Abstract
Background
Obesity is associated with chronic low-grade inflammation, which has been linked to increased morbidity. However, inflammation variably and unpredictably improves after bariatric surgery. This study aimed at (1) evaluating the relationship between amplitude of weight loss and variation of inflammatory parameters after bariatric surgery, and (2) identifying, among clinical and biological baseline parameters, predictive factors of variation in inflammatory parameters.
Methods
In a prospective cohort of patients who underwent bariatric surgery, serum concentrations of interleukin (IL)-6, IL-10, resistin, leptin, adiponectin chemerin, and C-reactive protein (CRP) were measured preoperatively and 1 year after surgery, and routine clinical and biochemical parameters were retrieved. Univariate and multivariate analyses (partial least square method) were performed to assess how parameters were associated with weight loss and to predict improvement of inflammatory parameters.
Results
Eighty-seven patients were included (mean weight ± SD 136.3 ± 3.2 kg, 35 gastric bypasses, 52 sleeve gastrectomies). In parallel with weight loss (39.5 ± 13.8 kg), pro-inflammatory markers (IL-6, CRP, leptin, resistin) significantly decreased, and anti-inflammatory markers (IL-10, adiponectin) increased. Multivariate analysis revealed a significant association between weight loss and improvement in inflammatory parameters. Among all the clinical and biological preoperative parameters, baseline chemerin level was the only parameter that was significantly associated with global improvement of the inflammatory status after surgery.
Conclusion
The amplitude of weight loss 1 year after bariatric surgery was strongly correlated with improvement of inflammatory profile, which could be predicted by baseline plasma level of chemerin. This suggests a key role of chemerin in obesity-driven inflammation, and a potential use as a biomarker.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prevalence of overweight and obesity has dramatically increased over the last decades, reaching currently one in three adults worldwide [1]. Moreover, obesity is associated with other conditions such as type 2 diabetes, dyslipidemia, and cardiovascular diseases [2], thus representing a complex disease. Development of such comorbidities seems to be clearly associated with the metabolic-derived inflammation that characterizes obese state [3]. Indeed, a chronic systemic low-grade inflammation has been demonstrated in patients with severe obesity [4, 5], which can be observed in daily practice by a moderate chronic elevation of C-reactive protein (CRP) [6, 7]. Moreover, independently of obesity, chronic low-grade inflammation has been associated with multiple medical conditions such as cancer, age-associated diseases, depression, neurodegenerative diseases, and atherosclerosis [8,9,10,11,12]. Pathophysiological links between obesity-associated comorbidities and chronic inflammation involve multiple cellular and molecular components of the immune system and complex interactions with metabolism [13] that are only partially understood. Homeostasis in adipose tissue derives from tight regulation between pro- and anti-inflammatory cytokines and adipokines, and in the lean state, the environment is maintained toward an anti-inflammatory profile [13, 14]. Thus, adiponectin, a key anti-inflammatory adipokine [15, 16]; leptin and resistin, major pro-inflammatory adipokines of obese status [14, 15, 17]; and chemerin, an adipokine involved in inflammation and metabolism modified by obese status [18,19,20,21,22] all deserve to be deeply explored in this context.
In patients with severe obesity, the most efficient and cost-effective procedure for weight loss is bariatric surgery [23, 24] that is able to provide a substantial benefit on health status along with a decrease of systemic inflammation in parallel to weight loss. In the literature so far, a global decrease in systemic inflammation parameters has been reported after surgery-induced weight loss [25, 26], but it is still unknown how this variation in inflammation is driven and what are the relationships between inflammatory profile and metabolic and clinical parameters.
Accordingly, we aimed to explore, in a cohort of patients with severe obesity who underwent bariatric surgery, first, the relation between amplitude of weight loss and variation of metabolic and inflammatory parameters, and second, the ability of clinical or metabolic/inflammatory baseline parameters to predict the variation in inflammation following bariatric surgery.
Methods
Patients
Patients selected for bariatric surgery were prospectively included in a monocentric observational cohort, from 2006 to 2012.
All patients fulfilled criteria for bariatric surgery, according to national and international guidelines [27,28,29] through the validation of a multidisciplinary team. Preoperative evaluation consisted in a comprehensive medical history assessment, a clinical examination with a particular focus on cardiovascular, nutritional, and inflammatory status.
Surgical procedures consisted in sleeve gastrectomies or Roux-en-Y gastric bypasses.
Eleven patients with a preoperative CRP level > 20 mg/L were not included in the study, considering that such level of systemic inflammation could not be exclusively attributed to chronic low-grade inflammation associated with obesity.
