Abstract
Purpose
Weight loss before bariatric surgery is not mandatory, but questions remain as to whether preoperative weight loss has an impact on weight loss after surgery. Most studies have small sample sizes. The objective was to evaluate the relationship between preoperative and successful postoperative weight loss defined as ≥25% total weight loss (TWL) at 1 and 2 years after primary bariatric surgery with regard to the obesity-related comorbidities.
Materials and Methods
Data were extracted from a large nationwide quality registry of patients who underwent a sleeve gastrectomy (SG) or gastric bypass (GBP) between January 2015 and January 2018. Patients with completed screening and preoperative and postoperative data were included. A multivariate logistic regression analysis was performed for each technique and follow-up years separately.
Results
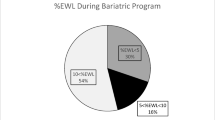
In total, 8751 were included in the analysis. Patients with preoperative weight loss were more likely to achieve ≥25% postoperative TWL in both procedures. Patients with higher preoperative weight loss of 5–10% had an increased likelihood for achieving 25% TWL compared to 0–5%, OR 1.79 (CI (1.42–2.25), p < 0.001) vs 1.25 (CI (1.08–1.46), p < 0.004) for the GBP group for year 2 postoperative. This was the same for the SG group at year 2, OR 1.30 (CI (1.03–1.64), p < 0.029) vs 1.14 (CI (0.94–1.38), p < 0.198).
Conclusion
Patients with preoperative weight loss were more likely to achieve ≥25% postoperative TWL at 1 and 2 years after surgery in both procedures; moreover, the extent of preoperative weight loss contributes to the significance and odds of this success.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Preoperative weight loss is not a definitive criterion to be eligible for bariatric surgery. Although reasonable attempts at other weight loss techniques are required, there is no demand for mandatory weight loss [1, 2]. The Enhanced Recovery After Surgery guideline reviewed preoperative weight loss in the literature published between January 1966 and January 2015 [3]. Summarizing the key findings, a systematic review by Cassie et al. and a large cohort study comprising 22,327 patients by Anderin et al. suggested that preoperative weight loss was associated with a reduction in postoperative complications [4, 5]. Additionally, preoperative weight loss appeared to be associated with postoperative weight loss in adults and adolescents [6, 7].
On the contrary, recent studies from 2019 demonstrated that patients receiving mandatory weight loss goals before surgery did not have improved weight loss or comorbidity resolution up to 4 years after surgery [8, 9].
Questions remain as to whether preoperative weight loss could have an impact on weight loss after surgery as well, and most studies have small sample sizes. In a large Swedish national bariatric registry data set comprising 9570 patients, preoperative weight loss was associated with sustained improved postoperative weight reduction and there was a relationship between the degree of pre- and postoperative weight loss [10]. However, Gerber et al. solely included the gastric bypass (GBP) procedure and it seemed unclear which comorbidities were taken into account.
The present nationwide retrospective cohort study evaluated the association between preoperative weight loss and successful postoperative weight loss defined as ≥25% total weight loss (TWL) in sleeve gastrectomy (SG) and GBP patients at 1 and 2 years after surgery with regard to the obesity-related comorbidities.
Methods
Registry
The Dutch Audit for Treatment of Obesity (DATO) is a nationwide mandatory quality registry for all bariatric procedures [11]. The database covers all 18 bariatric centers in the Netherlands with approximately 12,000 procedures being added annually. To be registered in this system and therefore suitable for bariatric surgery as approved by a multidisciplinary team, patients must be 18 years or older and have a body mass index (BMI) of ≥40 kg/m2 or a BMI ≥ 35 kg/m2 in combination with one of the 6 major obese-related comorbidities: diabetes mellitus, hypertension, dyslipidemia, obstructive sleep apnea syndrome (OSAS), gastroesophageal reflux disease (GERD), and musculoskeletal pain. The follow-up is recorded at 3, 6, 9, 12, 24, 36, 48, and 60 months. A retrospective cohort study was conducted by using the DATO database. No informed consent was needed as this is an opt-out registry. The study was performed in accordance with the ethical standards as stated in the Dutch law and the regulations of the Dutch Institute for Clinical Auditing (DICA).
