Abstract
Objective
Our aim was to conduct an up-to-date systematic review of randomised controlled trials (RCTs) to determine the benefits and harms of enhanced recovery after surgery (ERAS) programme in bariatric surgery.
Methods
MEDLINE, Embase, PubMed, CINAHL and the Cochrane Library were searched for RCTs on ERAS versus standard care (SC) until April 2020. The primary endpoint was the length of hospital stay (LOS).
Results
Five RCTs included a total of 610 procedures. ERAS adoption is capable of significantly reducing LOS (MD of − 0.51; 95% CI − 0.92 to − 0.10; P = 0.01) and postoperative nausea and vomiting (PONV) (OR 0.42; 95% CI 0.19 to 0.95; P = 0.04). No significant differences in terms of adverse events and readmissions.
Conclusions
The implementation of ERAS in bariatric surgery produces a significant reduction in LOS and PONV.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bariatric surgery represents the most effective and durable treatment for morbid obesity [1]. Laparoscopic sleeve gastrectomy (LSG) and laparoscopic Roux-en-Y gastric bypass (LRYGB) are the most popular bariatric surgical techniques worldwide [2]. Thanks to the widespread adoption of laparoscopic surgery and the implementation of perioperative care of obese patients, bariatric surgery stands out from other surgical fields as leading to lower morbidity and mortality and shorter lengths of hospital stay (LOS) [3,4,5]. Nevertheless, bariatric surgeons are still making efforts to further improve the care of bariatric patients through the application of enhanced recovery after surgery (ERAS) programmes in bariatric surgery. The objective of ERAS programmes is to incorporate evidence-based strategies into the preoperative, intraoperative and postoperative care plan with the aim of reducing patients’ surgical stress response and accelerating their functional recovery in order to improve quality of care, decrease complications and shorten hospital stays [6]. The application of ERAS to bariatric surgery is much more recent than in other surgical fields: ERAS Society guidelines for bariatric surgery date back only to 2016 [7]. To date, several observational studies comparing the adoption of ERAS versus standard of care (SC) in bariatric surgery have suggested that ERAS is safe and capable of shortening LOS [8,9,10,11]. Three earlier meta-analyses [12,13,14] widely based on observational studies and incorporating only two small and early RCTs [15, 16] also suggested that ERAS is safe and capable of shortening LOS. Due to the recent publication of three new RCTs [17,18,19] comparing ERAS versus SC in bariatric surgery, we considered it necessary to perform an up-to-date meta-analysis of RCTs to assess all available data.
Literature Search
Two authors conducted the online systematic bibliographic research on the following databases (up to April 2020): MEDLINE, Embase, PubMed, Cumulative Index to Nursing and Allied Health Literature (CINAHL), Cochrane Central Register of Controlled Trials and Cochrane Library. The following medical subject heading (MeSH) terms or words were used for the search: “enhanced recovery after surgery”, “ERAS”, “enhanced recovery after bariatric surgery”, “ERABS”, “fast track”, “perioperative protocol”, “clinical pathway”, “multimodal perioperative” and “enhanced pathway”. These MeSH terms or words were used for the search in various combinations with the following terms: “randomized clinical trial”, “bariatric surgery”, “weight loss surgery”, metabolic surgery”, “diabetes surgery”, “gastric banding”, “sleeve gastrectomy”, “one anastomosis gastric bypass”, “single anastomosis gastric bypass”, “mini gastric bypass”, “Roux-en-Y gastric bypass”, “double loop gastric bypass”, “duodenal switch” and “biliopancreatic diversion”.
A manual search of the Google Scholar database and of eight high impact journals was also conducted (Obesity Surgery, Surgery for Obesity and Related Disease, Annals of Surgery, British Journal of Surgery, Surgery, JAMA Surgery, Surgical Endoscopy and Journal of the American College of Surgeons). Additional articles were searched in the reference lists of the articles selected for full-text review. Only articles with full text in English were considered.
Study Selection
We planned to include in this systematic review only RCTs (irrespective of sample size or blinding) enrolling adult (age ≥ 18 years) obese (BMI ≥ 30 Kg/m2) patients undergoing bariatric/metabolic surgery (each surgical bariatric/metabolic procedure indicated in the 2019 IFSO consensus statement [22]) and comparing the use of an ERAS programme versus perioperative SC. We considered for inclusion only RCTs in which the ERAS pathways were composed of elements applied in the entirety of the patient’s pathway phases—preoperative, intraoperative and postoperative phases—and which reported at least one of the outcomes of interest. RCTs were excluded if they reported outcomes for an enhanced recovery programme not covering any of the patient’s pathway phases (preoperative, postoperative and intraoperative), if they did not report at least one of the outcomes of interest and if they enrolled non-obese patients or patients aged < 18 years.
Data Extraction
The abstracts of the articles retrieved through the bibliographic research were independently evaluated by three authors. Discrepancies were resolved after consensus between the three authors were reached. We retrieved the full text of the potentially eligible articles, which were independently assessed by the three authors in order to evaluate their eligibility with respect to the exclusion and inclusion criteria of the systematic review. Disagreements were discussed by the authors. In the event of overlapping institutions, authors or patient cohorts, the most recent article was selected.
The primary goal of this systematic review was to evaluate the benefits and drawbacks of the application of the ERAS programme in bariatric/metabolic surgery and to determine whether its adoption is capable of significantly reducing LOS compared with SC.