Measurements
Patients were assessed preoperatively and 1 year after bariatric surgery. In addition to the clinical examination, body mass index (BMI) and waist and hip sizes were measured for each patient, and lean and fat body mass percentages were evaluated using biphotonic X-ray absorptiometry (Discovery W, HOLOGIC, Marlborough, USA).
Venous blood samples (5 mL) were obtained in the morning after 12 h of fasting, were collected in heparin vacuum tubes for plasma preparation, and were centrifuged at 4 °C for 10 min at 2000g. Plasma aliquots were stored at − 80 °C until analyzed.
The measurement of the following parameters was performed: glucose, C-reactive protein, albumin, pre-albumin, high-density lipoprotein (HDL), low-density lipoprotein (LDL), and triglyceride (TG) (Beckman®) and insulin levels (CisbioAssay®). Insulin sensitivity was evaluated using the QUICKI (quantitative insulin sensitivity check index) formula based on insulinemia and fasting glycemia [30].
Plasma cytokine and adipokine concentrations were determined by using enzyme-linked immunosorbent assay (ELISA) assays from R&D system (IL-10 (reference D1000B); IL-6 (reference D6050); total adiponectin (reference DRP300); resistin (reference DRSN00); leptin (reference DLP00); chemerin (reference DCHM00)). The measurements were carried out according to the manufacturer’s protocol with an intra-assay coefficient of variation < 6%. The absorbance was measured at 450 nm, and concentrations were estimated referring to a standard reference range.
Statistical Analysis
Quantitative data are expressed as mean ± standard deviation (SD) unless otherwise indicated. Variation in weight loss before and after surgery was expressed as percentage of total weight loss (%TWL) and percentage of excess weight loss (%EWL, based on ideal weight for a BMI of 25 kg/m2). Clinical and biological quantitative variables were compared using t test, or,—when compared before and after bariatric surgery—using a paired Wilcoxon test. A p value < 0.05 was considered statistically significant. Correction for multiple tests was applied as appropriate (Bonferroni correction p = 0.05/n with n = number of parameters evaluated). When required, the quantitative parameters were transformed into qualitative variables as they were categorized as higher or lower than their median.
The first objective of this study was to explore the relation between amplitude of weight loss and variation of metabolic and inflammatory parameters; the second was the ability to predict the variation in inflammation following bariatric surgery using baseline pre-surgery clinical or metabolic/inflammatory parameters. According to these two objectives of the study, univariate and multivariate analyses were performed.
Univariate analysis was based on a volcano plot to highlight the most discriminant parameters associated with weight loss and with the variation of inflammatory parameters. For this purpose, the volcano plot was built on fold-change values and the threshold of significance using the non-parametric Wilcoxon test after adjustment for multiple test, using Metaboanalyst, version 2.1 (http://www.metaboanalyst.ca/faces/home.xhtml). The most relevant parameters were characterized by FC > 1.2 or < 0.8 and adjusted p < 0.2.
Multivariate models (partial least square, PLS) were performed for two objectives. The objective no. 1 aimed to explore the relation between the percentage of weight loss, expressed as %TWL and %EWL (defined as quartiles) and the variation of biological parameters before and 1 year after surgery. For objective no. 2, non-optimized multivariate models were built to predict the evolution of the inflammatory parameters (i.e., CRP, IL-10, IL-6, resistin, leptin, adiponectin, chemerin) most modified after surgery (i.e., more than 50% of variation between before and after surgery) from every baseline parameters. Then, optimized models were built to predict the variation of the inflammatory parameters after exclusion of the less discriminant variables and to keep less than 10 parameters in the final model. Finally, a Venn diagram was built to highlight the commonly shared baseline parameters able to predict the evolution of inflammatory parameters after bariatric surgery.
Statistical analyses were performed with Simca-P+-13 for the multivariate modeling and JMP statistical software version 7.0.2 (SAS Institute, Cary, NC) for the other analyses. Performance of the multivariate models was evaluated from p value of CV ANOVA (i.e., ANalysis Of VAriance testing of cross-validated predictive residuals was used as a diagnostic tool for assessing the reliability of PLS model.
Results
Patient Characteristics
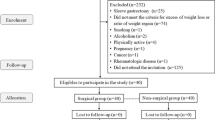
During the study period, 100 patients were screened for inclusion in the prospective cohort. Eleven patients were not included due to a preoperative CRP level > 20 mg/L, and two other patients due to missing data. Finally, 87 patients were included (23 males and 64 females—sex ratio 0.4) with an age of 40 ± 11 years and BMI of 49 ± 8 kg/m2 before surgery. Thirty-one (36%) patients had chronic hypertension, 19 (22%) had type 2 diabetes, and 29 (34%) had dyslipidemia. Bariatric surgical procedures consisted in Roux-en-Y gastric bypass for 35 patients (40.2%) and sleeve gastrectomy for 52 patients (59.8%).