Patient Selection
Patients who underwent primary sleeve gastrectomy or Roux-en-Y gastric bypass between January 2015 and January 2018 were extracted from the DATO database. Only patients with completed follow-up data 2 years after surgery were included. Patients who gained 5% of their total weight or lost more than 10% preoperatively were deemed as outliers, and thus excluded to adhere to the research question.
Collected patient characteristics were age, gender, weight, height, preoperative obese-related comorbidities, American Society of Anesthesiologists score (ASA), date of surgery, and type of surgery. Weight and height were measured at screening, day of surgery, and 1 and 2 years postoperatively. Screening was within 1 year of the operative intervention, and the annual follow-up had a range of ±3 months.
Preoperative weight loss was calculated as the percentage difference between weight at screening and weight at day of surgery. Postoperative weight loss was defined as the percentage difference between weight at screening and weight at 1 year or 2 years postoperative. Successful postoperative weight loss was defined as ≥25% TWL.
Statistical Analysis
Depending on the distribution, differences for continuous variables between types of surgery were measured using the one-way analysis of variance or the Mann-Whitney U test. Differences between types of surgery for categorical variables were analyzed using the chi-square test. Normally distributed, continuous variables were presented as mean and standard deviation. Not normally distributed data were reported as median and interquartile range (IQR). Categorical data were described in an absolute number as well as a percentage of the subgroup: SG or GBP.
Univariate logistic regression analysis was used to determine the association between the baseline characteristics, preoperative weight loss, and the dependent variable; successful postoperative weight loss was presented as the odds ratio (OR) with a 95% confidence interval (CI). Characteristics with a p < 0.05 or clinically relevant were included in the subsequent multivariable logistic regression analysis using stepwise backward elimination. Two multivariable logistic regression analyses were performed for the two different outcome variables: successful postoperative weight loss at 1 year after surgery and successful postoperative weight loss 2 years after surgery. Additionally, the logistic regression analyses were conducted for the SG and GBP cohort separately. Multicollinearity and effect modification were assessed for all included variables in the final multivariable logistic regression.
Statistical analyses were performed using SPSS software (version 26). Significance levels were set for p value <0.05.
Results
Of the 8751 patients included in the final analysis, 1739 (19.9%) were male and 7012 (80.1%) female, with a mean age of 45.2 ± 11.3 years and median baseline BMI of 41.7 kg/m2 (IQR 39.3–45 kg/m2). The majority underwent a Roux-en-Y gastric bypass procedure (n = 6178 (70.6%)).
Both cohorts lost a significant amount of preoperative weight (Table 1). This was achieved within a median of 18 weeks (IQR 13–28). Weight loss at 1 year was for both procedures substantial (SG −12.6 kg/m2, GBP −13.7 kg/m2). After 2 years of surgery, there was a slight weight regain in the SG cohort compared to year 1 with a median BMI increase of 0.4 kg/m2, whereas results for GBP were comparable at year 1 and year 2, median BMI from 27.6 to 27.5 kg/m2.
Impact of Preoperative Weight Loss
The multivariate analysis is demonstrated in Table 2. Preoperative weight loss remained significantly associated with successful postoperative weight loss (≥ 25% TWL) at 1 and 2 years for the gastric bypass cohort. After adjusting for confounders, the category 0–5% preoperative weight loss demonstrated an OR of 1.45, 95% CI (1.27–1.71), p < 0.001 at year 1 and OR 1.25 95% CI (1.08–1.46), p < 0.004 at year 2. This was also seen for the gastric sleeve procedure at year 1, OR for preoperative weight loss 0–5% 1.32 95% CI (1.07–1.62), p < 0.009. However, at year 2, preoperative weight loss was only significant if at least 5% was lost, OR 1.30 95% CI (1.03–1.64), p < 0.029.
Patients with higher preoperative weight loss of 5–10% had an increased likelihood for achieving 25% TWL compared to 0–5%, OR 2.13 (CI (1.64–2.77), p < 0.001) vs 1.45 (CI (1.23–1.71) p < 0.001) for the GBP group at year 1 and OR 1.79 (CI (1.42–2.25), p < 0.001) vs 1.25 (CI (1.08–1.46), p < 0.004) for year 2. This was the same for the SG group at year 1, OR 1.76 (CI (1.36–2.28), p < 0.001) vs 1.32 (CI (1.07–1.62), p < 0.009) and year 2 postoperatively, OR 1.30 (CI (1.03–1.64), p < 0.029) vs 1.14 (CI (0.94–1.38), p < 0.198).
