Abstract
Background
Melatonin has analgesic, anti-inflammatory, sedative, and anxiolytic properties. However, the relationship between endogenous melatonin levels and postoperative analgesic requirements has not been well elucidated in patients undergoing bariatric surgery. We studied endogenous melatonin levels, cortisol levels, body temperatures, and the relationship between the level of endogenous melatonin and postoperative morphine consumption.
Methods
The trial was conducted among 30 patients who were scheduled for laparoscopic bariatric surgery. Their ages were between 18 and 65 years and their BMIs were above 40 kg/m2. Secretion of melatonin, cortisol, and body temperature was monitored before the anesthetic induction, at 2 h intraoperatively, and at 2, 6, 10, (2:00 A.M.) and 24 h postoperatively. For each patient, morphine consumption was assessed at postoperative visits. The primary outcomes were to measure endogenous melatonin levels and to examine the relationship between these levels and morphine consumption. The secondary outcome was to observe the changes in cortisol and body temperature.
Results
There was a significant decrease in melatonin levels when preoperative melatonin levels were compared with intraoperative and all postoperative follow-up periods (p < 0.05). When the correlation between plasma melatonin levels and the postoperative morphine consumption of the patients was inspected, there was a significant correlation in all of the follow-up periods (p < 0.05). When preoperative cortisol levels were compared with intraoperative and postoperative cortisol levels, there was a significant difference in the follow-up periods, except two periods (p < 0.05). Body temperatures were similar in all measurement periods.
Conclusions
Endogenous melatonin secretion was significantly decreased in the intraoperative and postoperative periods. Furthermore, there was a significant inverse correlation between changes in endogenous melatonin levels and morphine consumption.
Trial Registration
Clinical Trial Number NCT03107702 from A service of the U.S. National Institutes of Health, clinicaltrials.gov
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hormones, all significant physiological functions, and the pharmacokinetics and pharmacodynamics of several drugs are associated with circadian rhythms. Biological rhythm has been shown to alter the effect and pharmacology of anesthetic medicines, such as local anesthetics, hypnotics, and muscle relaxants [1,2,3]. Major surgical operations are characterized by the endocrine-metabolic and surgical stress response during the postoperative period and lead to disturbance of the circadian rhythm [4, 5]. Many patients complain of pain, fatigue, drowsiness, reduced general well-being, and cognitive dysfunction after a major surgery [4].
Melatonin is a hormone secreted from the pineal gland that is responsible for regulating the endocrine rhythm and daily biorhythm [6]. Melatonin has a variety of functions, including regulation of circadian rhythm, antioxidant, oncostatic, anti-inflammatory, anticonvulsant, and good anesthetic properties, such as its anxiolytic, sedative and analgesic effects [3]. Disturbance of melatonin secretion induces several pathological states, including acute pain, idiopathic chronic pain syndrome, stroke, depressive disorder, and coronary artery disease [3, 4, 7, 8].
Obesity is a major public health issue that is related to morbidity and mortality. It has been reported that obesity and metabolic syndrome are associated with disorders of the circadian rhythm [9]. Disturbances in circadian rhythms promote glucose intolerance, obesity, and type-2 diabetes. Melatonin can be a beneficial add-on therapy for insulin resistance, dyslipidemia, and overweight in obese individuals [10]. However, preoperative, intraoperative, and postoperative endogenous melatonin levels have not been well elucidated in obese individuals undergoing laparoscopic bariatric surgery.
Previous studies showed that preoperative exogenous administration of melatonin provided an analgesic effect [11,12,13]; however, to our knowledge, the relationship between endogenous melatonin levels and postoperative analgesic requirements has not been well described. The aim of this study was to examine the relationship between the level of endogenous melatonin and postoperative morphine consumption and to describe the melatonin secretion profile in obese patients undergoing laparoscopic bariatric surgery.