The primary endpoint was to determine the LOS (in days) as a result of the ERAS programme.
The secondary outcomes included the following determinations:
-
Adverse events (all reported adverse events)
-
Major adverse events (grade ≥ 3 based on Clavien-Dindo classification of complications [23])
-
Readmissions
-
Anastomotic leak
-
Intra-abdominal bleeding
-
Mortality
-
Postoperative nausea and vomiting (PONV)
-
Postoperative pain, assessed using validated methods such as the visual analogue scale (VAS)
Assessment of Methodological Quality and Bias Risk of the Included Studies
The methodological quality of the included RCTs was assessed by two reviewers using the Jadad scale [24, 25]. The Jadad scale is a five-item scale assessing randomisation, blinding, withdrawals and dropouts of an RCT. The scale is composed by the following items/questions: (1) is the study described as randomised?; (2) is the methods of randomisation adequate?; (3) is the study described as double blinded?; (4) is the method of double-blinding appropriate?; and (5) was there a description of withdrawals and dropouts? For each question, one point is assigned for an affirmative response and zero point for a negative response. The score of the Jadad scale ranges from 0 (lowest quality) to 5 (highest quality). Good or very good methodological quality is considered for RCTs with scores from 3 to 5 points, while a score from 0 to 2 points indicates a poor study quality.
The Cochrane Collaboration’s tool was used by two authors to independently assess the risk of bias by evaluating the following bias domains: selection, performance, detection, attrition, selective reporting and other risks of bias.
Statistical Analysis
We used odds ratio (OR) or risk difference (RD) summary statistics with the Mantel-Haenszel method [26, 27] to analyse dichotomous variables. The RD as a summary statistic measure was used and reported in the result section in the events of trials reporting dichotomous endpoints with 0 events in both treatment groups (ERAS and SC) to also include them in the pooled estimated effect. Nevertheless, a sensitivity analysis using the OR was also performed in these cases.
The continuous variables were analysed using the mean difference (MD) as a summary statistic measure with the generic inverse variance method.
Pooled OR, RD and MD values were reported with 95% confidence intervals (CIs). A P value < 0.05 was considered statistically significant. In the event an included RCT reported continuous data in the form of medians with ranges, the mean and standard deviation was calculated using the method suggested by Hozo and colleagues [28]; if only the mean values were provided, standard deviations were calculated as described in the Cochrane Handbook [20]. If continuous data were reported as median and interquartile range (IQR), the mean and standard deviation was estimated using the method suggested by Wan et al. [29].
Statistical analysis was performed following the intent-to-treat principle. The χ2 test (with P < 0.05 indicating statistical significance) was used to assess statistical heterogeneity, while clinical heterogeneity was defined by calculating the I2 value, with a value > 50% indicating substantial clinical heterogeneity. If significant or substantial statistical or clinical heterogeneity was found, random-effects analysis was reported; otherwise, fixed-effects analysis was used for meta-analysis [30]. Funnel plots were used for investigating publication bias.
Meta-analysis was not performed if insufficient data (< 2 RCTs) were reported for a specific outcome. In this case, a descriptive analysis was carried out by indicating and describing the RCTs reporting a significant difference between the procedures (P < 0.05).
Subgroup analysis was performed, where possible, to assess the outcomes of interest in patients undergoing different bariatric surgical procedures (e.g. LSG, RYGB). Sensitivity analysis was conducted by excluding low-quality studies after their assessment with the Jadad scale.
Meta-analysis was performed using Review Manager version 5.3 (the Cochrane Collaboration, the Nordic Cochrane Centre, Copenhagen, Denmark).
Results
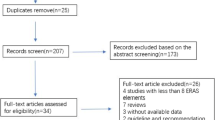
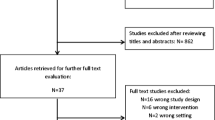
After bibliographic research, 245 records were identified (Fig. 1), of which 210 records were duplicates or did not meet the inclusion criteria after title or abstract assessment and were excluded. We then evaluated a total of 35 full-text articles, of which 30 did not meet the inclusion/exclusion criteria and were excluded. Ultimately, five RCTs [15,16,17,18,19] met the criteria for inclusion in this systematic review and were included in the quantitative and qualitative analysis. These five RCTs accounted for a total of 610 patients, of whom 306 (50.2%) were in the ERAS group and 304 (49.8%) were in the SC group.
Study Characteristics
In Table 1, we indicate the characteristics of the studies included in the systematic review. The included RCTs were monocentric and were conducted in Brazil [16], New Zealand [15], the Netherlands [17], Spain [18] and India [19]; additionally, they were published between 2013 and 2020. The sample size ranged from 10 to 220 patients. The RCT of Pimenta et al. [16] was conducted in 2012; the RCT of Lemanu et al. [15] was conducted between 2011 and 2012; the RCTs by Geubbels et al. [17] were carried out from 2013 to 2014; the RCT by Ruiz-Tovar et al. [18] was performed between 2016 and 2017; and the RCT of Prabhakaran et al. [19] was conducted from 2017 to 2018. In three of the included studies, the bariatric surgical procedure was the LSG [15, 16, 19]; in the remaining two RCTs, the bariatric surgical procedure was the LRYGB [17, 18]. All included studies reported the sample size calculation with a power analysis. The sample size calculation was based on the LOS primary outcome in four RCTs [15,16,17, 19] and on the postoperative pain 24 h after surgery (using the VAS score) primary endpoint in one of the included RCTs [18].
