.
For P. notoginseng the dynamics of saponin synthesis was dependent on culture condition. It may occur at the beginning of culture when there is a high sugar concentration in medium (60 g/l) content of total saponins (measured by colorimetric methods) was above 160 mg in 1 g of biomass (Zhang et al. 1996) or they are synthesized (above 900 mg/l for total saponin according to TLC colorimetry) simultaneously to a biomass growth at saccharose concentration of 30 g/l (Zhong et al. 1999) (Table 3).
It is interesting that the maximum content of saponins in the suspension culture investigated in this study (29 mg g−1 d.w.) was comparable to their contents in the leaves of 3-years-old plants of P. quinquefolium (24 mg g−1 d.w.) from a field cultivation (Kochan et al. 2008) which were the source of explants for our in vitro cultures initiation. The quantitative pattern of protopanaxadiol derivatives was the same: Rb2 > Rd > Rc.Rb1, however, opposite results were found for protopanaxatriol derivatives: Rg1 > Re for the suspension culture and Re > Rg1 for the leaves.
Comparing saponin contents in our suspension culture and in the roots from field cultivation, which are pharmaceutical raw materials. The roots of 3-years-old plant contained much more ginsenosides—about 56 mg g−1 d.w. (Kochan et al. 2008). Saponins pattern was entirely different in roots (Rb1 > Rc > Rd > Rb2 and Re > Rg1) than in our suspension culture (Rb2 > Rd > Rc > Rb1 and Rg1 > Re).
References
Akalezi CO, Liu S, Li QS, Yu JT, Zhong JJ (1999) Combined effects of initial sucrose concentration and inoculum size on cell growth and ginseng saponin production by suspension cultures of Panax ginseng. Process Biochem 34:639–642
Belegortsera N, Yoon JY, Kim KH (2000) Inhibition of Helicobacter pylori hemagglutination by polysaccharide fractions from roots of Panax ginseng. Planta Med 66:217–220
Keith I, Block MD (2003) Immune system effects of echinacea, ginseng and astralagus: a review. Integr Cancer Ther 2(3):247–267
Khodakovsaya MV, Bulgakov VP, Chernoded GK, Zhuravlev YN (1998) The approaches for the productivity improvement of the cultured ginseng cells. In: Ginseng in Europe. Proceedings of the 1st European ginseng congress. Magburg, pp 217–220
Kochan E, Kołodziej B, Gadomska G, Chmiel A (2008) Ginsenoside contents in Panax quinquefolium organs from field cultivation. Z Naturforsch 63c:91–95
Lee SH, Jung BH, Kim SY, Lee EH, Chung BCh (2006) The antistress effect of ginseng total saponin and ginsenoside Rg3 and Rb1 evaluated by brain polyamine level under immobilization stress. Pharmacol Res 54:46–49
Lian XY, Zhang Z, Stringer JL (2005) Protective effect of ginseng components in a rodent model of neurodegeneration. Ann Neurol 57(5):642–648
Liu S, Zhong JJ (1996) Effects of potassium ion on cell growth and production of ginseng saponin and polysaccharide in suspension cultures of Panax ginseng. J Biotechnol 52:121–126
Liu S, Zhong JJ (1997) Simultaneous production of ginseng saponin and polysaccharide by suspension cultures of Panax ginseng: nitrogen effects. Enzym Microb Technol 21:518–524
Mathur A, Shukla YN, Pal M, Ahuja PS, Uniyal GC (1994) Saponin production in callus and cell suspension cultures of Panax quinquiefolium. Phytochemistry 35(5):1221–1225
Naval MV, Gómez-Serranillos MP, Carretero ME, Villar AM (2007) Neuroprotective effect of a ginseng (Panax ginseng) root extract on astrocytes primary culture. J Ethnopharmacol 112:262–270
Predy Gn, Goel V, Lovlin R, Donner A, Stitt L, Basu TK (2005) Efficacy of an extract of North American ginseng containing poly-furanosyl-saccharides for preventing upper respiratory tract infections: a randomized controlled trial. Can Med Assoc J 173(9):1043–1048
Rai D, Bhatia G, Sen T, Palit G (2003) Anti-stress effect of Ginko biloba and Panax ginseng: a comparative study. J Pharmacol Sci 93:458–464
Zhang YH, Zhong JJ, Yu JT (1996) Enhancement of ginseng saponin production in suspension cultures of Panax notoginseng: manipulation of medium sucrose. J Biotechnol 51:49–56
Zhong JJ, Wang DJ (1996) Improvement of cell growth and production of ginseng saponin and polysaccharide in suspension cultures of Panax notoginseng Cu2+ effect. J Biotechnol 46:69–72
Zhong JJ, Wang SJ (1998) Effects of nitrogen source on the production of ginseng saponin and polysaccharide by cell cultures of Panax quinquefolium. Process Biochem 33:671–675
Zhong JJ, Chen F, Hu WW (1999) High density cultivation of Panax notoginseng cells in stirred bioreactors for the production of ginseng biomass and ginseng saponins. Process Biochem 35:491–496
Acknowledgments
The project was financed by Medical University in Łódź from its research grant no. 502-13-754 and by State Committee for Scientific Research grant no. 3PO5F01523.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by S. Werbrouck.
Rights and permissions
About this article
Cite this article
Kochan, E., Chmiel, A. Dynamics of ginsenoside biosynthesis in suspension culture of Panax quinquefolium . Acta Physiol Plant 33, 911–915 (2011). https://doi.org/10.1007/s11738-010-0619-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-010-0619-2