Methods
This prospective observational study was conducted after the approval of the Local Ethics Committee of the Inonu University Medical Faculty (Chairperson Prof R Karlıdag, 2015/212) on December 30, 2015, and registered with ClinicalTrials.gov (NCT03107702). Informed consent was obtained from all individual participants included in the study. The study was conducted from March 29 to July 30, 2017, in the Inonu University Medical Faculty, Turgut Ozal Medical Center.
The study was conducted with 30 patients who were scheduled for laparoscopic bariatric surgery, between the ages of 18 and 65, who were obese with a BMI above 40 kg/m2. Patients were excluded based on the following criteria: cardiovascular, neurological and psychiatric disorders, opioid tolerance, sleep disturbance, the use of hypnotics, neuroleptics, antidepressants, beta blockers and steroids, and a history of allergy to the drugs used in the study protocol.
The patients who were not administered premedication were accepted into the operating room and were informed about the patient-controlled analgesia (PCA) device and the visual analog scale (VAS) for pain. The electrocardiogram, arterial oxygen saturation, noninvasive blood pressure, and bispectral index (BIS) were monitored. All operations started between 8:00 A.M. and 8:30 A.M.
Standard general anesthesia was administered to all patients. Prior to anesthesia induction, preoxygenation was performed by providing 100% O2 for at least 3 min through a face mask. Anesthesia was induced with 2–3 mg kg−1 propofol and 1 μg kg−1 fentanyl, and 0.6 mg kg−1 rocuronium was given for muscle relaxation. Following the general anesthesia, an arterial catheter was inserted into the left radial artery. Blood samples in the intraoperative and postoperative periods were taken from this catheter. The patient’s eyes were closed with a band during the operative duration. The anesthetic was maintained with 1 MAC sevoflurane, 40% O2/60% air, and the BIS value was maintained between 40 and 60. Mechanical ventilation was continued under the pressure-controlled mode to keep the end-tidal CO2 concentration at 35–40 mmHg.
A private room was reserved for each patient during the study period; however, the patients were observed in an isolated room in the intensive care unit during the first 24 h, due to the routine procedures of our clinic. To provide a dark environment, the lights were turned off and the door of the room was closed between 11:00 P.M. and 6:00 A.M. However, optimal dark conditions could not be achieved because of the patient viewing window and monitors in the room. The lights were not turned on to take blood samples during the night. The light reflecting from the window of the room was sufficient to take blood samples and for the nurses to record their follow-ups.
For the postoperative analgesic management, 1 g IV paracetamol (Perfalgan® 1 g/100 ml vial) was applied at the end of the surgery for 30 min via slow infusion and every 6 h during the postoperative 24-h period. Additionally, all patients received morphine PCA (prepared with 10 ml of morphine sulfate (100 mg) + 90 ml of SF), 1 mg bolus and a 15-min lock-out time. During the 2-, 6-, 10-, (2:00 A.M.) and 24-h postoperative visits, the VAS levels and morphine consumption were recorded. Rescue analgesic with 0.5 mg kg−1 tramadol was planned when the VAS values were ≥ 4.
The primary outcome was to examine the relationship between the level of endogenous melatonin and postoperative morphine consumption. The secondary outcome was to observe the changes in the levels of endogenous melatonin, cortisol, and body temperature during the preoperative, intraoperative, and the 24-h postoperative periods.
For the melatonin and cortisol analysis, blood samples were taken before the anesthetic induction, at 2 h intraoperatively, and at 2, 6, 10, (2:00 A.M.) and 24 h postoperatively. Blood samples taken for melatonin were centrifuged, and the plasma content was separated and collected for analysis at − 20 °C.
A melatonin standard was purchased from Sigma-Aldrich (Steinheim, Germany). The stock solution of the melatonin standard (1 mg/ml) was prepared in a mixture of methanol: water (1:10, v/v). A calibration curve was generated from working solutions of the melatonin standard (10 ml) that ranged from 0.2 to 50 pg ml−1 and was stored at − 20 °C. The working solutions were prepared by appropriate dilution of the stock solution with deionized water produced by a Milli-Q system (Millipore, Bedford, MA, USA) and methanol. All other solvents and reagents were of analytical grade. Extraction columns, Oasis® HLB cartridges (3 ml/60 mg), were purchased from Waters (Milford, MA, USA).