Discussion
In this nationwide study comprised of 8751 participants, patients with preoperative weight loss were more likely to achieve ≥25% postoperative TWL at 1 and 2 years after primary bariatric surgery. Moreover, in the multivariable analysis, a higher threshold of preoperative weight loss led to higher likelihood for successful postoperative weight loss.
A recent review by Chinaka et al. investigated the effect of preoperative weight loss on postoperative weight loss and demonstrated a lot of controversial evidence [12]. They concluded that there is not enough evidence for a pre-specified weight loss practice and calls for a large-scale multi-center study. Additionally, in our study, baseline BMI was not only predictive in most cases for postoperative success, higher baseline BMIs also demonstrated higher odds for success. This finding supports the work of a large cohort study by Jain et al. (n = 4935) which demonstrates that BMI is an important factor regarding postoperative weight loss [13]. However, this result has not been previously described by other cohort studies [14,15,16]. A systematic review from 2019 concluded that although baseline BMI is highly correlated with long-term weight loss, preoperative factors for weight loss are inconsistent in reporting and show methodological flaws [17].
Male individuals are associated with less successful postoperative weight loss in the GBP cohort in our study, but are not significant for the SG cohort. Findings in the literature of gender on postoperative weight loss also remain controversial. Some studies found no difference [18, 19], others found greater weight loss in men [20, 21] and other authors suggested more benefit for females [22]. An explanation could be that predictors for weight loss are different for men and women [23].
In our study, older age was associated with less successful weight loss at 1 and 2 years after surgery. These results reflect those of previous research [14, 24, 25].
Obesity-related comorbidities demonstrated a lower likelihood in achieving 25% TWL, except for musculoskeletal pain in the GBP cohort with an OR of 1.35 (CI 1.15–1.60, p < 0.001) at year 1 and OR 1.37 (CI 1.19–1.58, p < 0.001) at year 2 postoperative. Although this finding was not significant for the SG cohort, contrary to expectations the OR was 0.87 (CI 0.72–1.05), p < 0.155 and 0.90 (CI 0.76–1.07), p < 0.247 at year 1 and 2, respectively, a decreased likelihood. Whereas some authors state pain and musculoskeletal comorbidities are common barriers to physical activity [26], others demonstrated that many of the perceived barriers to physical activity are not obesity related [27]. In addition, an older systematic review suggests that no study has tested the hypothesis that pain is a barrier to physical activity in bariatric surgery patients [28].
In our study, the presence of OSAS appeared to be significantly associated with unsuccessful weight loss in the GBP cohort. Although OSAS is a bidirectional association and not fully understood [29, 30], some authors suggested that sleep apnea could cause weight gain [31] and discontinuation of CPAP postoperative was associated with weight gain [32].
The only significant comorbidity for both cohorts at all time points in our study was diabetes mellitus. Consistent with the literature, diabetes was negatively associated with weight loss postoperative [33,34,35,36]. However, a large retrospective cohort study stated that preoperative use of insulin was associated with better long-term weight loss [16]. It is difficult to interpret these results as we did not distinguish between insulin- and non-insulin-dependent diabetes in our study.
Notably, between the SG and GBP cohorts, the variables gender, musculoskeletal pain, and baseline BMI are differently associated with the outcome successful postoperative TWL. SG and GBP are the most frequently performed procedures in bariatric surgery and consequently there is a lot of ongoing research on the potential different outcomes. Lee et al. stated based on their systematic review that GBP procedures resulted in greater loss of BMI compared to SG at year 1 and year 3 postoperative [37], whereas the authors of another meta-analysis concluded there was not any statistical difference between groups within this time period [38]. Although there is no consensus on the weight loss patterns between these different procedures yet, it could be an explanation to the differences in our findings.
There are several limitations to this study. It had a non-randomized, retrospective design and the quality registry consists of indirect source data. On the other hand, this mandatory registry is subject to an auditing system. Additionally, the time frame in which patients lost weight ranged from 1 week to 1 year and it remained unclear whether screening BMI was the highest BMI for a patient or after already losing weight by a preconditioning program. Furthermore, the method of achieving preoperative weight loss is unknown as it is not mandatory in the national guideline [39]. Nevertheless, this is one of the largest nationwide cohort studies evaluating the association of preoperative weight loss on successful postoperative weight loss including both sleeve gastrectomy and Roux-en-Y gastric bypass procedures.
