Abstract
The practical application of enzymes as a biocatalyst still faces serious challenges, namely inactivation and loss. Immobilized enzyme technology can significantly improve the recoverability, stability and environmental adaptability of enzymes. Magnetic nanocomposites are widely used in enzyme immobilization because of their magnetic properties and the composite properties of various nanomaterials. In this work, the research progress of magnetic nanocomposites as enzyme carriers is reviewed and the future development direction is prospected.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Enzymes are natural machinerys for molecular processing and powerful synthetic tools for building complex molecules in vivo (Van Beilen et al. 2002). Studying nature and ourselves is an important step forward in science and technology, so many researchers are committed to using enzymes for other purposes. Owing to their exceptional regio-, stereo- or chemospecificity and selectivity, enzymes can carry out very precise reactions (Van Beilen et al. 2002). Enzymes has been extensively used in numerous biochemical processing industries, including fine and bulk chemicals, organic syntheses, healthcare and pharmaceuticals, as well as cosmetics, foods, textiles, paper and pulp (Huang et al. 2022; Liang et al. 2021) (Fig. 1).
However, nothing can be perfect. Enzymes are expensive and easy to be inactivated, the three-dimensional structure of them is predominantly maintained by fragile non-covalent interactions, free enzymes are difficult to recover and susceptible to some extreme conditions (such as strong acid, strong alkali, high temperature), especially when the industrial processes are too complex and the processes often use the non-natural substrates of the biocatalysts (Sheldon et al. 2021; Zahirinejad et al. 2021). The recycling of free enzymes is also a big problem, they are often discharged from the reaction equipment with the product and difficult to separate (Gan et al. 2021). The above series of problems hinder a more widespread implementation of enzyme catalysts across different industries.
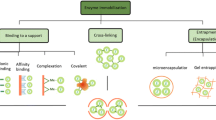
To solve the aforementioned drawbacks, serval strategies, such as artificial modification, directed evolution of enzymes, solvent engineering, protein and enzyme engineering and immobilization, have been adopted (Aggarwal et al. 2021). Enzyme immobilization is an enabling technology, which refers to enzymes directionally being embedded or fixed in the specific region of selected carriers via bonding, cross-linking, adsorption or encapsulation, the technology not only improve the operational stability and recoverability of enzymes, hence is instrumental in continuously reuse and cost savings, but also combine the catalytic reactions among them (Sharma et al. 2021a, b; Arana-Peña et al. 2021; Nunes et al. 2021).
Despite an array of various carrier materials (graphene oxide, chitosan and polyurethane foam, etc.) have be used for the enzyme immobilization, the exploration and development process of novel high-performance immobilization matrices are still endless (Zdarta et al. 2018). There are many entry points to the problem, one of which is how to improve the current 'false' recoverability. Most carriers still exist in nanometer or micron particles, separating them must be considered a hindrance, no company has the ability to provide nanoscale or micron-scale filters for effective enzyme separation (Homaei et al. 2013).
The most direct way to solve this problem is to make the carrier into millimeter or centimeter level, but this will inevitably have a negative impact on enzyme loading and catalytic reaction. Another strategy is to associate enzymes with magnetic nanocomposites, which can provide guiding force for drugs and biomolecules through external magnetic field, so as to achieve the purpose of fixing and separating magnetic labeled biological entities (Vaghari et al. 2016). Magnetic nanomaterials can achieve a facile separation of the biocatalytic system by the use of an external magnetic field, and therefore quickly terminating the enzymatic reactions and recovering the enzymes for continual uses (Bilal et al. 2018). In addition, magnetic nanocomposites are attracting great attention as promising carriers in enzyme immobilization due to the surface hydroxyl groups which enables their easy strong binding of the enzyme molecule and functionalization (Gennari et al. 2020). This review systematically analyzes the application of magnetic nanocomposites in enzyme immobilization, in order to provide reference for the application of enzyme immobilization.
Magnetic nanocomposites and their properties
Now there are still many shortcomings that limit the application of bare magnetic nanomaterials, such as poor chemical stability, high aggregation tendency, strong dipole–dipole attraction and low amount of adsorbed enzymes. (Cao et al. 2012, 2016). In order to solve the above problems, researchers have tried to use inorganic or organic materials to form magnetic nanocomposites with bare magnetic nanomaterials, which cannot only improve the chemical stability, but also delay the oxidation of magnetic nanomaterials (Table 1) (Feng et al. 2021).
Magnetic nanoparticles @ silica
Magnetic nanoparticles represented by Fe3O4 are easily oxidized and dissolved in acidic environments (Bellova et al. 2010). Silica (SiO2) has high stability, strong chemical inertness, rich surface groups and easy modification under air or acidic conditions (Jeelani et al. 2020). SiO2 are biocompatible and hydrophilic in many biosystems, so it is standard chemistry protocols can be followed to conjugate various biomolecules to its surface (Luckarift et al. 2004). All the above advantages make the magnetic nanoparticles @ silica favored by many researchers in enzyme immobilization.
Shanmugam et al. (2020) immobilized trichoderma asperellum laccase on Fe3O4@SiO2-chitosan nanosupport for delignification of lignocellulosic biomass and then utilized for biohydrogen production. With sweet sorghum stover as substrate, the immobilized laccase achieved higher delignification ability than the free one, and can maintain high activity in 8 cycles.
Li et al. (2021) used a magnetic host–guest cage structured magnetic nanoparticles@alginate@SiO2 with a specific enzyme embedding as its guest in the host matrix. The magnetic nanoparticles compensate for the collapsibility of the alginic acid internal structure, SiO2 enhances the swelling resistance of the carrier and inhibits the polysaccharides degradation in the natural environment (Fig. 2).
