Abstract
Twenty days’ exposure to 50 or 100 mM NaCl in the rooting medium substantially increased fresh and dry weights of seedling shoots of the recretohalophyte Limonium sinense while 200 or 300 mM were increasingly inhibitory. KCl treatment was only slightly stimulating (50 mM) or strongly inhibitory (100–300 mM). Lesser effects on leaf area were also seen. Diameter of foliar salt glands was significantly larger than that of controls in 100 and 200 mM NaCl with the effect being reversed at higher concentrations. Gland enlargement was also observed in the presence of 100 mM KCl, while larger concentrations reduced gland size. Generally, gland diameter was larger in the presence of NaCl than in KCl. NaCl and KCl also increased gland number per leaf and secretion rate per gland. At 100 and 200 mM NaCl or KCl, Na+ secretion per leaf from NaCl-treated plants exceeded K+ secretion rate from KCl-treated plants while at 200 mM, Na+ secretion per gland was significantly higher for Na+ than for K+. Evidence of cell death in leaves of salt-treated plants using Evans blue staining indicates that release of cell contents through loss of membrane integrity contributed to the secretion values. We conclude that the greater tolerance of L. sinenseto to NaCl compared to KCl is linked to the more effective secretion of Na+ than of K+ and, in turn, to a greater stimulation of salt gland formation and activity and larger gland diameter.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The most characteristic morphological features of recretohalophytes are salt-secreting structures (salt glands and salt bladders) that can excrete excessive salts (Karimi and Ungar 1989). Salt glands or salt bladders are prevalent on stem or leaf surfaces and, arguably, play an important role in regulating ion balance, maintaining or stabilizing osmotic pressure gradients and generally enhancing salt tolerance. Salt gland excretion rates can be significant, representing an important saline ion exclusion mechanism in salt tolerant grasses (Naidoo and Naidoo 1998; Marcum 2006). Berry and Thomson (1967), Berry (1970) and Thomson et al. (1969) found that the composition of the secreted salt was dependent on the salt composition of the root environment, with the predominant cation in the rooting medium being the one that is secreted the most. Waisel (1960) reported that preference of secretion in Tamarix aphylla was Na+ >Ca2+ >K+, while Pollack and Waisel (1970) reported a ranking in Aeluropus litoralis of Na+ >K+ >Ca2+. Reports on salt gland numbers in relation to external salt concentrations are also varied. Rozema et al. (1977) found increases in the number of salt glands in Glaux maritime as external salt concentrations increased, while Liphschitz et al. (1974) found the number of glands in Chloris gayana unchanged by 5-day exposure to salt. However, sprays of the synthetic cytokinin benzyl adenine (BA) can increase the number of glands in Chloris guayana and in maize, BA treatment increased the loss of sodium (Ramadan and Flowers 2004). In maize, this increased Na+ loss was related to greater numbers of microhairs and leaf area, but not to faster secretion per se. These inconsistencies may, in part, reflect species differences, and lead us to ask three pertinent questions needing resolution. First, variation in salt secretion rate may be attributed to the efficiency of the secretion process itself for each ion or to changes in the number of salt glands as salinity levels rise. Second, the salt glands of a given species may secrete some ions more effectively than others. It may also be the case that species differ in how effectively their glands secretes a given ion. A final question is whether salinity affects the size or frequency of salt glands and if this is related to any differences in overall amounts of secretion of the amounts each gland secretes.
We focus here on whether salt gland size and the number of salt glands per leaf of Limonium sinense change with the level of root-zone salinity and whether Na+ has a different effect than K+.
Materials and methods
Plant growth and treatments
Limonium sinense seeds were collected from native saline soil in Yellow River Delta area of China. After sterilizing with 0.1% HgCl2 for 10 min and washing with tap water, plump seeds were selected and sown in a plastic box filled with fine sand and irrigated with tap water. Seedlings were irrigated with half-strength Hoagland solution (pH 5.7) in a controlled growth chamber, for 1.5 months, under controlled conditions set to a 15-h photoperiod, photon with a flux density of 800 ± 100 μmol m−2 s−1, relative humidity of 70–80%, day temperature of 30 ± 3°C, and night temperature of 20 ± 3°C. When the fourth leaf appeared, uniform seedlings were transplanted to plastic pots (five plants per pot) and grown in a growth chamber. Control plants were irrigated with full-strength Hoagland’s solution. Salinity-stressed plants were irrigated with full-strength Hoagland’s solution containing NaCl or KCl. Concentrations of NaCl or KCl were increased by 50 mM every 12 h until the final concentrations of 50, 100, 200 and 300 mM were achieved. Plants were then irrigated with the same solutions once a day for 25 days. The experiment was terminated 25 days after the target salinity concentration was reached.