Conclusion
Patients with a preoperative weight loss of >5% were associated with successful weight loss defined as ≥25% TWL up to 2 years after surgery for both sleeve gastrectomy and Roux-en-Y procedures. The extent of preoperative weight loss contributes to the significance and odds of this success. No specific threshold of loss was identified to oblige a certain amount of preoperative weight loss; nevertheless, it should be encouraged firmly. Large randomized controlled studies with longer follow-up are needed to evaluate the sustainability of preoperative weight loss.
References
Fried M, Yumuk V, Oppert JM, Scopinaro N, Torres A, Weiner R, et al. Interdisciplinary European guidelines on metabolic and bariatric surgery. Obes Surg. 2014;24(1):42–55. Epub 2013/10/02. https://doi.org/10.1007/s11695-013-1079-8.
Mechanick JI, Apovian C, Brethauer S, Timothy Garvey W, Joffe AM, Kim J, et al. Clinical practice guidelines for the perioperative nutrition, metabolic, and nonsurgical support of patients undergoing bariatric procedures - 2019 update: cosponsored by American Association of Clinical Endocrinologists/American College of Endocrinology, The Obesity Society, American Society for Metabolic and Bariatric Surgery, Obesity Medicine Association, and American Society of Anesthesiologists. Obesity (Silver Spring). 2020;28(4):O1-O58. Epub 2020/03/24. doi: https://doi.org/10.1002/oby.22719.
Thorell A, MacCormick AD, Awad S, Reynolds N, Roulin D, Demartines N, et al. Guidelines for perioperative care in bariatric surgery: enhanced recovery after surgery (ERAS) society recommendations. World J Surg 2016;40(9):2065–83. Epub 2016/03/05. doi: 10.1007/s00268-016-3492-3.
Cassie S, Menezes C, Birch DW, Shi X, Karmali S. Effect of preoperative weight loss in bariatric surgical patients: a systematic review. Surg Obes Relat Dis 2011;7(6):760–7; discussion 7. Epub 2011/10/08. doi: 10.1016/j.soard.2011.08.011.
Anderin C, Gustafsson UO, Heijbel N, Thorell A. Weight loss before bariatric surgery and postoperative complications: data from the Scandinavian obesity registry (SOReg). Ann Surg 2015;261(5):909–13. Epub 2014/09/12. doi: 10.1097/SLA.0000000000000839.
Livhits M, Mercado C, Yermilov I, Parikh JA, Dutson E, Mehran A, et al. Preoperative predictors of weight loss following bariatric surgery: systematic review. Obes Surg 2012;22(1):70–89. Epub 2011/08/13. doi: 10.1007/s11695-011-0472-4.
Fennig U, Snir A, Halifa-Kurzman I, Sela A, Hadas A, Fennig S. Pre-surgical weight loss predicts post-surgical weight loss trajectories in adolescents enrolled in a bariatric program. Obes Surg 2019;29(4):1154–63. Epub 2019/01/04. doi: 10.1007/s11695-018-03649-8.
Monfared S, Athanasiadis DI, Furiya A, Butler A, Selzer D, Hilgendorf W, et al. Do mandated weight loss goals prior to bariatric surgery improve postoperative outcomes? Obes Surg 2020;30(3):889–94. Epub 2019/11/11. doi: 10.1007/s11695-019-04275-8.
Chinaka U, Fultang J, Ali A. Does preoperative weight loss predict significant postoperative weight loss among patients who underwent laparoscopic sleeve gastrectomy? Cureus. 2019;11(10):e5870. Epub 2019/11/26. doi: 10.7759/cureus.5870. PubMed PMID: 31763093; PubMed Central PMCID: PMCPMC6834096.
Gerber P, Anderin C, Gustafsson UO, Thorell A. Weight loss before gastric bypass and postoperative weight change: data from the Scandinavian obesity registry (SOReg). Surg Obes Relat Dis 2016;12(3):556–62. Epub 2016/02/29. doi: 10.1016/j.soard.2015.08.519.
Poelemeijer YQM, Liem RSL, Nienhuijs SW. A Dutch nationwide bariatric quality registry: DATO. Obes Surg. 2018;28(6):1602–10. Epub 2017/12/24. doi: 10.1007/s11695-017-3062-2. PubMed PMID: 29273926; PubMed Central PMCID: PMCPMC5973991.