Schematic diagram of nanoparticles@alginate@SiO2 preparation process (Li et al. 2021)
We all know porous materials can provide a better support for covalently binding or entrapping enzymes, while a current challenge facing magnetic nanoparticles@SiO2 composites is synthesizing and designing a ideal structure with suitable biocompatibility and properties (Zhang et al. 2020a, b; Zhang et al. 2019a, b, c). Unfortunately, up to now the synthesis of hierarchical mesoporous silica materials is complex in many ways (Feng et al. 2019). In addition, the low specific interaction between silica and enzymes leads to leaching, which affects the reusability and recovery (Poorakbar et al. 2018). Due to the diffusion restrictions and deactivation of enzymes during immobilization process, the catalytic performance of enzymes may be negatively affected.
Magnetic nanoparticles @ metal–organic frameworks
Metal–organic frameworks (MOF) is an organic–inorganic hybrid platform for enzyme immobilization, which has promising applications in the vast field drug/enzyme carriers (Nadar and Rathod 2018). It has some excellent properties such as adjustable pore sizes with well-defined cavities, large pore volumes, high surface areas, designable organic ligands and crystallographic structure (Huo et al. 2015). Especially, the large hierarchical surface area and ultra-high porosity lead to high loading capacity and strong affinity for enzyme molecules MOF to avert leaching, which is a perfect feature that does not share with SiO2 (Zhou et al. 2020).
In recent years, a novel strategie incorporating magnetic nanoparticles with enzyme and MOF composite (magnetic-enzyme-MOF) has received attention. These composites have both the diverse properties of MOFs and magnetic properties of metal or metal oxides (Lian et al. 2017). Wang et al. (2016) prepared a Fe3O4@MOF core–shell microsphere by growing MIL-100(Fe) and three-dimensional MOFs on Fe3O4 nanoparticles. The carrier has both large specific surface area and magnetic characteristics, the olive oil hydrolase immobilized on it showing high stability and recoverability (Fig. 3).
The synthetic route of Fe3O4@MIL-100(Fe) microspheres and the immobilization of lipase (Wang et al. 2016)
In addition, Magnetic nanoparticles@MOF may show structures and functions similar to natural enzymes, which are commonly referred as nanozymes (Huang et al. 2021). Nanozymes have some unique advantages such as impressive robustness and low-cost production with easy scale-up, which are ideal substitute for natural enzymes (Niu et al. 2020). Due to the presence of abundant redox sites, magnetic-MOF based nanozymes are regarded as a candidate in achieving high sensitivity for the detection of a target analytes (Zhang et al. 2019a, b, c). Wu et al. (2017) prepared a core–shell artificial peroxidase (Fe3O4@MIL-100(Fe)), which can significantly enhance peroxidase-like activity, the 3,3′,5,5′-tetramethylbenzidine was oxidized by H2O2 to blue product.
Although magnetic nanoparticles@MOF have many advantages, there are still many problems to be solved before the practical application. Firstly, the MOF synthetic methods depends on the organic and inorganic counterparts for biocatalytic applications (Gao et al. 2021). Some novel synthetic methods are necessary for designing multifunctional magnetic hybrid materials as a batter platform for enzyme immobilization. Secondly, most of the existing MOFs remain relatively unstable under some certain conditions (e.g., pH, solvents, water, etc.), which seriously hinders magnetic nanoparticles@MOF’s practical use (Chen et al. 2017). Therefore, we need to improve the structural stability of MOF to give full play to the potential of this immobilization strategy.
Magnetic nanoparticles @ polysaccharides
Due to the flexible chemical modification based on the properties of immobilization, polysaccharides (chitosan, cellulose, alginate, etc.) have become a highly concerned enzyme carrier and carrier intermediate (Sharma et al. 2021a, b). If the enantioselectivity, regional properties and chemical of the immobilized enzymes can be effectively regulated, it will obviously help to reverse any undesired reaction to increase the target end product (Sotelo et al. 2022). In addition, polysaccharides can enable the enzymes to be used in non-aqueous environments, especially considering that natural enzymes are only used in aqueous reaction media, which is very important (Bodakowska-Boczniewicz and Garncarek 2020).
Chitosan, a polysaccharide containing a large number of amino, hydroxyl and other functional groups, is an N-deacetylated product of chitin (the main component of crustacean and arthropod shells such as crab, lobster, squid and shrimp) (Jiang et al. 2005). Due to the presence of free amino groups on its molecular, the reactivity and solubility of chitosan are greater than chitin (Pospiskova and Safarik 2013).
Chitosan exhibits many significant biological and chemical properties, namely biodegradable, biocompatibility, hydrophilicity, availability of reactive functional groups for chemical modifications, mechanical stability, polycationic properties, regenerability, ease of preparation in different geometrical configurations suitable for a chosen biotransformation (Long et al. 2014; Yang et al. 2010; Zang et al. 2014). Especially, chitosan appears economically attractive to prepare low-cost carriers for large scale applications since chitin is the abundant natural polymer next to cellulos (Díaz-Hernández et al. 2018). Coating or surface modification magnetic nanoparticles with chitosan cannot only solve the problem of aggregation in liquid media, but also significantly improve the biocompatibility, so that they can be widely used in biomedical fields, such as protein immobilization, drug delivery systems, wound healing, tissue engineering and magnetic resonance imaging (Dal Magro et al. 2019).