For the solid-phase extraction (SPE) method, the plasma sample was mixed with 0.5 ml of sodium phosphate buffer (0.01 M, pH 7). The SPE Oasis HLB cartridge was conditioned by successive washing with 1 ml of methanol and 1 ml of water. Samples were loaded and eluted with 1 ml of 90% ethanol/water. The flow rate was maintained at 1–2 ml min−1. The eluate was collected in a 1.5-ml centrifuge tube, and the extract was evaporated to dryness in a Turbovap LV Evaporator (Zymark, Hopkinton, MA, USA) with a nitrogen stream at 35 °C. The residue was reconstituted with 100 μl of acetonitrile/water (50:50, v/v).
HPLC analysis was performed on a Shimadzu chromatography system that consisted of a 20 ADXR HPLC pump and scanning fluorescence detector. Separation was carried out with a Kromasil-100-5 column 5 μm (4.6 mm × 150 mm) and isocrotic elution at room temperature. The mobile phase consisted of 10 mM sodium acetate and acetonitrile 72:28 (pH = 5). The flow rate was set at 1.0 ml min−1, and the injection volume was 40 μl. Excitation and emission of the fluorescence detector were set to 275 and 345 nm, respectively (Fig. 1).
Quality control (QC) samples were prepared by spiking the plasma samples at three concentrations of the analyte. The limit of quantitation was 0.2 pg ml−1.
Cortisol serum samples were analyzed using an automated, non-isotopic immunoassay (Immulite 2000; Siemens Healthcare Diagnostics Product Ltd., Glyn Rhonwy, Lianberis, UK). Body temperatures were recorded at the same observational periods as the blood samples. Pharyngeal temperature measurements, using the heat probe in the anesthetics equipment, were used to monitor intraoperative body temperature. In all other observation periods, body temperature was monitored using a contactless infrared thermocouple (DT-8806, SIEMENS) on the forehead.
Pain severity was assessed using a VAS (scale of 1 to 10, 0: no pain, 10: worst possible pain). Nausea and pruritus were measured with a four-stage categorical scoring system (0: none, 1: light, 2: medium, 3: severe). Metoclopramide (10 mg) was used for treating nausea-vomiting.
Sleep was evaluated by using a 10-cm VAS scale (0: did not sleep at all, 10: slept perfectly), which is a subjective method, during the preoperative and postoperative periods.
Power analysis suggested at least 15 individuals with the change in melatonin levels of 34.5% (pg/ml), type I error of 0.05 and type II error of 0.10 (power = 0.90).
IBM SPSS Statistics version 24.0 was used for all statistical analyses. Data were expressed as the mean ± standard deviation based on the general variable distribution. Normality was assessed by using the Shapiro-Wilk test. The preoperative and postoperative data were compared by using the paired sample t test with Bonferroni adjustment. Plasma melatonin levels (pg ml−1) and postoperative morphine consumption (mg) of the patients were analyzed by the Pearson correlation coefficient. p values < 0.05 were accepted as significant.
Results
Forty patients were enrolled in the study. However, 10 patients were excluded from the study because of the following issues: 6 patients did not meet the inclusion criteria, 2 patients experienced bleeding during surgery and different surgical procedures, 1 patient experienced a malfunction in the PCA device, and 1 patient wished to leave the study (Fig. 2).
Demographic data are described in Table 1.