Chinaka U, Fultang J, Ali A, Rankin J, Bakhshi A. Pre-specified weight loss before bariatric surgery and postoperative outcomes. Cureus. 2020;12(12):e12406. Epub 2021/02/06. doi: https://doi.org/10.7759/cureus.12406. PubMed PMID: 33542862; PubMed Central PMCID: PMCPMC7849210.
Jain D, Sill A, Averbach A. Do patients with higher baseline BMI have improved weight loss with roux-en-Y gastric bypass versus sleeve gastrectomy? Surg Obes Relat Dis. 2018;14(9):1304–9. Epub 2018/07/26. https://doi.org/10.1016/j.soard.2018.05.014.
Nickel F, de la Garza JR, Werthmann FS, Benner L, Tapking C, Karadza E, et al. Predictors of risk and success of obesity surgery. Obes Facts. 2019;12(4):427–39. Epub 2019/08/16. doi: 10.1159/000496939. PubMed PMID: 31416073; PubMed Central PMCID: PMCPMC6758709.
Csendes A, Burgos AM, Martinez G, Figueroa M, Castillo J, Diaz JC. Loss and regain of weight after laparoscopic sleeve gastrectomy according to preoperative BMI : late results of a prospective study (78-138 months) with 93% of follow-up. Obes Surg 2018;28(11):3424–30. Epub 2018/06/30. doi: 10.1007/s11695-018-3356-z.
Wood GC, Benotti PN, Lee CJ, Mirshahi T, Still CD, Gerhard GS, et al. Evaluation of the association between preoperative clinical factors and long-term weight loss after roux-en-Y gastric bypass. JAMA Surg. 2016;151(11):1056–62. Epub 2016/08/18. https://doi.org/10.1001/jamasurg.2016.2334.
Cottam S, Cottam D, Cottam A. Sleeve gastrectomy weight loss and the preoperative and postoperative predictors: a systematic review. Obes Surg. 2019;29(4):1388–96. Epub 2019/01/21. https://doi.org/10.1007/s11695-018-03666-7.
Kennedy-Dalby A, Adam S, Ammori BJ, Syed AA. Weight loss and metabolic outcomes of bariatric surgery in men versus women - a matched comparative observational cohort study. Eur J Intern Med. 2014;25(10):922–5. Epub 2014/12/04. https://doi.org/10.1016/j.ejim.2014.10.020.
Al-Khyatt W, Ryall R, Leeder P, Ahmed J, Awad S. Predictors of inadequate weight loss after laparoscopic gastric bypass for morbid obesity. Obes Surg. 2017;27(6):1446–52. Epub 2016/12/13. https://doi.org/10.1007/s11695-016-2500-x.
Jambhekar A, Maselli A, Robinson S, Kabata K, Gorecki P. Demographics and socioeconomic status as predictors of weight loss after laparoscopic sleeve gastrectomy: a prospective cohort study. Int J Surg. 2018;54(Pt A):163–9. Epub 2018/04/24. doi: 10.1016/j.ijsu.2018.04.025.
Stroebele-Benschop N, Machado A, Milan FMP, Wössner C, Soz D, Bischoff SC. Gender differences in the outcome of obesity treatments and weight loss maintenance - a systematic review. J Obes Weight Loss Ther. 2013;3(4). doi: 10.4172/2165-7904.1000176.
Groth D, Wozniewska P, Olszewska M, Zabielski P, Ladny JR, Dadan J, et al. Gender-related metabolic outcomes of laparoscopic sleeve gastrectomy in 6-month follow-up. Wideochir Inne Tech Maloinwazyjne. 2020;15(1):148–56. Epub 2020/03/03. doi: 10.5114/wiitm.2019.86800. PubMed PMID: 32117498; PubMed Central PMCID: PMCPMC7020728.
Andersen JR, Aadland E, Nilsen RM, Vage V. Predictors of weight loss are different in men and women after sleeve gastrectomy. Obes Surg. 2014;24(4):594–8. Epub 2013/11/19. https://doi.org/10.1007/s11695-013-1124-7.
Cadena-Obando D, Ramirez-Renteria C, Ferreira-Hermosillo A, Albarran-Sanchez A, Sosa-Eroza E, Molina-Ayala M, et al. Are there really any predictive factors for a successful weight loss after bariatric surgery? BMC Endocr Disord. 2020;20(1):20. Epub 2020/02/07. doi: 10.1186/s12902-020-0499-4. PubMed PMID: 32024495; PubMed Central PMCID: PMCPMC7003414.