Díaz-Hernández et al. (2018) synthesized chitosan-coated Fe3O4 nanoparticles by a one-step alkaline precipitation method, and used the natural biological cross-linking agent genipin to cross-link the synthesized magnetic complex with xylanase and cellulase to prepare an enzyme reactor. The results showed that the synthesized magnetic composite nanoparticles had simple preparation process and superparamagnetism, and could be applied to the field of isomerase.
Hojnik Podrepšek et al. (2020) used different synthesis methods to functionalize maghemite with chitosan, and compared the properties of the product. The results show that the chitosan suspension cross-linking process (MC2) is most suitable for obtaining the highest activity of immobilized enzyme, and nanoparticles functionalized with chitosan using the covalent binding method (MC3) also have excellent properties (Fig. 4).
Schematic representation of the entire process of chitosan functionalization, activation and enzyme immobilization on a metal oxide nanoparticle, with chemistry mechanism (Hojnik Podrepšek et al. 2020)
However, pure chitosan in powder shape has low specific surface area and absence of porosity, which can result in inefficient enzyme immobilization, so researchers focused on chitosan based nanofibers (Zhao et al. 2011a, b; Hwang and Gu 2013). Due to the fiber diameter in the nanometric scale, the nanofibers have large surface area to volume ratio, excellent mechanical behavior and high porosity with interconnected voids (Huang et al. 2018). The poor stability of chitosan under acidic conditions is another concern, as the pH continues to decrease (lower than the pKa of chitosan), the electrostatic repulsion between the amino groups will increase, eventually leading to the dissolution of the carrier material (Demirkan et al. 2018).
The cross-linking method can overcome the above shortcomings to a certain extent (Gracida et al. 2019). The functional groups of chitosan can be used to form cross-linked structures via intermolecular linkages with cross-linkers (Gracida et al. 2019). In fact, these intermolecular linkages can improve the insolubility of material in acid medium; in addition to enhance its mechanical and thermal properties (Wang and Jiang 2019). The functional groups of chitosan can form cross-linked structures by intermolecular linkages with cross-linking agents, the intermolecular linkages cannot only improve the insolubility in acidic media, but also enhance the mechanical and thermal properties (Qiao et al. 2022).
As the most abundant renewable natural polysaccharide, cellulose has a series of advantages such as sustainability, low cost and biocompatibility (Gennari et al. 2020). Cellulose also exhibits other important features suitable for enzyme immobilization, such as chemically inert behavior and amphiphilic under physiological conditions (Cao et al. 2014).
The study of magnetic cellulose as a carrier for enzyme immobilization has been increasing in recent years. Researchers have tried to incorporate new modifications into these materials to improve their interaction with enzymes. Suo et al. (2020) prepared ionic liquids-modified magnetic carboxymethyl cellulose nanoparticles for lipase immobilization and achieved 2.81 times the specific activity of free enzyme. Water contact angle analysis showed that the introduction of ionic liquids increased the hydrophobicity of the carrier, which in turn induced the opening of the lid of the lipase, making its active sites easier to approach. In addition, the affinity between the lipase immobilized on the prepared carrier and the substrate was enhanced.
Alginate is one of the most profoundly exploited natural polysaccharides, which consists of alternating blocks of (1,4)-linked α-l-guluronate (G) and β-d-mannuronate (M) residues (Hou et al. 2015). Due to its low cost, non-toxicity, biocompatibility and the ability to gelate under mild conditions by adding divalent metal cations such as Ca2+, alginate plays a key role in the development of enzyme-immobilized carrier materials (Marjani et al. 2021).
Alginate can be encapsulated in different forms, covalently immobilized and adsorbed enzymes, such as microspheres, capsules and hydrogels (Ivanova et al. 2011). Zhang et al. (2020a, b) used in situ TYR-mediated dopamine polymerization and internal stereotype strategy-mediated magnetic alginate-polydopamine gelation reaction to co-immobilize double enzymes (tyrosinase (TYR) and β-glucosidase (β-Glu)) in magnetic alginate-polydopamine (PDA) microspheres. The double network cross-linking of alginate and PDA was induced by d-( +)-glucono-δ-lactone (GDL) and TYR, respectively, which significantly reduced the leakage of enzyme from alginate beads (Fig. 5).
Although most scholars recognize the application value of magnetic nanoparticles @ alginate in enzyme immobilization, some problems also exist objectively. Insufficient mechanical strength and enzyme leakage are two representative problems (Marjani et al. 2021). In order to solve these problems, sodium alginate is usually combined with gelatin, chitosan, starch, pectin and other biological polysaccharides and inorganic materials for enzyme immobilization (Cao et al. 2014).
Magnetic nanoparticles @ carbon-based materials
Immobilizing enzymes on organic materials can maintain their high activity, however, these materials do not have the appropriate chemical and thermal stability required for industrial use, and may even be toxic to enzymes. In order to overcome these shortcomings, inorganic materials, such as carbon-based materials, can be used, which have excellent heat resistance and better mechanical and microbial resistance than organic materials.
Carbon nanotubes are composed of graphite sheets, which are rolled into a cylindrical shape with a length of microns and a diameter of up to 100 nm. Carbon nanotubes exhibit extraordinary mechanical, electrical and thermal properties as well as biocompatibility, which makes enzyme immobilization a promising biotechnology application for carbon nanotubes.