In the ERAS patient group of the included trials, the mean preoperative BMI ranged from 42 [17] to 46.2 kg/m2 [15]; in the SC patients group, the mean preoperative BMI ranged from 41.4 [17] to 46.1 kg/m2 [15].
The proportion of female patients in the included studies was as follows: 70.5% in Lemanu et al.’s study [15], 90% in Pimenta et al.’s study [16], 87.2% in Geubbels et al.’s study [17], 72.2% in Ruiz-Tovar et al.’s study [18] and 67.8% in Prabhakaran et al.’s study [19].
Of the patients in Lemanu et al.’s study [15], 60.2% were affected by type 2 diabetes mellitus (T2DM). The corresponding percentages for Geubbels et al.’s study [17], Ruiz-Tovar et al.’s study [18] and Prabhakaran et al.’s study [19] were 16.1%, 28.3% and 39.2%, respectively. The study by Pimenta et al. [16] did not report data about the proportion of T2DM patients.
The proportion of patients affected by obstructive sleep apnea (OSA) was 21.8% in Lemanu et al.’s study [15], 6.8% in Geubbels et al.’s study [17], 64.4% in Ruiz-Tovar et al.’s study (also including patients with sleep apnea-hypopnea syndrome (SAHS)) [18] and 8.9% in Prabhakaran et al.’s study [19]. The study by Pimenta et al. [16] did not report data about the proportion of OSA patients.
The actual ERAS guidelines for bariatric surgery published by the ERAS Society in 2016 reported a total of 21 items and 36 recommendations for the application of ERAS in bariatric surgery [7]. In Table 2, we summarised the items and recommendations indicating the adoption of any recommendations in the included RCTs. The assessment of the adoption of any recommendations in the included studies was extrapolated by analysing the ERAS protocols reported or described in any published studies. Based on this assessment, the number of ERAS recommendations found to be adopted in the included RCTs was 12/36 (33.3%) in Lemanu et al. [15], 10/36 (27.7%) in Pimenta et al. [16], 15/36 (41.6%) in Geubbels et al. [17], 20/36 (55.5%) in Ruiz-Tovar et al. [18] and 17/36 (47.2%) in Prabhakaran et al. [19]. Only one trial [15] assessed compliance with the planned ERAS items in the randomised patients.
Assessment of Methodological Quality and Bias Risk
All of the included RCTs had good methodological quality, obtaining at least three points after assessment with the Jadad scale [24], as indicated in Table 1.
The assessment of the risk of bias in the included RCTs is reported in Table 3. All of the included RCTs reported the use of an appropriate randomisation process. In three studies [15, 17, 19], concealment of allocation was performed using appropriate methods, whereas in two studies [16, 18], the methods were not described. Four studies [15, 17,18,19] stated no blinding for patients, while in one study, the blinding of patients was unclear [16]. Two studies reported no blinding of outcome assessors [15, 17], while another two studies reported a blinding of outcome assessors [18, 19]; in one study, the blinding of outcome assessment was unclear [16]. In four of the included trials, the risk for attrition bias was low [15,16,17,18]; in one study, data about patient follow-ups and attrition were not clearly reported or discussed [19]. None of the included trials suffered from reporting bias. The risk for other bias was judged low in two RCTs [15, 17] and uncertain in the remaining 3 trials [16, 18, 19].
The overall risk of bias was considered low for four RCTs [15, 17,18,19] and was unclear for one RCT [16]. The methodological assessment with the Jadad scale and the judgement of risk of bias was based on a consideration of whether, in the ERAS studies, blinding was impossible to apply to patients and staff due to the nature of ERAS itself, but only to outcome data assessors.
Meta-Analysis Results
The meta-analysis results for the dichotomous and continuous endpoints are reported in Table 4.
Length of Hospital Stay (LOS)
All trials included in the analysis reported data about the LOS [15,16,17,18,19]. The LOS was significantly shorter in the ERAS group than in the SC treatment (P = 0.01) with a pooled MD of − 0.51 (− 0.92 to − 0.10). The analysis shows significant heterogeneity (χ2 = 18.43, I2 = 78%) (Fig. 2). The funnel plot was symmetrical (figure not shown).
Adverse Events
All studies investigated adverse events [15,16,17,18,19]. The pooled RD is 0.01 (− 0.04 to 0.06) in favour of the SC group (P = 0.74) without heterogeneity (χ2 = 0.49, I2 = 0%) (Fig. 3). The funnel plot was symmetrical (not shown).
Major Adverse Events
It was possible to extract the rate of postoperative major adverse events from the five included RCTs [15,16,17,18,19]. The pooled RD is 0.01 (− 0.02 to 0.04) in favour of SC (P = 0.41), without heterogeneity (χ2 = 2.11, I2 = 0%) (Fig. 4). The funnel plot was symmetrical (figure not shown).
Readmissions
It was possible to extract the number of readmissions from all of the included RCTs [15,16,17,18,19]. The pooled RD for readmissions is 0.01 (− 0.03 to 0.04) in favour of SC (P = 0.76), without significant heterogeneity (χ2 = 3.49, I2 = 0%) (Fig. 5). The funnel plot was symmetrical (figure not shown).