One year after surgery (± 2 months), BMI decreased to 35 ± 8 kg/m2 (p < 0.001), corresponding to a %TWL of 29.3 ± 9% and a %EWL of 63.8 ± 23.3%. Main clinical and biological characteristics of the patients before and after surgery are reported in Table 1. Plasma CRP concentrations significantly decreased after surgery, along with LDL cholesterol and triglyceride levels (p < 0.0005), while insulinemia, fasting glycaemia, and HDL cholesterol concentration decreased after surgery (p < 0.0001).
Variation of cytokines and adipokines levels before and after surgery is shown in Fig. 1: anti-inflammatory cytokine (IL-10) and adipokine (adiponectin) significantly increased after surgery while pro-inflammatory cytokines (IL-6) and adipokines (leptin, resistin, and chemerin) significantly decreased after surgery (p < 0.0001 for all).
Comparison of mean plasmatic concentrations of cytokines and adipokines before and 1 year after bariatric surgery. a Pro-inflammatory cytokines and adipokines. b Anti-inflammatory cytokine and adipokine. I bars represent standard error of the mean. ***p < 0.001, after paired Wilcoxon test and correction (Bonferroni) for multiple tests
Comparison of the two surgical technics employed (sleeve gastrectomy versus gastric bypass) revealed no significant difference in %TWL and %EWL, and in variation of the clinical and biological parameters before and after surgery.
Relationship Between Variation of Biological Parameters Before and After Bariatric Surgery and Weight Loss
The model was correct (p = 0.002) for explaining the percentage of weight loss (%TWL) defined as quartiles from the biological parameters. However, with the use of %EWL, the model was not reliable (p > 0.05) because of the use of an estimated theoretical variable (estimated weight for a BMI of 25 kg/m2) and, consequently, was not employed.
The score scatter plot showed a repartition of patients along the X-axis according to the quartiles of %TWL (Fig. 2a). The loading scatter plot highlights parameters that are most linked with %TWL (Fig. 2b): a higher decrease of insulin, LDL, resistin, leptin, CRP, and IL-6 levels with %TWL could be observed, and, conversely, the QUICKI was less improved in patients with lower %TWL (opposite points on the loading plot).
Significant relationship between variation of biological parameters before and after bariatric surgery and weight loss. Multivariate model (partial least square method) was used to explore the relation between the percentage of weight loss (percentage of total weight loss, %TWL) defined by quartiles, and the variation of biological parameters before and 1 year after surgery. a Score scatter plot showing the repartition of patients along the X-axis according to the quartiles of weight loss variation (represented as a four-color scale). b Loading scatter plot revealing parameters most linked with weight loss. CRP, C-reactive protein; HDLc, high-density lipoprotein cholesterol; LDLc, low-density lipoprotein cholesterol; QUICKI, quantitative insulin sensitivity check index; TG, triglyceride
According to univariate analysis, 3 parameters with FC > 1.2 or FC < 0.8 and adjusted p value < 0.2 were identified: QUICKI (adjusted p = 0.03, FC = 1.7), insulin (adjusted p = 0.047, FC = 0.75), and LDL (adjusted p = 0.18, FC = 0.64) (Figure S2), that is highly consistent with multivariate model.
Using Baseline Clinical and Biological Parameters to Predict Variation of Inflammatory Parameters After Surgery
Among the inflammatory parameters, those that were most affected by bariatric surgery were IL-6 (− 72.2 ± 27.1%); CRP (− 62.6 ± 45.9%), resistin (− 58.9 ± 26.0%), and adiponectin (+ 145.3 ± 105.4%) and were thus used for the following analyses.
In univariate analysis, baseline parameters that were significantly associated with variation of the selected inflammatory parameters after surgery were only observed for variations of IL-6 and adiponectin. Variation in IL-6 level after surgery was thus significantly associated with baseline IL-6 level (adjusted p = 0.002, FC = 3.3), and baseline resistin level (p = 0.007, FC = 1.9) (Figure S3A). Adiponectin variation before and after surgery was significantly associated with baseline adiponectin level (p = 0.036 and FC = 0.81) and baseline lean mass (adjusted p = 0.026, FC = 0.78) (Figure S3A).