Zhao et al. (2011a, b) prepared magnetic carbon nanotubes (MCNTs) with necklace-like nanostructures by hydrothermal method. Based on the Michael addition of methyl acrylate and the amidation reaction of the generated ester with a large amount of excessive ethylenediamine (EDA), hyperbranched polyamide-amine (PAMAM) was grafted on the surface of MCNTs, which can achieve generation growth under this uniform step-by-step reaction. The-NH2 group at the end of the dendritic PAMAM reacts with different functional groups to form functionalized MCNTs. Subsequently, glucoamylase was immobilized on the functionalized MCNTs by adsorption, covalent bond and metal ion affinity interaction. The results showed that in addition to being easily recovered by magnetic separation, the immobilized glucoamylase on functionalized MCNTs provided excellent stability and reusability without compromising the substrate specificity of free glucoamylase.
Biochar is a stable, porous and light solid product obtained by thermal decomposition of biomass under anaerobic conditions. It is mainly composed of 70% C and P, and may also contain elements such as Ca, Mg and Si. Biology has high surface area, good physical and chemical tolerance, rich surface oxygen-containing functional groups, good dispersion and biocompatibility, which can be used for stable and high-load enzyme immobilization.
Magnetic biochar derived from waste has become an attractive enzyme immobilization carrier due to its low cost, easy availability, lack of enzyme inhibition and porosity. Zhang et al. (2020b) prepared magnetic biochar from biosolids under hydrothermal conditions for laccase immobilization. The specific activity of immobilized laccase was 47.1% higher than that of free laccase. After 10 times of washing, the immobilized enzyme retained 79.3% activity. In addition, MBC has low acute toxicity and is relatively benign from an environmental perspective.
Graphene exhibits a two-dimensional structure of honeycomb carbon lattice, with a large specific surface area, abundant oxygen-containing functional groups, biocompatibility and excellent structural, electrical, mechanical and thermal properties (Li et al. 2018). In addition, due to its excellent antioxidant properties, graphene can promote the removal of free radicals and protect the enzymes from biocatalytic inactivation. The above series of excellent characteristics make graphene an excellent carrier for loading magnetic nanoparticles and immobilized enzymes (Heidarizadeh et al. 2017). Due to the severe functionalization of the conjugated network, graphene sheets have the characteristics of insulation, the Magnetic nanoparticles@graphene composites not only has a unique magnetic two-dimensional structure, but also has the characteristics of simple production, low toxicity, strong surface modification and large enzyme loading, which has attracted great attention in enzyme engineering research (Badoei-Dalfard et al. 2019; Wang et al. 2020).
Ariaeenejad et al. (2021) reported bi-functional applications of a novel immobilized enzyme on the modified magnetic graphene oxide for degradation of dyes in water. The results showed that the immobilized process provides more enzyme protection and dye adsorption/degradation ability even in the concentrated dye solutions (Fig. 6).
Schematic representation of plausible mechanism for the reduction of methylene blue by immobilized enzyme nano-biocatalyst (Ariaeenejad et al. 2021)
Rouhani et al. (2018) prepared a graphene oxide modified by CuFe2O4 magnetic nanoparticles and immobilized the laccase by covalent interaction. The results showed that the immobilized laccase has a wider temperature and pH applicability than the free one, there was no significant activity loss after storage for 30 days or repeated 10 times (Fig. 7).
Synthesis steps of GO-CuFe2O4-Laccase (Rouhani et al. 2018)
Now some key points still need to be solved. Firstly, the effects of graphene on the structure and function of enzymes (or other proteins) need to be further studied, which is important for optimizing nanobiocatalytic systems (Baghayeri et al. 2019). Secondly, the development of simple and low-cost methods for designing and creating novel graphene-based materials with tailored physicochemical properties and surface functionality is an urgent task (Li et al. 2022). Finally, it is crucial to develop graphene-based nanomaterials with high biocompatibility and negligible environmental impact, which will expand the application of graphene-based nano biocatalytic systems in the fields of biocatalytic conversion, enzyme engineering, biofuel and energy production (Paz-Cedeno et al. 2021).
Magnetic nanoparticles @ Au
The surface of Au nanoparticles can be modified to obtain abundant functional groups (such as thiol group and disulfide group), which can provide a good carrier for the enzymes (Zhang et al. 2019a, b, c). The Magnetic nanoparticles@Au nanocomposites have excellent electrical conductivity and biocompatibility, providing an appropriate microenvironment for maintaining enzyme activity (Mohammadi et al. 2019).
Peng et al. (2013) reversibly immobilized trypsin on Fe3O4 nanoparticles by using Au nanoparticles as intermediate ligands. The results showed that trypsin was immobilized on magnetic AuNP@Fe3O4 to form an excellent biocatalyst, which could digest the protein very quickly. After repeated times, it could still maintain high activity and some enzymes could be regenerated (Fig. 8).
Facile preparation of novel core–shell enzyme-Au-polydopamine-Fe3O4 magnetic bionanoparticles for glucosesensor (Peng et al. 2013)
Majouga et al. (2015) synthesized core–shell NPs having magnetite cores and gold shells modified with various sulfur containing ligands, the carboxylic groups displayed at the surface of the NPs were utilized for NP conjugation with a model enzyme, the enzyme activity can be manipulated by a remote magnetic field using the proposed enzymes-superparamagnetic NP hybrid structures (Fig. 9).