Anastomotic Leak
The proportion of anastomotic leak was reported or was possible to extract in four of the included trials [15, 16, 18, 19]. The pooled RD for anastomotic leak is − 0.00 (− 0.03 to 0.03) in favour of the ERAS group (P = 0.97), without heterogeneity (χ2 = 0, I2 = 0%) (Fig. 6). The funnel plot was symmetrical (figure not shown).
Intra-abdominal Bleeding
Four RCTs reported data about intra-abdominal bleeding [15, 16, 18, 19]. The pooled RD for intra-abdominal bleeding was − 0.00 (− 0.03 to 0.03) in favour of the ERAS group (P = 0.97), without heterogeneity (χ2 = 0, I2 = 0%). The funnel plot was symmetrical (figure not shown).
Mortality
There were no deaths reported in any of the included RCTs [15,16,17,18,19]. The pooled RD for death was 0.00 (− 0.01 to 0.01), P = 1.00, without heterogeneity (χ2 = 0, I2 = 0%). The funnel plot was not available because no events were reported in any of the included trials.
Postoperative Nausea and Vomiting
Three RCTs reported data about postoperative nausea and vomiting [16, 18, 19]. The pooled OR for postoperative nausea and vomiting is 0.42 [0.19 to 0.95] in favour of the ERAS group (P = 0.04), without heterogeneity (χ2 = 1.67, I2 = 0%) (Fig. 7). The funnel plot was symmetrical (figure not shown).
Postoperative Pain
Only three of the included trials reported data about postoperative pain, but in a format not amenable for pooled analysis [17,18,19]. In the study by Geubbels et al., the time needed to achieve control of postoperative pain (visual analogue scale, VAS score ≤ 4) was significantly (P < 0.009) shorter in the ERAS group (median 1.2 h) than in the SC group (median 2 h) [17]. In the study by Ruiz-Tovar et al., the mean postoperative pain was assessed with the VAS score 24 h after surgery. The 24-h postoperative mean VAS score was significantly lower (P < 0.001) in the ERAS group (mean 16 mm) with respect to the SC group (mean 37 mm) [18]. In the study by Prabhakaran et al., postoperative pain scores were measured with a VAS score at 1, 2, 4, 8, 12, 16, 20 and 24 h [19]. The postoperative VAS pain score was significantly lower in the ERAS group with respect to the SC group only at 4 h (mean 2.39 versus 2.13, P = 0.003) and 8 h (mean 1.49 versus 2.11, P = 0.013).
Results of the Subgroup Analysis for LSG and LRYGB Procedures and of Sensitivity Analysis
Sensitivity analysis was not carried out as none of the included RCTs was judged to be of low methodological quality on the basis of the Jadad scale assessment.
The results of the subgroup analysis for LSG and LRYGB are reported in Table 4.
For each evaluated outcome in the systematic review, the subgroup analysis for the LSG and LRYGB procedures did not significantly improve statistical and clinical heterogeneity between RCTs. Moreover, effect measure estimates and relative P values were not significantly affected for each of the analysed endpoints except those for LOS and PONV.
The LOS was significantly shorter in the ERAS group versus the SC group for LSG (3 RCTs [15, 16, 19]) procedures alone, with a pooled MD of − 0.56 (− 0.78 to − 0.34) in favour of the ERAS group (P < 0.00001) without heterogeneity (χ2 = 0.44, I2 = 0%). Conversely, the LOS for LRYGB procedures was not significantly different between the ERAS and SC groups (2 RCTs [17, 18]), with a pooled MD of − 0.49 (− 1.56 to 0.58) in favour of the ERAS group (P = 0.37) but still with high heterogeneity (χ2 = 7.86, I2 = 87%).
In both the LSG and LRYGB subgroup analysis, PONV occurrence was not statistically different between the ERAS and SC groups. In the LSG subgroup analysis (2 RCTs [16, 19]), the pooled OR was 0.55 (0.21 to 1.45) in favour of the ERAS group (P = 0.23) but now with moderate heterogeneity (χ2 = 1.64, I2 = 39%). The PONV rates for LRYGB procedures were reported in only one of the included RCTs [18], with an OR of 0.23 (0.05 to 1.13) in favour of the ERAS group (P = 0.07).
Discussion
To our knowledge, this is the first systematic review and meta-analysis in the literature based on RCTs to evaluate the benefits and drawbacks of ERAS in bariatric surgery compared with SC. We found that the adoption of the ERAS programmes in bariatric surgery is capable of significantly reducing the LOS by about half a day (MD − 0.51, P = 0.01) and the occurrence of postoperative nausea and vomiting (6.4% versus 13.4%, OR 0.42, P = 0.04) with respect to SC. No statistically significant differences were found in terms of overall adverse events, major adverse events, mortality or readmission. Data about postoperative pain were reported in a format not amenable for pooling in the meta-analysis.