Determination of inorganic ions
To determine the quantity of Na+ and K+ secreted from the surfaces, ions were collected according to Zhou and Zhao (2003). The adaxial surface of the fourth leaf, counting from the base was thoroughly washed with distilled water. These same leaves were washed again with a fixed volume of distilled water (10 ml) 5 days later, and the washings retained for analysis. The concentrations of Na+ and K+ were measured with a flame photometer (Flame Photometer 410, Sherwood Scientific Ltd, Cambridge, UK). To Estimate leaf area, whole leaves were excised from the plant, traced onto scale paper, cut out and area determined arithmetically from its weight knowing the weight per unit area of the scale paper.
Preparation of leaf samples for scanning electron microscopy
Leaf samples were prepared according to Lu (1995). Fresh harvested leaves were cut into 0.2 cm × 0.2 cm pieces, air-dried and fixed for 24 h in 2.5% glutaraldehyde at room temperature, washed with 0.1 mM phosphate buffer, fixed with 1% OsO4 for 1.5 h and washed again with distilled water, before being dehydrated in an ethanol series (30, 50, 70, 80, 95% each for 20 min and finally in 100% alcohol for 3 h). After an infiltration for 24 h with isoamyl acetate, samples were embedded in the same isoamyl acetate and polymerized for 24 h at 30, 45, and 60°C sequentially. The material was dried with common critical point drier and platinized with ion sputter coater (IB-5), then observed with a Hitachi H-580 scanning electron microscope.
Calculation of salt secretion rate and density of salt glands
Leaf-prints were prepared according to Hilu and Randall (1984). Clear nail varnish was applied to the leaf surface. After 1 h, the dry film was peeled off using ultra clear adhesive ‘sellotape’ and placed on a clean slide. Salt glands were counted with a light microscope (Olympus BX51) using 100-fold magnification of 0.0254 mm2. The diameter of salt glands was measured on the dry films by light microscopy (Olympus DP70) at 200-fold magnification. Salt glands were counted in ten fields selected randomly. The density of salt glands was calculated according to the average of these ten fields.
The total number of salt glands (N) on adaxial surfaces of L. sinense leaves was calculated from the leaf area and the density of salt glands. The total Na+ or K+ secreted by salt glands on adaxial surface of the leaves for 5 days (M) was calculated from Na+ or K+ concentrations in washings. Na+ or K+ secretion rate per salt gland (V) was calculated as nmol per gland day−1 from these values (V = M/5 N).
Measurement of cell death
Cell death, indicated as loss of plasma membrane integrity, was measured by Evans blue staining of detached leaves (Wright et al. 2000). Detached leaves, completely submerged in a 0.1% (w/v) aqueous solution of Evans blue (Sigma, St Louis, MN), were subjected to two 5-min cycles of vacuum followed by a 20-min continuous vacuum. The leaves were then washed by vacuum infiltration of phosphate-buffered saline plus 0.05% (v/v) Tween for 3 × 15 min. Leaves were mounted on glass slide and viewed under a light microscope (Olympus BX51). Uptake of blue die by cells indicates death.
Statistical analysis
Data were subjected to one-way ANOVA using SAS™ software (SAS Institute, 1989).
Results
Different growth responses of L. sinense seedlings to NaCl and KCl
Plant fresh and dry weight increased with NaCl concentration up to 100 mM and then declined with further increases in concentration up to 300 mM (Fig. 1). At 300 mM NaCl, growth was severely inhibited with fresh weight decreasing to 35% of controls and only 16.8% of the weight of plants treated with 100 mM NaCl (Fig. 1a). Seedling growth was less strongly promoted by KCl than by NaCl and optimum seedling fresh or dry weight size was achieved in 50 mM. KCl with stronger solutions being markedly inhibitory and giving much smaller plants than equivalent concentrations of NaCl. At 300 mM KCl, final fresh weight was 27.3% of control values and only 20.3% of plants in 50 mM KCl.