Synthesis, purification and modification of core–shell magnetite–gold nanoparticles (Majouga et al. 2015)
Immobilized enzymes bioreactors utilizing a magnetic field
Although the research of magnetic nanocomposites as enzyme carriers is not complete, it is necessary to fully understand the development status of their supporting bioreactors. Now the research on immobilized enzymes bioreactors utilizing a magnetic field has made some progress, mainly aiming at realizing the simple recovery of magnetic nanocomposites and inhibiting the agglomeration between nanoparticles (Al-Qodah et al. 2017). In the magnetic field, the induced magnetic force stabilizes the magnetic particles and makes them uniformly arranged along the magnetic field line (Al-Qodah et al. 2017). Another advantage of this stability is the suppression of the formation of large bubbles, which helps to avoid poor contact between different phases (De Cuyper 2018). Other studies have shown that the introduction of magnetic field is beneficial to prolong the life of enzymes on magnetic nanocomposites, because mutual collision and aggregation will not only lead to enzyme shedding but also destroy the microenvironment of enzyme survival (Wang et al. 2022).
Some specific issues have to be considered when building the above bioreactors (even in the laboratory). Magnetic particle size is the first parameter, too large or too small is wrong. Too large particle size will affect the mass transfer efficiency, and too small will make the agglomeration difficult to control (Takei et al. 2018). The next question is the direction and strength of the magnetic field. At present, the axial magnetic field is still the first choice, because it is beneficial for the magnetic field to exert a uniform influence on the whole reactor (Darwesh et al. 2019). The magnetic field strength should also be controlled within a reasonable range, because studies have shown that high magnetic field strength can easily lead to agglomeration (Sotelo et al. 2022).
Some forward-looking issues also have to be considered. Firstly, it should be noted that the magnetic field can affect the activity of the enzymes by affecting the three-dimensional structure of the active sites, this effect can be positive or negative (Armenia et al. 2019). Therefore, it is better to test the effect of magnetic field on the target enzyme before building the bioreactors. In addition, based on the results of the laboratory to enlarge and scale is the inevitable problem in future. It is therefore an important preparation to try to model these systems to correlate the reactor and particle size with the required conversion rate.
Conclusion and future perspectives
Magnetic nanocomposites have shown obvious advantages in enzyme immobilization due to their easy separation and recovery by magnetic fields, larger surface area and high mass transfer capacity. In this work, the research progress of magnetic nanocomposites as enzyme carriers is reviewed and the future development direction is prospected.
-
(1)
A variety of inorganic or organic materials have been used to form magnetic nanocomposites with bare magnetic nanomaterials to improve some inherent defects (poor chemical stability, high aggregation tendency, strong dipole–dipole attraction and low adsorption enzyme amount, etc.). Easy preparation, high specific surface area, low cost, strong stability and excellent biocompatibility are the basic requirements for the organic or inorganic materials. Unfortunately, it is difficult to find materials that meet the above conditions at the same time. In fact, our research group does not believe that there is a universal material suitable for all application scenarios. What the front-line engineers need to do is to find the relative best choice according to the actual needs.
-
(2)
Some novel synthetic methods are necessary for designing multifunctional magnetic hybrid materials as a batter platform for enzyme immobilization. The novel synthesis methods must be able to fully combine magnetic nanoparticles with one or more other inorganic or organic materials under the premise of cost control. The interaction between the composite materials and the enzymes is also a matter of concern. In addition to providing an attachment platform for the enzymes, the specific interaction should promote the enzyme activity.
-
(3)
With the continuous development of magnetic nanocomposites as enzyme carriers, bioreactors based on them are also an inevitable demand. Now the research on immobilized enzymes bioreactors utilizing a magnetic field has made some progress, mainly aiming at realizing the simple recovery of magnetic nanocomposites and inhibiting the agglomeration between nanoparticles. Obviously, a series of emerging issues are worthy of attention. For example, it should be noted that the magnetic field can affect the activity of the enzymes by affecting the three-dimensional structure of the active sites, this effect can be positive or negative.
-
(4)
Moreover, the current research still faces some problems. For example, the focus of research is limited to the immobilization of single enzyme and ignores the study of immobilized multi-enzyme system; there is a lack of effective control methods for the interface interaction between carrier and enzyme molecules; there are few studies on the synergistic catalysis of magnetic carrier and enzyme. In addition, concerted efforts are required to analyze the nanoparticles-enzyme binding sites, surface–function relationship and the involvement of conformational changes in the immobilization process.
In a word, the research on enzyme immobilization involves the cross integration of many disciplines such as material science, biological science, catalytic science and process science, and its related research achievements play an important role in promoting the development of many industries such as biology, pharmacy, energy, environment and food.