Optimized PLS models to predict percentages of variation of the four selected inflammatory parameters—IL-6, IL-10, CRP, resistin, and adiponectin—from baseline parameters were robust as they were all significant (p = 0.007; 0.0016; 0.018 and 4.4 × 10−5, respectively) from a reduced number of parameters (less than 8). The most discriminant parameters for the prediction of IL-6, IL-10, CRP, resistin, and adiponectin variations were included on a Venn diagram to highlight the shared baseline parameters. The Venn diagram revealed that chemerin was the only parameter associated with variation of the four selected parameters (Fig. 3).
Using baseline clinical and biological parameters to predict variation of inflammatory status after surgery: Venn diagram showing shared baseline parameters associated with improvement of inflammatory parameters. Multivariate modeling was performed using all clinical and biological parameters to determine the most discriminant to predict variations of IL-6, IL-10, CRP, resistin, and adiponectin plasmatic concentrations before and after surgery (noted “Δ”), see text for details. Shared parameters of the most discriminant parameters for each cytokine and adipokine are thus shown on the Venn diagram. (Reviewer no. 3, Q no. 3) CRP, C-reactive protein; HDLc, high-density lipoprotein cholesterol; LDLc, low-density lipoprotein cholesterol; QUICKI, quantitative insulin sensitivity check index; TG, triglyceride
Data about the non-optimized models to predict the group of parameter variations defined as less than or greater than the median are provided on supplementary data (Figure S3B).
Given that presence or absence of metabolic comorbidities (medical history of hypertension, diabetes, and dyslipidemia) were not directly included in the analyses, we also explored variation of inflammatory parameters according to the presence or absence of these comorbidities, independently of their potential associated variables at baseline (i.e., QUICKI; fasting glycaemia; insulinemia for diabetes; and LDL, TG, HDL levels for dyslipidemia). For this purpose, we grouped patients as either “metabolically healthy” if they had no medical history of diabetes (n = 33), hypertension, and dyslipidemia before surgery, or as “metabolically unhealthy” if they had one or more of this condition (n = 54) [31]. There was no significant difference in variations of the four selected inflammatory parameters between the two groups (p = 0.12 for CRP, p = 0.24 for IL-6, p = 0.41 for adiponectin, and p = 0.18 for resistin). Similarly, we found no significant difference in variation of the four inflammatory parameters between male and female (p = 0.13 for CRP, p = 0.93 for IL-6, p = 0.54 for adiponectin, and p = 0.82 for resistin).
Discussion
This study shows that improvements in both metabolic and inflammatory profiles were strongly correlated with the amplitude of weight loss—expressed as %TWL—in patients with severe obesity at 1 year following bariatric surgery. Moreover, baseline level of chemerin in plasma was identified as the most reliable parameter to predict this decrease in inflammatory status.
As expected, weight reduction after surgery has been followed by a significant improvement of metabolic profile, as shown by lower LDL and TG levels, higher HDL level, lower fasting glycaemia, and lower insulin level. Regarding the improvement of systemic inflammatory profile, a significant decrease in inflammatory CRP level was observed in this study, in accordance with previous studies [32,33,34,35,36,37,38,39,40]. Interestingly, multivariate data also showed a strong correlation between the amplitude of weight loss and the decrease in plasma CRP. Such correlation tends to strengthen the hypothesis of a direct link between weight loss and a reduction in systemic inflammation.
Improvement in Pro-inflammatory Profile 1 Year After Surgery
This study revealed that pro-inflammatory cytokine IL-6, along with resistin, leptin, and chemerin, significantly decreased after surgery, while anti-inflammatory cytokine IL-10 and anti-inflammatory adiponectin significantly increased. These results are consistent with previous reports exploring variation of inflammatory parameters after bariatric surgery [32, 37, 38, 41,42,43,44,45]. Interestingly, this work shows that the decrease of these inflammatory markers was highly correlated to the magnitude of weight loss in the multivariate model, except for IL-10. These findings support the concept of a progressive return to homeostasis after surgery, with a reduction in the imbalance of pro- and anti-inflammatory response seen in obese state.
Baseline Chemerin Level to Predict Improvement of Inflammatory Status After Surgery
During obese state, chronic inflammation is probably the common denominator between insulin resistance and cardiovascular diseases [3, 46]. Thus, being able to predict the improvement of inflammatory profile before surgery could be clinically relevant. In the model used here, it appeared that baseline level of chemerin was the only parameter associated with the improvement in inflammatory markers such as IL-6, CRP, resistin, adiponectin, and IL-10. In other words, the more the plasma chemerin level is elevated at baseline, the more the IL-6, CRP, and resistin will decrease after surgery and adiponectin and IL-10 will increase after bariatric surgery. These data strongly encourage further study to determine and validate whether—and how—chemerin could be used as a biomarker, to screen patients who could drastically benefit from bariatric surgery in terms of inflammation reduction.