Three previously published meta-analyses widely based on observational studies focused on the implementation of ERAS in bariatric surgery and assessed LOS with respect to SC [12,13,14]. The meta-analysis by Ahmed et al. found a significant (P < 0.01) reduction (MD − 1.5 days) in the LOS in favour of ERAS [14]. Also, the meta-analysis of Singh et al. found a significant (P < 0.001) reduction in the LOS in ERAS compared with SC (MD − 1.56 days) [12], as did the meta-analysis of Malczak et al., in which LOS was significantly (P = 0.002) shorter in the ERAS group (SMD − 2.39) [13]. In our analysis, the reduction in LOS of 0.51 days in the ERAS group, although statistically significant, was less marked than in the previous systematic review described above. This is possibly related to the influence of selection bias in the observational studies analysed in the earlier meta-analysis, which implied the inclusion of the patients with the worst baseline clinical and demographic characteristics in the SC group rather than in the ERAS group. Moreover, in some of the RCTs included in our meta-analysis, such as those by Geubbels et al. [17], a fast-track protocol was implemented as early as the ERAS protocol in the institution, and this possibly contributed to consistently reducing the LOS in the SC group, meaning that the difference with the ERAS group was less than expected. In the subgroup analysis of LOS in LRYGB procedures, we found that ERAS had no significant benefit with respect to SC. However, this evidence should be interpreted with caution because only two of the included studies reported data on LOS for RYGB procedures [17, 18]. And, in one of these RCTs [17], as underlined by the authors, the patients in the SC group had an unexpectedly short LOS, which was possibly related to the fact that a fast-track protocol was, as mentioned above, already used before the introduction of ERAS in their institution.
The ERAS Society emphasises that in the ERAS philosophy, “the key surgical end point is the quality, rather than speed of recovery”, also focusing on improving functional status, reducing variabilities of care and complications and improving patients’ satisfaction [6]. We decided to investigate LOS as a primary outcome in our study because LOS notably represents the most adopted surrogate endpoint for the assessment of patient’s functional status recovery in the ERAS literature [31, 32]. This is because the eligibility for hospital discharge is correlated with the ability to resume basic activities of daily living (ADL), recovery of physiological gastrointestinal functions and with adequate pain control. Unfortunately, these aspects of functional recovery are not or poorly investigated in the included studies, and therefore, the only way to assess patient’s functional recovery in our analysis was to use LOS. Moreover, all RCTs included in the present meta-analysis, except those of Ruiz-Tovar et al. [18], assessed the LOS as a primary endpoint. Despite LOS after bariatric surgery is already quite short even outside ERAS programmes, our meta-analysis demonstrated a statistically significant advantage in the reduction of LOS with the adoption of ERAS (only about half a day) suggesting an improved patient’s functional recovery.
The significant reduction in LOS with ERAS in some surgical fields, such as in colorectal surgery, is strongly linked with a significant reduction in postoperative complications, such as wound infections and postoperative ileus [33, 34]. In our analysis, we failed to detect any significant difference between ERAS and SC in the incidence of overall adverse events or major adverse events. In our opinion, this is partly due to the fact that postoperative complications in bariatric surgery (such as bleeding, leaks, strictures, infections and venous thromboembolism) are, even outside ERAS management, quite low when compared with other surgical fields, and therefore, a larger sample size is needed to detect possible differences between the two treatments (ERAS versus SC) with adequate statistical power [4, 5, 35, 36]. Conversely, we know that PONV represented the main cause of hospital readmission and delayed discharge after bariatric surgery [35,36,37,38]. Interestingly, we found a significant reduction in the proportion of PONV in the ERAS group with respect to the SC group (6.4% versus 13.4%, OR 0.42, P = 0.04). PONV was not investigated in previous meta-analyses of ERAS in bariatric surgery but was assessed in the three included RCTs [16, 18, 19] in our analysis. The significant reduction in PONV in the ERAS group is likely related to the fact that in all of the included RCTs except one [15], multimodal or structured strategies for PONV prevention were adopted in the ERAS groups. We speculate that based on our analysis, the reduction of PONV in the ERAS patients likely contributes to the observed shortened LOS.
The subgroup analysis conducted in this meta-analysis of patients who underwent LSG and LRYGB failed to confirm the significant reduction of PONV in the ERAS groups. However, we hypothesise that these subgroup analysis results are not consistent due to the small number of RCTs addressing PONV in LSG (2 RCTs) and LRYGB (1 RCTs) patients, making the results prone to type 2 statistical error.
The PONV prevention strategies adopted in each of the included RCTs for the ERAS patients are highly variable and are reported in Table 5. It should be highlighted that although it is difficult to determine, in light of the available evidence, the ERAS item/s with the greatest influence in determining the reduction in LOS, low oral fluid intake and a high intravenous volume of fluids administered in the postoperative phase were founded to be significant risk factors for prolonged LOS after bariatric surgery [39] and were both linked with PONV.
Earlier meta-analysis of observational studies investigating ERAS in bariatric surgery found a reduction in LOS without any significant increase in readmission [12,13,14]. In the present systematic review of RCTs, we can confirm such a finding, calculating a readmission rate of 4.9% in the ERAS group versus a rate of 4.2% in the SC group (RD 0.01, P = 0.76). This evidence along with those demonstrating no increase in adverse events confirmed that ERAS adoption in bariatric surgery is safe.