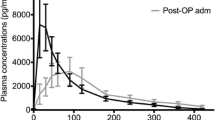
The mean preoperative plasma melatonin level was 78.96 ± 39.82 pg ml−1. There was a significant decrease in melatonin levels when preoperative melatonin levels were compared with intraoperative and all postoperative follow-up periods (p < 0.05) (Table 2). When the correlation of plasma melatonin levels and postoperative morphine consumption of the patients was inspected, there was a significant correlation in all of the follow-up periods (p < 0.05). The descriptive results of plasma melatonin levels and postoperative morphine consumption of the patients were given in Table 3.
The cortisol secretion was in circadian rhythm during the postoperative 24-h period. When preoperative cortisol levels were compared with intraoperative and postoperative cortisol levels, there was a significant difference in the follow-up periods, except for the 6- and 24-h postoperative periods (p < 0.05) (Fig. 3).
Body temperatures were normothermic and similar in all measurement periods.
Postoperative pain assessed using a VAS score is presented in Fig. 4. No patient needed rescue analgesia.
Severe nausea was observed in three patients in the 2- and 6-h postoperative periods.
A light pruritus was observed in one patient during the 2-h postoperative period, in two patients at the 6-h period, and in one patient at the 10-, 18-, and 24-h periods. No patient required any intervention for pruritus.
The preoperative and postoperative sleep VAS (median (min-max)) were 7 (3–9) and 5 (1–7) respectively. There was a significant difference between the preoperative sleep VAS and the postoperative sleep VAS (p < 0.001).
Discussion
In this prospective observational study of obese patients undergoing laparoscopic bariatric surgery, the secretion of melatonin was found to show a diurnal rhythm, but compared with the preoperative period, plasma melatonin levels tended to decrease during the intraoperative and postoperative periods. A significant relationship was determined between the melatonin levels and the consumption of morphine. Cortisol was also secreted in a diurnal rhythm. Cortisol levels were found to significantly increase up to the 6-h postoperative period and to significantly decrease between the 6- and 24-h postoperative periods. Body temperature changes were similar during all follow-up periods.
Treatment with exogenous melatonin, with its sedative, analgesic, anti-inflammatory, and antioxidant effects including circadian rhythm regulation, may become an attractive option for patient premedication. Caumo et al. [11] observed clinically significant anxiolytic and analgesic effects with 5 mg of exogenous oral melatonin, which was given before the night of the operation and 1 h before the operation. In another study, Caumo et al. [12] found that compared to the placebo group, anxiety and postoperative pain levels were significantly reduced with melatonin or clonidine treatment. Additionally, postoperative morphine consumption was significantly lower in the treatment groups. A previous study has also demonstrated that melatonin premedication improved overall recovery, as well as improved sleep and lower pain levels in patients undergoing bariatric surgery. It was emphasized melatonin was safe and improved quality of recovery in bariatric patients and can be an alternative premedication agent [14]. We did not administer exogenous melatonin to the patients in our study. However, we observed that compared to preoperative levels, endogenous melatonin levels decreased in the intraoperative and postoperative periods. We found a negative correlation between the reduction in melatonin levels and postoperative morphine consumption, which can be explained as follows: melatonin had a dose-dependent antinociception and may increase the β-endorphin induced by opioid receptor agonists [15, 16]. Moreover, melatonin could inhibit the release of pro-inflammatory cytokines [17]. Reduction in melatonin levels might diminish these beneficial analgesic effects of melatonin; therefore, the postoperative morphine consumption could increase in this situation.
Many mediators, including met-enkephalin, β-endorphin, bradykinin, 5-hydroxytryptamine, glutamate, nitric oxide, substance P, and cytokines, play a role in pain and fluctuate over a 24-h period. Therefore, it is not surprising that a circadian expression of pain was reported [18]. Aya et al. [19] reported that labor pain perception showed a circadian change. Graves et al. [20] reported that morbid obesity did not have a significant effect on the morphine requirement. They found a daily rhythm in morphine requirements; the most analgesic used was at 9:00 A.M., and the lowest used was at 3:00 A.M. Postoperative opioid requirements had a circadian rhythm. Auvil-Novak et al. [21] showed that the period with the greatest morphine requirement was between 8:00 A.M. and 12:00 P.M., while the minimum needed was between 12:00 A.M. and 4:00 A.M. We achieved similar results in our study. The consumption of morphine was observed to be the lowest at 2:00 A.M., and the morphine consumption increased in the following hours.