El Moussaoui I, Van Vyve E, Johanet H, Dabrowski A, Piquard A, Delaunay T, et al. Laparoscopic sleeve gastrectomy for morbid obesity in a Belgian-French prospective multicenter study: outcomes and predictors weight loss failure. Acta Chir Belg. 2020:1–7. Epub 2020/10/23. https://doi.org/10.1080/00015458.2020.1841485.
Joseph PL, Bonsignore A, Kunkel GF, Grace SL, Sockalingam S, Oh P. Benefits and barriers to exercise among individuals with class III obesity. Am J Health Behav. 2019;43(6):1136–47. Epub 2019/10/31. https://doi.org/10.5993/AJHB.43.6.11.
Zabatiero J, Hill K, Gucciardi DF, Hamdorf JM, Taylor SF, Hagger MS, et al. Beliefs, barriers and facilitators to physical activity in bariatric surgery candidates. Obes Surg 2016;26(5):1097–109. Epub 2015/09/02. doi: 10.1007/s11695-015-1867-4.
Speck RM, Bond DS, Sarwer DB, Farrar JT. A systematic review of musculoskeletal pain among bariatric surgery patients: implications for physical activity and exercise. Surg Obes Relat Dis. 2014;10(1):161–70. Epub 2013/11/05. https://doi.org/10.1016/j.soard.2013.08.001.
Kuvat N, Tanriverdi H, Armutcu F. The relationship between obstructive sleep apnea syndrome and obesity: a new perspective on the pathogenesis in terms of organ crosstalk. Clin Respir J. 2020;14(7):595–604. Epub 2020/03/01. https://doi.org/10.1111/crj.13175.
Romero-Corral A, Caples SM, Lopez-Jimenez F, Somers VK. Interactions between obesity and obstructive sleep apnea: implications for treatment. Chest. 2010;137(3):711–9. Epub 2010/03/06. doi: 10.1378/chest.09-0360. PubMed PMID: 20202954; PubMed Central PMCID: PMCPMC3021364.
Unnikrishnan D, Jun J, Polotsky V. Inflammation in sleep apnea: an update. Rev Endocr Metab Disord. 2015;16(1):25–34. Epub 2014/12/17. doi: 10.1007/s11154-014-9304-x. PubMed PMID: 25502450; PubMed Central PMCID: PMCPMC4346503.
Collen J, Lettieri CJ, Eliasson A. Postoperative CPAP use impacts long-term weight loss following bariatric surgery. J Clin Sleep Med. 2015;11(3):213–7. Epub 2014/12/18. doi: 10.5664/jcsm.4528. PubMed PMID: 25515283; PubMed Central PMCID: PMCPMC4346641.
Ortega E, Morinigo R, Flores L, Moize V, Rios M, Lacy AM, et al. Predictive factors of excess body weight loss 1 year after laparoscopic bariatric surgery. Surg Endosc. 2012;26(6):1744–50. Epub 2012/01/12. https://doi.org/10.1007/s00464-011-2104-4.
Kitamura R, Chen R, Trickey A, Eisenberg D. Positive and negative independent predictive factors of weight loss after bariatric surgery in a veteran population. Obes Surg. 2020;30(6):2124–30. Epub 2020/02/06. https://doi.org/10.1007/s11695-020-04428-0.
Barhouch AS, Padoin AV, Casagrande DS, Chatkin R, Sussenbach SP, Pufal MA, et al. Predictors of excess weight loss in obese patients after gastric bypass: a 60-month follow-up. Obes Surg. 2016;26(6):1178–85. Epub 2015/10/05. https://doi.org/10.1007/s11695-015-1911-4.
Still CD, Wood GC, Chu X, Manney C, Strodel W, Petrick A, et al. Clinical factors associated with weight loss outcomes after Roux-en-Y gastric bypass surgery. Obesity (Silver Spring). 2014;22(3):888–94. Epub 2013/06/28. doi: 10.1002/oby.20529. PubMed PMID: 23804287; PubMed Central PMCID: PMCPMC3819407.