To obtain quick functional recovery after surgery is of fundamental importance the application of multimodal, opioid-sparing pain control strategies as part of the ERAS programme. All of the included RCTs reported the application of a standardised analgesia protocol in the ERAS patients, but these protocols were very heterogeneous in terms of drugs and local anaesthetic infiltration. Only the studies by Geubbels et al. [17], Ruiz-Tovar et al. [18] and Prabhakaran et al. [19] used local anaesthetic infiltration of surgical wounds in the operating theatre: The study by Prabhakaran et al. [19] used the ultrasound-guided bilateral subcostal and posterior TAP blocks, while Lemanu et al.’s study [15] reported the installation of intraperitoneal local anaesthetic. The studies by Geubbels et al. [17], Prabhakaran et al. (tramadol) [19], Pimenta et al. (morphine) [16] and Ruiz-Tovar et al. (morphine) [18] permitted opioid use only as a rescue treatment. The study by Lemanu et al. [15] did not indicate whether opioids were allowed for analgesia in the postoperative period in ERAS patients.
Unfortunately, postoperative pain was assessed in only three of the included RCTs [17,18,19], in which data were reported in a format not amenable for pooling in the meta-analysis. From qualitative assessment of these data, it emerged that the adoption of ERAS seems to be related to better control of postoperative pain, as reported in the results section.
Compliance with at least 70% to 80% of the elements of the ERAS protocols was considered critical to improving outcomes [6, 40] in bariatric surgery as well [11, 41].
In this systematic review, we assessed the ERAS protocols described in the included RCTs to extract the ERAS elements recommended in the actual guidelines of the ERAS Society for bariatric surgery [7].
In the ERAS guidelines for bariatric surgery [7], a total of 26 ERAS elements were strongly recommended, while the remaining 10 received a weak recommendation (total of 36 recommendations). The proportion of ERAS elements with a strong recommendation reported in the RCTs included in this systematic review varied between 34.6 and 65.4% of the total reported in the guidelines, with an average of 46.1% (Table 2). This translates into evidence that, in none of the included trials, compliance with at least 70% of the elements of the current ERAS guidelines [7] has been reached. In fact, it should be noted that based on the 2016 ERAS guidelines [7], the overall number of recommendations reported in the ERAS protocols of the included RCTs ranged between 10/36 (27.7%) and 20/36 (55.5%) as indicated in Table 2. Only the RCT by Lemanu et al. [15] reported an overall adherence rate to its ERAS protocol elements of 85%, but it should be noted that this study indicated in its ERAS protocol a total of only 12 recommendations among the 36 reported in the 2016 guidelines [7].
Thus, we can argue that the effects of ERAS estimated in the present meta-analysis, and in the selected RCTs, for the different outcomes are likely underestimated due to the poor adoption of the ERAS elements of the current ERAS guidelines for bariatric surgery, in particular those graded as strong recommendations. Moreover, it should be underlined that the ERAS protocols in each RCT are still very heterogeneous and that the less-reported ERAS elements were smoking and alcohol cessation, airway management, ventilation strategies, neuromuscular block, monitoring of anaesthetic depth, early postoperative nutrition, postoperative oxygenation and non-invasive positive pressure ventilation. Contrariwise the following recommendations were indicated to be adopted in all the included RCTs: preoperative counselling, preoperative administration of glucocorticoids, PONV prevention strategies, adoption of multimodal systemic medication and local anaesthetic infiltration for pain control, adoption of laparoscopic approach and avoidance of nasogastric tube. However, it was not possible to find any recommendations that were consistent across the included studies because of their heterogeneity especially in terms of timing of application, dosages and types of administrated drugs or adopted techniques as well as their poor definition in the analysed trials. It should also be noted that our speculations about the ERAS elements described in the included RCTs were based on data extracted from the included studies and should therefore be considered with caution. This is because some authors may have reported only some of the items effectively used in their ERAS protocol.
Based on our analysis, a standardisation of ERAS protocols in bariatric surgery is still lacking, despite publication in 2016 of international guidelines. Thus, national and international ERAS societies should strive to make ERAS protocols more homogeneous among institutions, thereby reducing variability in ERAS care—one of the goals of ERAS itself. This would allow the evaluation of the endpoints of ERAS in bariatric surgery with greater accuracy and precision, thus reducing statistical and clinical heterogeneity.
Despite our systematic review of ERAS in bariatric surgery being the first to be exclusively based on RCTs in the literature, it has some limitations. First, the number of RCTs selected for analysis was quite low. Nevertheless, it should be noted that all of the included RCTs except one [18] provided a power analysis for sample size calculation of the LOS endpoint that represented the primary outcome of the present systematic review, making the results obtained for this outcome reliable from a statistical point of view. It should also be considered that the well-conducted systematic reviews and meta-analyses published to date in the literature in the field of ERAS in bariatric surgery included, respectively, a total of 5 [12], 11 [13] and 13 [14] studies, of which only two were RCTs [15, 16]. Moreover, a large proportion of the included observational studies in some of these systematic reviews did not apply enhanced programmes with elements distributed in the preoperative, intraoperative and postoperative phases of the patient’s pathways (no true ERAS protocols). Although of limited quantity, the RCTs included in this systematic review have a good methodological quality; additionally, four out of five RCTs were judged as having a low risk of bias, while one had an unclear risk of bias. Two relevant objectives of the ERAS pathway are to improve patients QoL (quality of life) and reduce costs. Unfortunately, only the RCT by Geubbels et al. [17] assessed the QoL failing to detect differences between the ERAS and SC groups. Only the RCT by Lemanu et al. [15] assessed costs indicating a significant reduction in terms of total costs (calculated in New Zealand dollars, NZD) for patients in the ERAS group with respect to those in the SC group (mean 14.836 NZD versus mean 15.566 NZD, respectively). Since the surgical bariatric procedures performed in the included trials were LSG and RYGB, the results of this systematic review are generalisable only to patients who underwent these specific surgical procedures; moreover, in all of the RCTs, the primary aim of surgery is weight loss, not diabetes/metabolic surgery. To date in the literature, no trials have evaluated the use of ERAS in diabetes/metabolic surgery, which is relevant because diabetes/metabolic surgery is now considered a distinct surgical discipline with a different primary aim (treat diabetes or metabolic disease despite obesity) with respect to weight loss surgery [42]. Rubino et al. demonstrated that patients selected for diabetes/metabolic surgery presented with different demographical and clinical factors than those selected for weight loss surgery, thereby needing dedicated care and management with a relevant impact also in the implementation of dedicated ERAS pathways [42].