References
Aggarwal S, Chakravarty A, Ikram S (2021) A comprehensive review on incredible renewable carriers as promising platforms for enzyme immobilization & thereof strategies. Int J Biol Macromol 167:962–986
Al-Qodah Z, Al-Shannag M, Al-Busoul M et al (2017) Immobilized enzymes bioreactors utilizing a magnetic field: a review. Biochem Eng J 121:94–106
Arana-Peña S, Carballares D, Morellon-Sterlling R et al (2021) Enzyme co-immobilization: always the biocatalyst designers’ choice… or not? Biotechnol Adv 51:107584
Ariaeenejad S, Motamedi E, Salekdeh GH (2021) Application of the immobilized enzyme on magnetic graphene oxide nano-carrier as a versatile bi-functional tool for efficient removal of dye from water. Biores Technol 319:124228
Armenia I, Bonavia MVG, De Matteis L et al (2019) Enzyme activation by alternating magnetic field: Importance of the bioconjugation methodology. J Colloid Interface Sci 537:615–628
Badoei-Dalfard A, Karami Z, Malekabadi S (2019) Construction of CLEAs-lipase on magnetic graphene oxide nanocomposite: an efficient nanobiocatalyst for biodiesel production. Biores Technol 278:473–476
Baghayeri M, Alinezhad H, Tarahomi M et al (2019) A non-enzymatic hydrogen peroxide sensor based on dendrimer functionalized magnetic graphene oxide decorated with palladium nanoparticles. Appl Surf Sci 478:87–93
Beilen V, Jan B, Li Z (2002) Enzyme technology: an overview. Curr Opin Biotechnol 13(4):338–344
Bellova A, Bystrenova E, Koneracka M et al (2010) Effect of Fe3O4 magnetic nanoparticles on lysozyme amyloid aggregation. Nanotechnology 21(6):065103
Bilal M, Zhao Y, Rasheed T et al (2018) Magnetic nanoparticles as versatile carriers for enzymes immobilization: a review. Int J Biol Macromol 120:2530–2544
Bodakowska-Boczniewicz J, Garncarek Z (2020) Immobilization of naringinase from aspergillus niger on a magnetic polysaccharide carrier. Molecules 25(12):2731
Cao M, Li Z, Wang J et al (2012) Food related applications of magnetic iron oxide nanoparticles: enzyme immobilization, protein purification, and food analysis. Trends Food Sci Technol 27(1):47–56
Cao SL, Li XH, Lou WY et al (2014) Preparation of a novel magnetic cellulose nanocrystal and its efficient use for enzyme immobilization. J Mater Chem B 2(34):5522–5530
Cao Y, Wen L, Svec F et al (2016) Magnetic AuNP@Fe3O4 nanoparticles as reusable carriers for reversible enzyme immobilization. Chem Eng J 286:272–281
Chen S, Wen L, Svec F et al (2017) Magnetic metal–organic frameworks as scaffolds for spatial co-location and positional assembly of multi-enzyme systems enabling enhanced cascade biocatalysis. RSC Adv 7(34):21205–21213
De Cuyper M (2018) Applications of magnetoproteoliposomes in bioreactors operating in high-gradient magnetic fields. In: Handbook of nonmedical applications of liposomes (pp. 325–342). CRC Press, Boca Raton.
Dal Magro L, de Moura KS, Backes BE et al (2019) Immobilization of pectinase on chitosan-magnetic particles: influence of particle preparation protocol on enzyme properties for fruit juice clarification. Biotechnol Reports 24:e00373
Darwesh OM, Matter IA, Eida MF (2019) Development of peroxidase enzyme immobilized magnetic nanoparticles for bioremediation of textile wastewater dye. J Environ Chem Eng 7(1):102805
Demirkan E, Avci T, Aykut Y (2018) Protease immobilization on cellulose monoacetate/chitosan-blended nanofibers. J Ind Text 47(8):2092–2111
Díaz-Hernández A, Gracida J, García-Almendárez B E et al (2018) Characterization of magnetic nanoparticles coated with chitosan: a potential approach for enzyme immobilization. J Nanomater.
Feng Y, Zhong L, Jia S et al (2019) Acid-resistant enzyme@ MOF nanocomposites with mesoporous silica shells for enzymatic applications in acidic environments. J Biotechnol 306:54–61
Feng Y, Hu H, Wang Z et al (2021) Three-dimensional ordered magnetic macroporous metal-organic frameworks for enzyme immobilization. J Colloid Interface Sci 590:436–445
Gan JS, Bagheri AR, Aramesh N et al (2021) Covalent organic frameworks as emerging host platforms for enzyme immobilization and robust biocatalysis-a review. Int J Biol Macromol 167:502–515
Gao X, Zhai Q, Hu M et al (2021) Hierarchically porous magnetic Fe3O4/Fe-MOF used as an effective platform for enzyme immobilization: a kinetic and thermodynamic study of structure–activity. Catal Sci Technol 11(7):2446–2455
Gennari A, Führ AJ, Volpato G et al (2020) Magnetic cellulose: versatile support for enzyme immobilization-a review. Carbohyd Polym 246:116646
Gracida J, Arredondo-Ochoa T, García-Almendárez BE et al (2019) Improved thermal and reusability properties of xylanase by genipin cross-linking to magnetic chitosan particles. Appl Biochem Biotechnol 188(2):395–409
Heidarizadeh M, Doustkhah E, Rostamnia S et al (2017) Dithiocarbamate to modify magnetic graphene oxide nanocomposite (Fe3O4-GO): a new strategy for covalent enzyme (lipase) immobilization to fabrication a new nanobiocatalyst for enzymatic hydrolysis of PNPD. Int J Biol Macromol 101:696–702
Hojnik Podrepšek G, Knez Ž, Leitgeb M (2020) Development of chitosan functionalized magnetic nanoparticles with bioactive compounds. Nanomaterials 10(10):1913
Homaei AA, Sariri R, Vianello F et al (2013) Enzyme immobilization: an update. J Chem Biol 6(4):185–205
Hou C, Qi Z, Zhu H (2015) Preparation of core-shell magnetic polydopamine/alginate biocomposite for Candida rugosa lipase immobilization. Colloids Surf, B 128:544–551
Huang WC, Wang W, Xue C et al (2018) Effective enzyme immobilization onto a magnetic chitin nanofiber composite. ACS Sustain Chem Eng 6(7):8118–8124
Huang X, Zhang S, Tang Y et al (2021) Advances in metal–organic framework-based nanozymes and their applications. Coord Chem Rev 449:214216
Huang C, Jiang X, Shen X et al (2022) Lignin-enzyme interaction: a roadblock for efficient enzymatic hydrolysis of lignocellulosics. Renew Sustain Energy Rev 154:111822
Huo J, Aguilera-Sigalat J, El-Hankari S et al (2015) Magnetic MOF microreactors for recyclable size-selective biocatalysis. Chem Sci 6(3):1938–1943
Hwang ET, Gu MB (2013) Enzyme stabilization by nano/microsized hybrid materials. Eng Life Sci 13(1):49–61
Ivanova V, Petrova P, Hristov J. (2011). Application in the ethanol fermentation of immobilized yeast cells in matrix of alginate/magnetic nanoparticles, on chitosan-magnetite microparticles and cellulose-coated magnetic nanoparticles. arXiv preprint arXiv:1105.0619.