Chemerin as a Potential Key Player in Immunopathology of Obese State
Chemerin is a pro-inflammatory adipokine that has been reported to be linked to obesity and inflammation [19]. It is produced essentially by adipocytes under pro-inflammatory stimuli such as IL-1β and TNF-α and, once secreted, recruits and activates immune cells such as macrophages, dendritic cells, and natural killer cells [35, 47, 48]. Adipocytes also express chemerin receptors, suggesting an autocrine property of chemerin on adipocyte production [49]. During obese state, it has been demonstrated that serum levels of chemerin are elevated [50], and decrease after weight loss [43, 50], which is consistent with the present findings. Moreover, Chakaroun et al. [50] found, in their cohort of obese patients, a strong correlation between chemerin production and inflammation. Given these data and results of this study, it could be speculated that chemerin might have an important role in maintaining the pro-inflammatory environment that characterizes adipose tissue in obese state. Indeed, initial pro-inflammatory signals in the adipose tissue can stimulate adipocytes to produce chemerin. Chemerin release will increase recruitment and activation of macrophages in the white adipose tissue. The pro-inflammatory environment in which macrophages arrive will further drive them into the production of inflammatory cytokines, which can, in turn, stimulate production of chemerin by adipocytes. Thus, a pro-inflammatory vicious circle could be locally triggered if massive food intake is persistent over time, in which chemerin could be a key player. Equally interesting, it has been shown that obesity and various chronic inflammatory diseases, such as psoriasis, rheumatoid arthritis, and inflammatory bowel diseases, are associated with elevated systemic level of chemerin [51,52,53]. At the end, given these data and the fact that patients in this study with the highest preoperative level of chemerin are those who had the greatest regulation of inflammation after surgery, strongly supports the hypothesis of a key role of chemerin in perpetuation of obesity-induced inflammation. Indeed, one could speculate that reduction in food intake after surgery leads to a reduction in stress and inflammatory signals in adipose tissue, thus decreasing chemerin release, and eventually limiting activation and recruitment of pro-inflammatory macrophages. Figure 4 summarizes this putative pathophysiological loop.
Putative patholophysiological mechanisms involving chemerin in obesity-driven inflammation. Chronic increased food intake (a) leads to danger signal release, hypoxia, and adipocyte necrosis that turns adipose tissue micro-environment into pro-inflammatory, leading to chemerin release by adipocytes. Chemerin thus favors macrophage recruitment and activation in adipose tissue, that, in turn, release pro-inflammatory cytokines that further enhance chemerin production by adipocytes. Bariatric surgery possibly break this vicious circle (b), by reducing food intake, with subsequent dampening of pro-inflammatory signals in adipose tissue, leading to a reduction in chemerin production, and, eventually helps to phenotypic switch of macrophages to alternatively anti-inflammatory-activated macrophages
Limitations
This study has several limitations. First, regarding extrapolation of the results, it is important to stress out that statistical correlations and associations made in this study cannot be interpreted as causal direct links between, first, amplitude of weight loss and amplitude of improvement of inflammation, and second, preoperative level of chemerin and improvement of inflammation. However, as discussed above, pathophysiological rationale regarding potential role of chemerin is strong, strengthening importance for further studies.
Second, the study did not include a control group. Indeed, we only considered variation of inflammatory parameters from baseline to 1 year after surgery, and not possible to return to “normal” level of these parameters. It would have been of interest to explore parameters associated with return to a normal range of inflammatory markers. However, difficulty raises with the definition of “normal” range regarding cytokine and adipokine levels, but, a control group of sex- and age-matched healthy and lean subjects might have allowed at least an intra-study baseline.
Last, outcomes explored in this study did not include relevant clinical outcome for caregivers such as long-term morbidity, mortality, or health-related quality of life. It would be of importance to further explore the link between importance of improvement of inflammatory status and these clinical outcomes. Moreover, given the role of chronic low-grade inflammation in multiple diseases such as cardiovascular events, age-related comorbidities, depression, or degenerative diseases of the central nervous system [8,9,10,11,12], it would be highly relevant to specifically explore these conditions in correlation with variation of inflammatory status after surgery. In the same line, comorbidities at 1 year were not explicitly retrieved and reassessed in this study (for example: need for medication and appropriate control of diabetes and hypertension), so we could not explore potential correlations between improvement in inflammatory status and improvement of these conditions in terms of clinical management.