Further high-quality randomised trials are needed to better investigate some aspects of ERAS in bariatric surgery and diabetes/metabolic surgery. In particular, in designing these future trials, authors should consider that bariatric surgery (a type of upper GI surgery) is different from colorectal surgery (lower GI surgery), for which ERAS was developed. The assessment of ERAS in bariatric patients requires a focus on relevant outcomes for this specific field that are in large part different from the most relevant outcome used to assess ERAS in colorectal surgery, such as the assessment of PONV, dehydration, inability to tolerate liquid diets, pain control with opioid-sparing strategies and psychological aspects. Further, adequately powered RCTs are also needed to better investigate some patient-related outcomes (PROs) as a primary endpoint, instead of LOS, such as PONV, postoperative pain and fatigue as well as outcomes linked to functional status. Functional status should be assessed investigating the resumption of patients’ function in their daily lives, the physical activity-related outcomes and the global postoperative recovery with validated instruments [43]. Moreover, the impact of existing or new ERAS items should be better assessed in obese patients with comorbidities, such as T2DM, gastroesophageal reflux or OSAS, that have a high frequency in the bariatric population. We should keep in mind that even at present, most of the elements of actual ERAS guidelines in bariatric surgery are recommended on the basis of evidence derived from the application of ERAS in other surgical fields, especially colorectal surgery [7].
References
Colquitt JL, Pickett K, Loveman E, et al. Surgery for weight loss in adults. Cochrane Database Syst Rev. 2014;8(8):CD003641.
Angrisani L, Santonicola A, Iovino P, et al. IFSO worldwide survey 2016: primary, endoluminal, and revisional procedures. Obes Surg. 2018;28(12):3783–94.
Cardoso L, Rodrigues D, Gomes L, et al. Short- and long-term mortality after bariatric surgery: a systematic review and meta-analysis. Diabetes Obes Metab. 2017;19(9):1223–32.
Longitudinal Assessment of Bariatric Surgery C, Flum DR, Belle SH, et al. Perioperative safety in the longitudinal assessment of bariatric surgery. N Engl J Med. 2009;361(5):445–54.
Aminian A, Brethauer SA, Kirwan JP, et al. How safe is metabolic/diabetes surgery? Diabetes Obes Metab. 2015;17(2):198–201.
Ljungqvist O, Scott M, Fearon KC. Enhanced recovery after surgery: a review. JAMA surgery. 2017;152(3):292–8.
Thorell A, MacCormick AD, Awad S, et al. Guidelines for perioperative care in bariatric surgery: enhanced recovery after surgery (ERAS) society recommendations. World J Surg. 2016;40(9):2065–83.
Mannaerts GH, van Mil SR, Stepaniak PS, et al. Results of implementing an enhanced recovery after bariatric surgery (ERABS) protocol. Obes Surg. 2016;26(2):303–12.
Barreca M, Renzi C, Tankel J, et al. Is there a role for enhanced recovery after laparoscopic bariatric surgery? Preliminary results from a specialist obesity treatment center. Surg Obes Relat Dis. 2016;12(1):119–26.
Meunier H, Le Roux Y, Fiant AL, et al. Does the implementation of enhanced recovery after surgery (ERAS) guidelines improve outcomes of bariatric surgery? A propensity score analysis in 464 patients. Obes Surg. 2019;29(9):2843–53.
Brethauer SA, Grieco A, Fraker T, et al. Employing enhanced recovery goals in bariatric surgery (ENERGY): a national quality improvement project using the metabolic and bariatric surgery accreditation and quality improvement program. Surg Obes Relat Dis. 2019;15(11):1977–89.
Singh PM, Panwar R, Borle A, et al. Efficiency and safety effects of applying ERAS protocols to bariatric surgery: a systematic review with meta-analysis and trial sequential analysis of evidence. Obes Surg. 2017;27(2):489–501.
Malczak P, Pisarska M, Piotr M, et al. Enhanced recovery after bariatric surgery: systematic review and meta-analysis. Obes Surg. 2017;27(1):226–35.
Ahmed OS, Rogers AC, Bolger JC, et al. Meta-analysis of enhanced recovery protocols in bariatric surgery. J Gastrointest Surg. 2018;22(6):964–72.
Lemanu DP, Singh PP, Berridge K, et al. Randomized clinical trial of enhanced recovery versus standard care after laparoscopic sleeve gastrectomy. Br J Surg. 2013;100(4):482–9.
Pimenta GP, Capellan DA, de Aguilar-Nascimento JE. Sleeve gastrectomy with or without a multimodal perioperative care. A randomized pilot study. Obes Surg. 2015;25(9):1639–46.