Area of the fourth leaf was affected less than plant fresh or dry weight by salinity (Fig. 1c). A concentration of 100 mM was optimal for both NaCl. At 300 mM NaCl, the leaf area was 67.9% of the control values. KCl had no stimulating effect on leaf area at any concentration, but at 300 mM it reduced area by almost 27%.
Salt secretion
Na+ secretion rate per leaf increased as NaCl in the medium increased (Fig. 2). At 100 mM, Na+ secretion rate per leaf was 3.4 times greater than that of controls. When NaCl concentration was raised to 300 mM, Na+ secretion rate per leaf was 7.9 times faster than that of controls. Likewise, K+ secretion rate per leaf increased markedly with KCl concentration (Fig. 2). At 100 mM KCl, K+ secretion rate per leaf was 7.2 times greater than that of control and at 300 mM secretion was 23.6 times faster. In addition, Na+ secretion rate per salt gland increased markedly when NaCl concentrations were raised (Fig. 3). At 100 mM, Na+ secretion rate per gland was similar to that of controls but was 2.9 times higher at 300 mM NaCl. Similar results were found in response to KCl (Fig. 3). At 100 mM KCl, K+ secretion rate per salt gland was already 3.4 times faster than that of controls, and 6.5 times higher at 300 M KCl. However, broadly, Na+ excretion on a per leaf basis or on a per gland basis always exceeded that for K+.
Effects of NaCl and KCl on the rate of salt-secretion by the adaxial surface of the fourth leaf of L. sinense plants treated with NaCl and KCl for 25 days. Means within NaCl or KCl treatment that have the same letters are not significantly different at P < 0.05. Vertical bars represent standard deviations (n = 5)
NaCl and KCl treatment and plasma membrane damage
Electrolyte leakage, which reflects damage to cellular membranes (Goodman 1968), has been shown to occur under severe NaCl or KCl stress. To analyse plasma membrane damage to cells under NaCl or KCl treatment we used Evans blue, a dye that is excluded by membranes of living cells but diffuses into dead cells (Gaff and Okong ‘O-Ogola 1971). With the increase of NaCl and KCl, progressively more blue dye appeared in more cells, indicating compromised membrane integrity. At 300 mM NaCl or KCl, Evans blue labelling was observed in most of the epidermal cells of the adaxial surface of leaf 4 (Fig. 4).
Evans blue staining on adaxial surface of the fourth leaves of L. sinense plants treated with NaCl and KCl for 25 days. Blue cells indicate loss of viability and membrane integrity. (a, b) Control (c) 100 mM NaCl treatment (d) 100 mM KCl treatment (e) 200 mM NaCl treatment (f) 200 mM KCl treatment (g) 300 mM NaCl treatment (h) 300 mM KCl treatment. Scale bar 200 μm
Effects of NaCl and KCl on development of salt glands
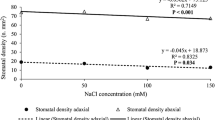
Effects of salinity on secretion rate may be affected by gland size or their density per unit leaf area. Limonium sinense glands have a “flower-like” structure of 5–8 secreting cells surrounding a central pore (Fig. 5). At 100 and 200 mM NaCl, the diameter of salt glands was significantly enlarged compared with the control, but a reduction back to the control size was seen at 300 mM (Fig. 6). A similar pattern was seen in KCl-treated plants. But generally, KCl-treated plants had smaller glands than NaCl plants at equivalent concentrations (Fig. 6). Leaves from plants given 100 mM NaCl showed a higher number of glands than those from plants treated with the same concentration of KCl. The increase in NaCl was almost threefold compared to controls. No significant difference was observed between plants treated with 200 mM NaCl and 200 mM KCl while at 300 mM NaCl the number of glands per leaf was significantly lower than at 300 mM KCl (Fig. 7).