Conclusion
In this study, the amplitude of weight loss in patients with severe obesity 1 year following bariatric surgery was strongly correlated with improvement in both metabolic and inflammatory profiles. Moreover, baseline level of chemerin in plasma was the most robust parameter to predict such improvement in inflammatory status: patients with the highest preoperative level of chemerin were those who had the most drastic improvement in inflammatory parameters at 1 year (CRP, IL-6, adiponectin, resistin). This latter result suggests that first, chemerin may be a key player in obesity-driven inflammation, possibly by a vicious circle—that bariatric surgery could alleviate—of adipocyte overproduction induced by inflammatory signals of immune cells recruited through chemerin release itself. Second, chemerin could be next explored as a potential biomarker to predict improvement in inflammatory status after surgery.
References
Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. Elsevier. 2014;384(9945):766–81
Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet Elsevier. 2005;365(9468):1415–28.
Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444(7121):860–7.
de Heredia FP, Gómez-Martínez S, Marcos A. Obesity, inflammation and the immune system. Proc Nutr Soc Cambridge University Press. 2012;71(02):332–8.
Rodríguez-Hernández H, Simental-Mendía LE, Rodríguez-Ramírez G, et al. Obesity and Inflammation: epidemiology, risk factors, and markers of inflammation. Int J Endocrinol. Hindawi Publishing Corporation. 2013;2013(10):1–11.
Visser M, Bouter LM, McQuillan GM, et al. Elevated C-reactive protein levels in overweight and obese adults. JAMA. 1999;282(22):2131–5.
Park H, Park Y, Yu R. Relationship of obesity and visceral adiposity with serum concentrations of CRP, TNF-a and IL-6. Diabetes Res Clin Pract. 2005;69:1–7.
Heneka MT, Kummer MP, Latz E. Innate immune activation in neurodegenerative disease. Nat Rev Immunol. Nature Publishing Group. 2014;14(7):463–77.
Gisterå A, Hansson GK. The immunology of atherosclerosis. Nat Rev Nephrol. 2017;13(6):368–80.
Mantovani A, Allavena P, Sica A, et al. Cancer-related inflammation. Nature. Nature Publishing Group. 2008;454(7203):436–44.
Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol. 2015;16(1):22–34.
Franceschi C, Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol A Biol Sci Med Sci. 2014;69(Suppl 1):S4–9.
Lackey DE, Olefsky JM. Regulation of metabolism by the innate immune system. Nat Rev Endocrinol. 2016;12(1):15–28.
Brestoff JR, Artis D. Immune regulation of metabolic homeostasis in health and disease. Cell. Elsevier. 2015;161(1):146–60.
Ouchi N, Parker JL, Lugus JJ, et al. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11(2):85–97.
Ohashi K, Ouchi N, Matsuzawa Y. Anti-inflammatory and anti-atherogenic properties of adiponectin. Biochimie. 2012;94(10):2137–42.
Cao H. Adipocytokines in obesity and metabolic disease. J Endocrinol. BioScientifica. 2014;220(2):T47–59.
Ernst MC, Sinal CJ. Chemerin: at the crossroads of inflammation and obesity. Trends Endocrinol Metab. Elsevier Ltd. 2010;21(11):660–7.
Helfer G, Wu Q-F. Chemerin: a multifaceted adipokine involved in metabolic disorders. J Endocrinol. Bioscientifica Ltd. 2018;238(2):R79–94.
Rourke JL, Dranse HJ, Sinal CJ. Towards an integrative approach to understanding the role of chemerin in human health and disease. Obes Rev. 2012;14(3):245–62.
Chang S-S, Eisenberg D, Zhao L, et al. Chemerin activation in human obesity. Obesity. 2016;24(7):1522–9.
Li Y, Shi B, Li S. Association between serum chemerin concentrations and clinical indices in obesity or metabolic syndrome: a meta-analysis. Villa E, editor. PLoS ONE. Public Library of Science; 2014;9(12):e113915–14.
Colquitt JL, Pickett K, Loveman E, et al. Surgery for weight loss in adults. In: Colquitt JL, editor. Cochrane Database Syst Rev, vol. 8. Chichester: John Wiley & Sons, Ltd; 2014. p. CD003641.
Picot J, Jones J, Colquitt JL, et al. The clinical effectiveness and cost-effectiveness of bariatric (weight loss) surgery for obesity: a systematic review and economic evaluation. Health Technol Assess. 2009;13(41):1. –190–215–357–iii–iv
Frikke-Schmidt H, O’Rourke RW, Lumeng CN, et al. Does bariatric surgery improve adipose tissue function? Obes Rev. 2016;17(9):795–809
Goktas Z, Moustaid-Moussa N, Shen C-L, et al. Effects of bariatric surgery on adipokine-induced inflammation and insulin resistance. Front Endocrinol. Frontiers. 2013;4:69.