Geubbels N, Evren I, Acherman YIZ, et al. Randomized clinical trial of an enhanced recovery after surgery programme versus conventional care in laparoscopic Roux-en-Y gastric bypass surgery. BJS open. 2019;3(3):274–81.
Ruiz-Tovar J, Garcia A, Ferrigni C, et al. Impact of implementation of an enhanced recovery after surgery (ERAS) program in laparoscopic Roux-en-Y gastric bypass: a prospective randomized clinical trial. Surg Obes Relat Dis. 2019;15(2):228–35.
Prabhakaran S, Misra S, Magila M, Kumar SS, Kasthuri S, Palanivelu C, Raj PP. Randomized Controlled Trial Comparing the Outcomes of Enhanced Recovery After Surgery and Standard Recovery Pathways in Laparoscopic Sleeve Gastrectomy. Obes Surg. 2020;30(9):3273-3279
Higgins JPT TJ, Chandler J, Cumpston M, et al. (editors). Cochrane handbook for systematic reviews of interventions version 60 (updated July 2019) Cochrane, 2019.Available from www.training.cochrane.org/handbook.
Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100.
Bhandari M, Fobi MAL, Buchwald JN, Bariatric metabolic surgery standardization working G. Standardization of bariatric metabolic procedures: world consensus meeting statement. Obes Surg 2019;29(Suppl 4):309–345.
Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250(2):187–96.
Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1–12.
Balasubramanian SP, Wiener M, Alshameeri Z, et al. Standards of reporting of randomized controlled trials in general surgery: can we do better? Ann Surg. 2006;244(5):663–7.
Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22(4):719–48.
Greenland S, Robins JM. Estimation of a common effect parameter from sparse follow-up data. Biometrics. 1985;41(1):55–68.
Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13.
Wan X, Wang W, Liu J, et al. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135.
Friedman HP, Goldberg JD. Meta-analysis: an introduction and point of view. Hepatology. 1996;23(4):917–28.
Day RW, Fielder S, Calhoun J, et al. Incomplete reporting of enhanced recovery elements and its impact on achieving quality improvement. Br J Surg. 2015;102(13):1594–602.
Neville A, Lee L, Antonescu I, et al. Systematic review of outcomes used to evaluate enhanced recovery after surgery. Br J Surg. 2014;101(3):159–70.
Greco M, Capretti G, Beretta L, et al. Enhanced recovery program in colorectal surgery: a meta-analysis of randomized controlled trials. World J Surg. 2014;38(6):1531–41.
Zhuang CL, Ye XZ, Zhang XD, et al. Enhanced recovery after surgery programs versus traditional care for colorectal surgery: a meta-analysis of randomized controlled trials. Dis Colon Rectum. 2013;56(5):667–78.
Aman MW, Stem M, Schweitzer MA, et al. Early hospital readmission after bariatric surgery. Surg Endosc. 2016;30(6):2231–8.
Berger ER, Huffman KM, Fraker T, et al. Prevalence and risk factors for bariatric surgery readmissions: findings from 130,007 admissions in the metabolic and bariatric surgery accreditation and quality improvement program. Ann Surg. 2018;267(1):122–31.
Groene P, Eisenlohr J, Zeuzem C, Dudok S, Karcz K, Hofmann-Kiefer K. Postoperative nausea and vomiting in bariatric surgery in comparison to non-bariatric gastric surgery. Wideochir Inne Tech Maloinwazyjne. 2019;14(1):90-95
Suh S, Helm M, Kindel TL, Goldblatt MI, Gould JC, Higgins RM. The impact of nausea on post-operative outcomes in bariatric surgery patients. Surg Endosc. 2020;34(7):3085-3091
Major P, Wysocki M, Torbicz G, et al. Risk factors for prolonged length of hospital stay and readmissions after laparoscopic sleeve gastrectomy and laparoscopic Roux-en-Y gastric bypass. Obes Surg. 2018;28(2):323–32.
Gustafsson UO, Oppelstrup H, Thorell A, et al. Adherence to the ERAS protocol is associated with 5-year survival after colorectal cancer surgery: a retrospective cohort study. World J Surg. 2016;40(7):1741–7.
Malczak P, Wysocki M, Twardowska H, et al. Impact of adherence to the ERAS(R) protocol on short-term outcomes after bariatric surgery. Obes Surg. 2020;30(4):1498–505.
Rubino F, Shukla A, Pomp A, et al. Bariatric, metabolic, and diabetes surgery: what’s in a name? Ann Surg. 2014;259(1):117–22.
Myles PS, Weitkamp B, Jones K, et al. Validity and reliability of a postoperative quality of recovery score: the QoR-40. Br J Anaesth. 2000;84(1):11–5.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Dr. Amilcare Parisi discloses the following relationship: Research grant from Ethicon Endo-Surgery. Dr. Jacopo Desiderio, Prof. Roberto Cirocchi and Dr. Stefano Trastulli declare that they have no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Statement of Informed Consent
Informed consent does not apply to this study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Parisi, A., Desiderio, J., Cirocchi, R. et al. Enhanced Recovery after Surgery (ERAS): a Systematic Review of Randomised Controlled Trials (RCTs) in Bariatric Surgery. OBES SURG 30, 5071–5085 (2020). https://doi.org/10.1007/s11695-020-05000-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-020-05000-6