Scanning electron microscopy of salt glands on adaxial surface of the fourth leaves of L. sinense plants treated with NaCl and KCl for 25 days. a The distribution of salt glands on the leaf surface at 100 mM NaCl, ×100. Single salt gland in 0 (b), 100 (c), 200 (e) and 300 mM NaCl (g), ×500, respectively. Single salt gland in 100 (d), 200 (f), 300 mM KCl (h), ×500, respectively
Effects of NaCl and KCl on the number of salt glands on adaxial surfaces of the fourth leaves of L. sinense plants treated with NaCl and KCl for 25 days. Means within NaCl or KCl treatment that have the same letters are not significantly different at P < 0.05. Vertical bars represent standard deviations (n = 5)
Discussion
It is clear from earlier work that ion secretion by salt glands is increased with external concentration of salt and time of exposure (Liphschitz et al. 1974; Marcum and Murdoch 1992; Pollak and Waisel 1970). However, it is not clear whether the change in secretion is due to increased efficiency of the secretion process per salt gland or to a change in the number of salt glands on a given leaf. From the present research with L. sinense seedlings we found an increase in salt secretion by leaf 4 with the increase of NaCl and KCl concentrations in the rooting medium from 100 to 300 mM. In 100 mM NaCl, the number of salt glands per leaf also increased significantly. However, salt secretion rate per salt gland did not increase significantly at this concentration indicating that the promotion of secretion was largely attributable to increased gland number. However, at 200 and 300 mM NaCl an additional contribution from increased salt secretion rate per gland is indicated since both the number of glands and their individual activity both increased. In all KCl-treated plants (100, 200 and 300 mM) both secretion rate per gland and gland number were increased suggesting both effects contributed to the faster ion secretion per leaf. At similar concentrations of applied NaCl and KCl Na+ secretion rate per leaf exceeded that of K+, suggesting a more effective secretion mechanism for Na+ than for K+ operates in L. sinense. This is probably best attributed to more active glands since when NaCl and KCl were both supplied at 200 mM, Na+ secretion rate per glands was statistically significantly higher than that for K+. A further mechanism for the loss of ions by leaves could be loss of membrane integrity as cells are injured by excessive salt. The Evans blue staining of leaf epidermal cells indicated that extensive cell death, especially at the higher salt levels. Thus some secretion may be better attributed to unregulated release caused by salt-induced death of leaf cells.
There was no significant difference in the number of salt glands on adaxial surface per leaf when NaCl concentration increased from 100 to 300 mM; surprisingly, this number did increase when KCl concentrations were raised from 100 to 300 mM, with a significant difference in the numbers between Na+ and K+. There was no significant difference in the number of salt glands on adaxial surface per leaf between plants under 200 mM NaCl treatment and those under 200 mM KCl treatment. The number of salt glands on adaxial surface per leaf under 300 mM NaCl treatment was significantly lower than that under 300 mM KCl treatment. These results suggest that salt gland formation was more sensitive to changes in K+ concentration than changes in Na+. Such an interpretation would also explain differences in the preference ranking of Na+ and K+ secretion between per salt gland and salt glands per leaf.
The size of salt bladders in Atriplex triangularis was shown to increase with an increase in salinity (Karimi and Ungar 1989), but reports on the effects of different ions and salinity levels on the development of salt glands are missing. Based on our analysis of the sizes, distribution and number and the activity of salt glands in L. sinense (Figs. 6, 7), we suggest that modest NaCl or KCl treatments may enhance the development of salt glands in newly developing leaves, while severe NaCl or KCl stresses tend to increasingly inhibit their development. Generally, gland diameter was larger in the presence of NaCl than in KCl.
In conclusion, modest NaCl and KCl treatments significantly stimulated the secretion of salt from leaves and salt glands of L. sinense due to an increase in glands number and secretion rate per gland. This response is considered to be of considerable adaptive significance. Release of ions by leaves may also have been enhanced under salinity stress by an increased incidence of cell death. The overall secretion/release mechanism was more effective for Na+ than for K+. Further research on the ultrastructure of salt glands and proteins that excrete Na+ and K+ in L. sinense will further enhance our understanding of how salinity in the root environment can enhance salt-secretion by leaves and salt glands in this well-adapted recretohalophyte.