Sauerland S, Angrisani L, Belachew M, et al. Obesity surgery: evidence-based guidelines of the European Association for Endoscopic Surgery (EAES). Surgical endoscopy. 2005;19(2):200–21
Laville M, Romon M, Chavrier G, et al. Recommendations regarding obesity surgery. Obes Surg. Springer-Verlag. 2005;15(10):1476–80.
Haute Autorité de Santé. Obésité: prise en charge chirurgicale de l’adulte [Internet]. 2009. Available from: http://www.has-sante.fr
Katz A, Nambi SS, Mather K, et al. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab. 2000;85(7):2402–10.
Iacobini C, Pugliese G, Fantauzzi CB, et al. Metabolically healthy versus metabolically unhealthy obesity. Metabolism Elsevier Inc. 2019;92(C):51–60.
Arismendi E, Rivas E, Agustí A, Ríos J, Barreiro E, Vidal J, et al. The systemic inflammome of severe obesity before and after bariatric surgery. Thatcher TH, editor. PLoS ONE. Public Library of Science; 2014;9(9):e107859.
Iannelli A, Anty R, Schneck AS, et al. Evolution of low-grade systemic inflammation, insulin resistance, anthropometrics, resting energy expenditure and metabolic syndrome after bariatric surgery: a comparative study between gastric bypass and sleeve gastrectomy. J Visc Surg. Elsevier Masson SAS. 2013;150(4):269–75.
Gumbau V, Bruna M, Canelles E, et al. A prospective study on inflammatory parameters in obese patients after sleeve gastrectomy. Obes Surg. Springer US. 2014;24(6):903–8.
Catalán V, Gómez-Ambrosi J, Rodríguez A, et al. Increased levels of chemerin and its receptor, chemokine-like receptor-1, in obesity are related to inflammation: tumor necrosis factor-α stimulates mRNA levels of chemerin in visceral adipocytes from obese patients. Surg Obes Relat Dis. Elsevier Inc. 2013;9(2):306–14.
Miller GD, Nicklas BJ, Fernandez A. Serial changes in inflammatory biomarkers after Roux-en-Y gastric bypass surgery. Surg Obes Relat Dis. Elsevier Inc. 2011;7(5):618–24.
Netto BDM, Bettini SC, Clemente APG, et al. Roux-en-Y gastric bypass decreases pro-inflammatory and thrombotic biomarkers in individuals with extreme obesity. Obes Surg. 2014;25(6):1010–8.
Dalmas E, Rouault C, Abdennour M, et al. Variations in circulating inflammatory factors are related to changes in calorie and carbohydrate intakes early in the course of surgery-induced weight reduction. Am J Clin Nutr. American Society for Nutrition. 2011;94(2):450–8.
Vilarrasa N, Vendrell J, Sánchez-Santos R, et al. Effect of weight loss induced by gastric bypass on proinflammatory interleukin-18, soluble tumour necrosis factor-α receptors, C-reactive protein and adiponectin in morbidly obese patients. Clin Endocrinol. Blackwell Publishing Ltd. 2007;67(5):679–86.
Morínigo R, Casamitjana R, Delgado S, et al. Insulin resistance, inflammation, and the metabolic syndrome following Roux-en-Y gastric bypass surgery in severely obese subjects. Diabetes Care. American Diabetes Association. 2007;30(7):1906–8.
Marantos G, Daskalakis M, Karkavitsas N, et al. Changes in metabolic profile and adipoinsular axis in morbidly obese premenopausal females treated with restrictive bariatric surgery. World J Surg. 2011;35(9):2022–30.
Ress C, Tschoner A, Engl J, et al. Effect of bariatric surgery on circulating chemerin levels. Eur J Clin Investig. Wiley/Blackwell (10.1111). 2010;40(3):277–80.
Sell H, Divoux A, Poitou C, et al. Chemerin correlates with markers for fatty liver in morbidly obese patients and strongly decreases after weight loss induced by bariatric surgery. J Clin Endocrinol Metab. 2010;95(6):2892–6.
Terra X, Auguet T, Guiu-Jurado E, et al. Long-term changes in leptin, chemerin and ghrelin levels following different bariatric surgery procedures: Roux-en-Y gastric bypass and sleeve gastrectomy. Obes Surg. Springer US. 2013;23(11):1790–8.
Freitas WR, Oliveira LVF, Perez EA, et al. Systemic inflammation in severe obese patients undergoing surgery for obesity and weight-related diseases. Obes Surg Obesity Surgery. 2018;307(5):1–12.
Debnath M, Agrawal S, Agrawal A, et al. Metaflammatory responses during obesity: pathomechanism and treatment. Obes Res Clin Pract. Asia Oceania Association for the Study of Obesity. 2015;10(2):1–11.