Jeelani PG, Mulay P, Venkat R et al (2020) Multifaceted application of silica nanoparticles. a review Silicon 12(6):1337–1354
Jiang DS, Long SY, Huang J et al (2005) Immobilization of Pycnoporus sanguineus laccase on magnetic chitosan microspheres. Biochem Eng J 25(1):15–23
Li Y, Jing T, Xu G et al (2018) 3-D magnetic graphene oxide-magnetite poly (vinyl alcohol) nanocomposite substrates for immobilizing enzyme. Polymer 149:13–22
Li J, Yang Y, Han Z et al (2021) Degradation of tetrachloroguaiacol by an enzyme embedded in a magnetic composite cage structure of MNPs@ ALG@ SiO2. Biochem Eng J 170:107924
Li Y, Wang Q, Ding Z et al (2022) A functionalized magnetic graphene-based MOFs platform as the heterogeneous mimic enzyme sensor for glucose detection. Catal Lett 152(8):2375–2385
Lian X, Fang Y, Joseph E et al (2017) Enzyme-MOF (metal-organic framework) composites. Chem Soc Rev 46(11):3386–3401
Liang W, Wied P, Carraro F et al (2021) Metal-organic framework-based enzyme biocomposites. Chem Rev 121(3):1077–1129
Long J, Jiao A, Wei B et al (2014) A novel method for pullulanase immobilized onto magnetic chitosan/Fe3O4 composite nanoparticles by in situ preparation and evaluation of the enzyme stability. J Mol Catal B Enzym 109:53–61
Luckarift HR, Spain JC, Naik RR et al (2004) Enzyme immobilization in a biomimetic silica support. Nat Biotechnol 22(2):211–213
Majouga A, Sokolsky-Papkov M, Kuznetsov A et al (2015) Enzyme-functionalized gold-coated magnetite nanoparticles as novel hybrid nanomaterials: synthesis, purification and control of enzyme function by low-frequency magnetic field. Colloids Surf, B 125:104–109
Marjani A, Zare MH, Sadeghi MH et al (2021) Synthesis of alginate-coated magnetic nanocatalyst containing high-performance integrated enzyme for phenol removal. J Environ Chem Eng 9(1):104884
Mohammadi M, Mokarram RR, Ghorbani M et al (2019) Inulinase immobilized gold-magnetic nanoparticles as a magnetically recyclable biocatalyst for facial and efficient inulin biotransformation to high fructose syrup. Int J Biol Macromol 123:846–855
Nadar SS, Rathod VK (2018) Magnetic-metal organic framework (magnetic-MOF): a novel platform for enzyme immobilization and nanozyme applications. Int J Biol Macromol 120:2293–2302
Niu X, Li X, Lyu Z et al (2020) Metal-organic framework based nanozymes: promising materials for biochemical analysis. Chem Commun 56(77):11338–11353
Nunes YL, de Menezes FL, de Sousa IG et al (2021) Chemical and physical Chitosan modification for designing enzymatic industrial biocatalysts: how to choose the best strategy? Int J Biol Macromol 181:1124–1170
Paz-Cedeno FR, Carceller JM, Iborra S et al (2021) Magnetic graphene oxide as a platform for the immobilization of cellulases and xylanases: ultrastructural characterization and assessment of lignocellulosic biomass hydrolysis. Renewable Energy 164:491–501
Peng HP, Liang RP, Zhang L et al (2013) Facile preparation of novel core-shell enzyme-Au-polydopamine-Fe3O4 magnetic bionanoparticles for glucosesensor. Biosens Bioelectron 42:293–299
Poorakbar E, Shafiee A, Saboury AA et al (2018) Synthesis of magnetic gold mesoporous silica nanoparticles core shell for cellulase enzyme immobilization: improvement of enzymatic activity and thermal stability. Process Biochem 71:92–100
Pospiskova K, Safarik I (2013) Low-cost, easy-to-prepare magnetic chitosan microparticles for enzymes immobilization. Carbohyd Polym 96(2):545–548
Qiao W, Zhang Z, Qian Y et al (2022) Bacterial laccase immobilized on a magnetic dialdehyde cellulose without cross-linking agents for decolorization. Colloids Surf, A 632:127818
Rouhani S, Rostami A, Salimi A et al (2018) Graphene oxide/CuFe2O4 nanocomposite as a novel scaffold for the immobilization of laccase and its application as a recyclable nanobiocatalyst for the green synthesis of arylsulfonyl benzenediols. Biochem Eng J 133:1–11
Shanmugam S, Krishnaswamy S, Chandrababu R et al (2020) Optimal immobilization of Trichoderma asperellum laccase on polymer coated Fe3O4@SiO2 nanoparticles for enhanced biohydrogen production from delignified lignocellulosic biomass. Fuel 273:117777
Sharma A, Gupta G, Ahmad T et al (2021a) Enzyme engineering: current trends and future perspectives. Food Rev Intl 37(2):121–154
Sharma A, Thatai KS, Kuthiala T et al (2021b) Employment of polysaccharides in enzyme immobilization. React Funct Polym 167:105005
Sheldon RA, Basso A, Brady D (2021) New frontiers in enzyme immobilisation: robust biocatalysts for a circular bio-based economy. Chem Soc Rev 50(10):5850–5862
Sotelo DC, Ornelas-Soto N, Osma JF (2022) Novel magnetic polymeric filters with laccase-based nanoparticles for improving congo red decolorization in bioreactors. Polymers 14(12):2328
Suo H, Xu L, Xue Y, Suo H, Xu L, Xue Y et al (2020) Ionic liquids-modified cellulose coated magnetic nanoparticles for enzyme immobilization: improvement of catalytic performance. Carbohyd Polym 234:115914