Guo et al. [22] investigated melatonin and cortisol levels in patients at the 1-, 2-, and 3-day perioperative and postoperative follow-up periods in patients undergoing coronary artery bypass grafting and found that compared to baseline values, plasma melatonin concentrations in all patients decreased at the perioperative period and immediately after the operation. Furthermore, they showed that the circadian rhythm was established at the 2- and 4-day postoperative periods. We also observed in our study that melatonin secretion was in a circadian rhythm during the 24-h period (preoperative, intraoperative, and postoperative first day) and that the melatonin levels decreased. However, the levels of cortisol in our study were different from Guo et al., who did not observe a significant difference in the intraoperative plasma cortisol concentration. Furthermore, compared with the basal values, the cortisol concentrations were significantly higher than the immediate postoperative period, at 12:00 A.M., 3:00 A.M., and 6:00 A.M. In our study, a probable reason for this difference may be the metabolic and surgical stress response between the laparoscopic bariatric surgery and the cardiopulmonary bypass graft operation-induced thoracotomy, the cardiopulmonary bypass technique and hypothermia.
The results of our study were similar to Ram et al. [23], who investigated the changes in melatonin and cortisol levels in patients undergoing laparoscopic surgery. Plasma melatonin levels were found to decrease on the night of surgery, and cortisol levels were found to increase. This difference can be explained by the negative correlation between cortisol and melatonin [24].
We believe that acute or chronic stress could not increase melatonin secretion, as reported by Cronin et al. [25, 26]. It was noted that the reduction in melatonin secretion more likely disrupted circadian rhythm or directly decreased sleep. A possible cause of the decrease in melatonin levels in our study may have been the sleep disturbances indicated by Cronin et al. Additionally, we could not achieve an optimal dark environment in the intensive care unit in which we monitored our patients, which could also have affected the result [27].
Gögenur et al. [5] found that body temperature was significantly altered in the perioperative period, and the mean temperature significantly increased at night on postoperative days 1 and 2. In contrast, in our study, we found that the body temperature did not change during any of the follow-up periods. This was most likely due to the method of monitoring body temperature. Body temperature was postoperatively monitored using a contactless infrared thermocouple in the study.
Limitation of the Study
There were some limitations in the present study. First, baseline values of melatonin and cortisol secretion values were not measured 24 h prior to the surgery or at the 2- and 3-day postoperative period. However, due to our clinic’s routine procedures, the patients were admitted to the hospital the morning of the operation and monitored in the intensive care unit during the first 24 h. Second, the optimal darkness and quiet environment could not be achieved in the intensive care unit due to the structure of the isolated room. Third, the VAS sleep assessment used in the present study is a subjective report. For technical reasons, objective sleep measurement, like an actigraphy, could not be used. Further, VAS sleep assessment was used alone in many previous reported studies [28, 29].
Conclusion
Compared to the preoperative levels in obese patients undergoing laparoscopic bariatric surgery, endogenous melatonin secretion was significantly decreased in the intraoperative and postoperative periods. Furthermore, there was a significant inverse correlation between changes in endogenous melatonin levels and morphine consumption. Melatonin and cortisol secretion showed a diurnal rhythm.
References
Chassard D, Bruguerolle B. Chronobiology and anesthesia. Anesthesiology. 2004;100:413–27.
Wager-Smith K, Kay SA. Circadian rhythm genetics: from flies to mice to humans. Nat Genet. 2000;26:23–7.
Hansen MV. Chronobiology, cognitive function and depressive symptoms in surgical patients. Dan Med J. 2014;61:B4914.
Gögenur I. Postoperative circadian disturbances. Dan Med Bull. 2010;57:B4200.