Parlee SD, Ernst MC, Muruganandan S, et al. Serum chemerin levels vary with time of day and are modified by obesity and tumor necrosis factor-α. Endocrinology. 2010;151(6):2590–602.
Bondue B, Wittamer V, Parmentier M. Chemerin and its receptors in leukocyte trafficking, inflammation and metabolism. Cytokine Growth Factor Rev. Elsevier Ltd. 2011;22(5-6):331–8.
Mattern A, Zellmann T, Beck-Sickinger AG. Processing, signaling, and physiological function of chemerin. IUBMB Life. 2014;66(1):19–26.
Chakaroun R, Raschpichler M, Klöting N, et al. Effects of weight loss and exercise on chemerin serum concentrations and adipose tissue expression in human obesity. Metabolism. 2012;61(5):706–14.
Del Prete A, Salvi V, Sozzani S. Adipokines as potential biomarkers in rheumatoid arthritis. Mediat Inflamm. Hindawi. 2014;2014(4):425068–11.
Bai F, Zheng W, Dong Y, et al. Serum levels of adipokines and cytokines in psoriasis patients: a systematic review and meta-analysis. Oncotarget. 2018;9(1):1266–78.
Buechler C. Chemerin, a novel player in inflammatory bowel disease. Cell Mol Immunol. Nature Publishing Group. 2014;11(4):315–6.
Acknowledgments
We would like to thank Véronique Théret, Carole Sauvestre, Patricia Cosson, Marie-Céline Bourgoin, and Dr. David Jacobi. Some of the figures were created using the vector image bank of Servier Medical Art (http://smart.servier.com/). Servier Medical Art by Servier is licensed under a Creative Commons Attribution 3.0 Unported License (https://creativecommons.org/licenses/ by/3.0/).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict interest.
Ethical Approval
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards All patients included in the study gave written consent for scientific and anonymous use of their data, and the study was approved by the institutional ethic committee.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
Volcano plot representation of association between weight loss and variation of inflammatory and metabolic parameters, before and after bariatric surgery. Univariate analysis was performed to highlight the most discriminant parameters associated with weight loss. Results are represented as a volcano plot built on fold-change values and the threshold of significance using the non-parametric Wilcoxon test after adjustment for multiple test. (Reviewer #3, Q#3) The most relevant parameters were characterized by FC> 1.2 or <0.8 and adjusted p<0.2. (difference between baseline and one-year levels, noted Δ). Parameters with adjusted p=0.2 and fold change (FC) >1.2 or < 0.8 were considered as significant and are showed on the graph: variation of insulinemia, QUICKI and LDLc. QUICKI: quantitative insulin sensitivity check index; LDLc: low density lipoprotein cholesterol. (PDF 220 kb)
ESM 2
Predicting improvement of inflammatory parameters after surgery using baseline clinical and biological parameters. (A): In univariate analysis, among the selected inflammatory cytokines and adipokines, only IL-6 and adiponectin variations revealed significant association with baseline parameters. Parameters with adjusted p=0.2 and fold change (FC) >1.2 or < 0,8 were considered as significant. Thus, IL-6 variation before and after surgery was significantly associated with baseline IL-6 level (adjusted p =0.002, FC=3.3), and baseline resistin level (p=0.007, FC=1.9). Adiponectin variation before and after surgery was significantly associated with baseline adiponectin level (p=0.036 and FC=0.81) and baseline lean mass (adjusted p=0.026, FC=0.78). Volcano plots were next built for representations of association between fold change of variation of IL-6 and adiponectin before and after surgery (difference between baseline and one-year levels, noted “Δ”) and baseline parameters. Significant associations are mentioned on the graphs. (B): Results of multivariate analysis using non-optimized partial least square technic to explore association between baseline clinical and biological parameters and variation of selected inflammatory parameters (Resistin, CRP, Adiponectin and IL-6) before and after surgery (noted Δ), defined as < or > to the median. The two important measures in PLS-DA are represented: the variable importance in projection (VIP) and the weighted sum of absolute regression coefficients (the colored boxes on the right indicate the relative concentrations of the corresponding variable in each group under study). CRP: C-reactive protein; HDLc: high density lipoprotein cholesterol; LDLc: low density lipoprotein cholesterol; TG: Triglyceride. (PDF 1192 kb)
Rights and permissions
About this article
Cite this article
Jouan, Y., Blasco, H., Bongrani, A. et al. Preoperative Chemerin Level Is Predictive of Inflammatory Status 1 Year After Bariatric Surgery. OBES SURG 30, 3852–3861 (2020). https://doi.org/10.1007/s11695-020-04584-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-020-04584-3