Takei T, Sakoguchi S, Yoshida M (2018) Efficient mixing of microliter droplets as micro-bioreactors using paramagnetic microparticles manipulated by external magnetic field. J Biosci Bioeng 126(5):649–652
Vaghari H, Jafarizadeh-Malmiri H, Mohammadlou M et al (2016) Application of magnetic nanoparticles in smart enzyme immobilization. Biotech Lett 38(2):223–233
Wang D, Jiang W (2019) Preparation of chitosan-based nanoparticles for enzyme immobilization. Int J Biol Macromol 126:1125–1132
Wang J, Zhao G, Yu F (2016) Facile preparation of Fe3O4@ MOF core-shell microspheres for lipase immobilization. J Taiwan Inst Chem Eng 69:139–145
Wang F, Feng Y, He S et al (2020) Nickel nanoparticles-loaded three-dimensional porous magnetic graphene-like material for non-enzymatic glucose sensing. Microchem J 155:104748
Wang M, Li J, Ning S et al (2022) Simultaneously enhanced treatment efficiency of simulated hypersaline azo dye wastewater and membrane antifouling by a novel static magnetic field membrane bioreactor (SMFMBR). Sci Total Environ 821:153452
Wu Y, Ma Y, Xu G et al (2017) Metal-organic framework coated Fe3O4 magnetic nanoparticles with peroxidase-like activity for colorimetric sensing of cholesterol. Sens Actuators, B Chem 249:195–202
Yang K, Xu NS, Su WW (2010) Co-immobilized enzymes in magnetic chitosan beads for improved hydrolysis of macromolecular substrates under a time-varying magnetic field. J Biotechnol 148(2–3):119–127
Zahirinejad S, Hemmati R, Homaei A et al (2021) Nano-organic supports for enzyme immobilization: scopes and perspectives. Colloids Surf, B 204:111774
Zang L, Qiu J, Wu X et al (2014) Preparation of magnetic chitosan nanoparticles as support for cellulase immobilization. Ind Eng Chem Res 53(9):3448–3454
Zdarta J, Meyer AS, Jesionowski T et al (2018) A general overview of support materials for enzyme immobilization: characteristics, properties, practical utility. Catalysts 8(2):92
Zhang X, Li G, Wu D et al (2019a) Recent progress in the design fabrication of metal-organic frameworks-based nanozymes and their applications to sensing and cancer therapy. Biosens Bioelectron 137:178–198
Zhang Y, Wang D, Yue S et al (2019b) Sensitive multicolor visual detection of exosomes via dual signal amplification strategy of enzyme-catalyzed metallization of Au nanorods and hybridization chain reaction. ACS Sensors 4(12):3210–3218
Zhang Y, Yue Q, Zagho MM et al (2019c) Core-shell magnetic mesoporous silica microspheres with large mesopores for enzyme immobilization in biocatalysis. ACS Appl Mater Interfaces 11(10):10356–10363
Zhang H, Lu M, Jiang H et al (2020a) Tyrosinase-mediated dopamine polymerization modified magnetic alginate beads for dual-enzymes encapsulation: preparation, performance and application. Colloids Surf, B 188:110800
Zhang T, Huang B, Elzatahry AA et al (2020b) Synthesis of podlike magnetic mesoporous silica nanochains for use as enzyme support and nanostirrer in biocatalysis. ACS Appl Mater Interfaces 12(15):17901–17908
Zhao G, Li Y, Wang J et al (2011a) Reversible immobilization of glucoamylase onto magnetic carbon nanotubes functionalized with dendrimer. Appl Microbiol Biotechnol 91:591–601
Zhao LM, Shi LE, Zhang ZL et al (2011b) Preparation and application of chitosan nanoparticles and nanofibers. Braz J Chem Eng 28:353–362
Zhou Z, Gao Z, Shen H et al (2020) Metal–organic framework in situ post-encapsulating DNA-enzyme composites on a magnetic carrier with high stability and reusability. ACS Appl Mater Interfaces 12(6):7510–7517
Acknowledgments
The work was financially supported by Hebei Natural Science Foundation [Grant Number E2020208054].
Funding
Supported by Hebei Natural Science Foundation (E2020208054).
Author information
Authors and Affiliations
Contributions
Conceptualization was contributed by WZ; methodology was contributed by HJ; software was contributed by WG; validation was contributed by RL; formal analysis was contributed by BX; investigation was contributed by WZ; resources were contributed by CH; data curation was contributed by CL; writing—original draft preparation, was contributed by CL; writing—review and editing, was contributed by QZ; visualization was contributed by ZN; supervision was contributed by GL; project administration was contributed by CL; funding acquisition was contributed by ZL.
Corresponding authors
Ethics declarations
Conflict of interest
No competing financial interests exist.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zaixing, L., Chao, L., Qinqin, Z. et al. Magnetic nanocomposites as multifunctional carriers for enzymes immobilization: a review. Chem. Pap. 78, 1353–1365 (2024). https://doi.org/10.1007/s11696-023-03173-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-023-03173-9