References
Berry WL (1970) Characteristics of salts secreted by Tamarix aphylla. Am J Bot 57:1226–1230. doi:10.2307/2441362
Berry WL, Thomson WW (1967) Composition of salt secreted by salt glands of Tamarix aphylla. Can J Bot 45:1774–1775. doi:10.1139/b67-187
Gaff DF, Okong ‘O-Ogola O (1971) The use of nonpermeating pigments for testing the survival of cells. J Exp Bot 22:756–758. doi:10.1093/jxb/22.3.756
Goodman RN (1968) The hypersensitive reaction in tobacco: a reflection of changes in host cell permeability. Phytopathol 58:872–873
Hilu KW, Randall JL (1984) Convenient method for studying grass leaf epidermis. Taxon 33:413–415
Karimi SH, Ungar IA (1989) Development of Epidermal Salt Hairs in Atriplex triangularis Willd. in Response to Salinity, Light Intensity, and Aeration. Bot Gaz 150:68–71. doi:10.1086/337749
Liphschitz N, Waisel Y (1974) Existence of salt glands in various genera of the Gramineae. New Phytol 73:507–513. doi:10.1111/j.1469-8137.1974.tb02129.x
Lu JM, Li JD, Hu AL, Li XL (1995) Observation of secretory salt structure in Limonium bicolor leaf. Chin J Appl Environ Biol 6:355–358
Marcum KB (2006) Use of saline and non-potable water in the turfgrass industry: constraints and developments. Agric Water Manage 80:132–146. doi:10.1016/j.agwat.2005.07.009
Marcum KB, Murdoch CL (1992) Salt tolerance of the coastal salt marsh grass, Sporobolus virginicus (L.) Kunth. New Phytol 120:281–288. doi:10.1111/j.1469-8137.1992.tb05665.x
Naidoo Y, Naidoo G (1998) Salt tolerance in Sporobolus virginicus: the importance of ion relations and salt secretion. Flora 193:337–344
Pollack G, Waisel Y (1970) Salt secretion in Aeluropus litoralis (willd.) Parl. Ann Bot (Lond) 34:879–888
Ramadan T, Flowers TJ (2004) Effects of salinity and benzyl adenine on development and function of microhairs of Zea mays L. Planta 219:639–648. doi:10.1007/s00425-004-1269-7
Rozema J, Riphagen I (1977) Physiology and ecologic relevance of salt secretion by salt glands of Glaux maritima L. Oecologia 29:349–357. doi:10.1007/BF00345808
Thomson WW, Berry WL, Liu LL (1969) Localization and secretion of salt by the salt glands of Tamarix phylla. Proc Natl Acad Sci USA 63:310–317. doi:10.1073/pnas.63.2.310
Waisel Y (1960) Ecological studies on Tamarix aphylla (L.) Karst.III. The salt economy. Plant Soil 13:356–364. doi:10.1007/BF01394647
Wright KM, Duncan GH, Pradel KS, Carr F, Wood S, Oparka KJ, Cruz SS (2000) Analysis of the N gene hypersensitive response induced by a fluorescently tagged tobacco mosaic virus. Plant Physiol 123:1375–1386. doi:10.1104/pp.123.4.1375
Zhou S, Zhao KF (2003) Discussion on the problem of salt gland of Glycine soja. Acta Bot Sin 45:574–580
Acknowledgments
We are grateful for financial support from the NSFC (National Natural Science Research Foundation of China, project No. 30670177 and No. 30070069), Specialized Research Fund for the Doctoral Program of Higher Education (SRFDP, 20050445003). We thank Prof. Hans Bohnert (Department of Plant biology, University of Illinois at Urbana-Champaign) and Prof. T.J. Flowers (School of Biological Sciences, University of Sussex, UK) for critically reading and revising the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Jackson.
Rights and permissions
About this article
Cite this article
Ding, F., Song, J., Ruan, Y. et al. Comparison of the effects of NaCl and KCl at the roots on seedling growth, cell death and the size, frequency and secretion rate of salt glands in leaves of Limonium sinense . Acta Physiol Plant 31, 343–350 (2009). https://doi.org/10.1007/s11738-008-0240-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-008-0240-9