Lee Y, Doumouras AG, Yu J, Aditya I, Gmora S, Anvari M, et al. Laparoscopic sleeve gastrectomy versus laparoscopic roux-en-Y gastric bypass: a systematic review and meta-analysis of weight loss, comorbidities, and biochemical outcomes from randomized controlled trials. Ann Surg. 2021;273(1):66–74. Epub 2019/11/07. https://doi.org/10.1097/SLA.0000000000003671.
Yang P, Chen B, Xiang S, Lin XF, Luo F, Li W. Long-term outcomes of laparoscopic sleeve gastrectomy versus roux-en-Y gastric bypass for morbid obesity: results from a meta-analysis of randomized controlled trials. Surg Obes Relat Dis. 2019;15(4):546–55. Epub 2019/03/04. https://doi.org/10.1016/j.soard.2019.02.001.
Mechanick JI, Apovian C, Brethauer S, Garvey WT, Joffe AM, Kim J, Kushner RF, Lindquist R, Pessah-Pollack R, Seger J, Urman RD, Adams S, Cleek JB, Correa R, Figaro MK, Flanders K, Grams J, Hurley DL, Kothari S, Seger MV, Still CD. Clinical practice guidelines for the perioperative nutrition, metabolic, and nonsurgical support of patients undergoing bariatric procedures - 2019 update: cosponsored by American Association of Clinical Endocrinologists/American College of Endocrinology, the Obesity Society, American Society for Metabolic & Bariatric Surgery, Obesity Medicine Association, and American Society of Anesthesiologists - executive summary. Endocr Pract. 2019 Dec;25(12):1346–1359. doi: https://doi.org/10.4158/GL-2019-0406. Epub 2019 Nov 4.
Acknowledgements
E.J. Hazebroek, MD, PhD (Rijnstate Hospital, Arnhem); L.M. de Brauw, MD, PhD (MC Slotervaart, Amsterdam); A. Demirkiran, MD, PhD (Red Cross Hospital, Beverwijk); M. Dunkelgrün, MD, PhD (Franciscus Gasthuis & Vlietland, Rotterdam); I.F. Faneyte, MD, PhD (ZGT Hospital, Hengelo); J.W.M. Greve, MD, PhD (Zuyderland MC, Heerlen); M.J. Wiezer, MD, PhD (St. Antonius Hospital, Nieuwegein); E.H. Jutte, MD (MC Leeuwarden, Leeuwarden); R.A. Klaassen, MD (Maasstad Hospital, Rotterdam); E.A.G.L. Lagae, MD (ZorgSaam Zorggroep Zeeuws-Vlaanderen, Terneuzen); B. Lamme, MD, PhD (Albert Schweitzer Hospital, Dordrecht); R.S.L. Liem, MD (Groene Hart Hospital, Gouda); J.K. Maring, MD, PhD (Elisabeth-TweeSteden Hospital, Tilburg); S.W. Nienhuijs, MD, PhD (Catharina Hospital, Eindhoven); R. Schouten, MD, PhD (MC Zuiderzee, Lelystad); D.J. Swank, MD, PhD (Dutch Obesity Clinic West, The Hague); G. van ‘t Hof, MD (Dutch Obesity Clinic South-West Netherlands, Bergen op Zoom); R.N. van Veen, MD, PhD (OLVG Hospital, Amsterdam); and M.W.J.M. Wouters, MD, PhD (Netherlands Cancer Institute-Antoni van Leeuwenhoek Hospital, Amsterdam).
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study formal consent is not required.
Conflict of Interest
The authors declare no competing interests.
Informed Consent
Informed Consent does not apply as this is an opt-out registry and the study has been performed in accordance with the ethical standards as stated in Dutch law and the regulations of the Dutch Institute for Clinical Auditing (DICA).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key points
• Preoperative weight loss is associated with achieving ≥ 25% TWL postoperative.
• More preoperative weight loss leads to higher odds of achieving ≥ 25% TWL.
• Diabetes mellitus and sleep apnea decrease the odds of achieving ≥ 25% TWL.
This manuscript was written on behalf of the Dutch Audit for Treatment of Obesity (DATO) Group, listed in the “Acknowledgements.”
Rights and permissions
About this article
Cite this article
Lodewijks, Y., Akpinar, E., van Montfort, G. et al. Impact of Preoperative Weight Loss on Postoperative Weight Loss Revealed from a Large Nationwide Quality Registry. OBES SURG 32, 26–32 (2022). https://doi.org/10.1007/s11695-021-05760-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-021-05760-9