Gögenur I, Ocak U, Altunpinar O, et al. Disturbances in melatonin, cortisol and core body temperature rhythms after major surgery. World J Surg. 2007;31:290–8.
Claustrat B, Brun J, Chazot G. The basic physiology and pathophysiology of melatonin [physiological review]. Sleep Med Rev. 2005;9:11–24.
Naguib M, Gottumukkala V, Goldstein PA. Melatonin and anesthesia: a clinical perspective. J Pineal Res. 2007;42:12–21.
Naguib M, Hammond DL, Schmid 3rd PG, et al. Pharmacological effects of intravenous melatonin: comparative studies with thiopental and propofol. Br J Anaesth. 2003;90:504–7.
Garaulet M, Madrid JA. Chronobiology, genetics and metabolic syndrome. Curr Opin Lipidol. 2009;20:127–34.
Szewczyk-Golec K, Woźniak A, J. Reiter RJ. Inter-relationships of the chronobiotic, melatonin, with leptin and adiponectin: implications for obesity. J Pineal R Volume 2015; 59: 277–291.
Caumo W, Torres F, Moreira Jr NL, et al. The clinical impact of preoperative melatonin on postoperative outcomes in patients undergoing abdominal hysterectomy. Anesth Analg. 2007;105:1263–71.
Caumo W, Lavandovski R, Hidalgo MP. Preoperatve anxiolytic effect of melatonin and clonidine on postoperative pain and morphine consumption in patients undergoing abdominal hysterectomy: a double-blind, randomized, placebo controlled study. J Pain. 2009;10:100–8.
Ismail SA, Mowafi HA. Melatonin provides anxiolysis, enhances analgesia, decreases intraocular pressure, and promotes better operating conditions during cataract surgery under topical anesthesia. Anesth-Analg. 2009;108:1146–51.
Ivry M, Goitein D, Welly W, et al. Melatonin premedication improves quality of recovery following bariatric surgery—a double blind placebo controlled prospective study. Surg Obes Relat Dis. 2017;13:502–6.
Pang CS, Tsang SF, Yang JC. Effects of melatonin, morphine and diazepam on formalin-induced nociception in mice. Life Sci. 2001;68:943–51.
Pertovaara A, Hämäläinen MM, Kauppila T, et al. Dissociation of the alpha 2-adrenergic antinociception from sedation following microinjection of medetomidine into locus caeruleus in rats. Pain. 1994;57:207–15.
Cuzzocrea S, Zingarelli B, Gilad E, et al. Protective effect of melatonin in carrageen-induced models of local inflammation: relationship to its inhibitory effect on nitric oxide production and its peroxynitrite scavenging activity. J Pineal Res. 1997;23:106–16.
Kerdelue´ B, Palkovitz M, Karteszi M, et al. Circadian variations in substance P, luberin (LHRH) and thyreoliberin (TRH) contents in hypothalamic and extrahypothalamicbrain nuclei of adult male rats. Brain Res. 1981;206:406–14.
Aya AG, Vialles N, Mangin R, et al. Chronobiology of labour pain perception: an observational study. Br J Anaesth. 2004;93:451–3.
Graves D, Batenhorst R, Bennett R, et al. Morphine requirements using patient-controlled analgesia: influence of diurnal variation and morbid obesity. Clin Pharm. 1983;2:49–53.
Auvil-Novak SE, Novak R, Smolensky MH, et al. Twenty-four hour variation in self-administration of morphine sulfate and hydromorphone by postsurgical gynecologic cancer patient. Ann Rev Chronopharmacol. 1988;5:343–6.
Guo X, Kuzumi E, Charman SC, et al. Perioperative melatonin secretion inpatients undergoing coronary artery bypass grafting. Anesth Analg. 2002;94:1085–91.
Ram E, Vishne TH, Weinstein T, et al. General anesthesia for surgery influences melatonin and cortisol levels. World J Surg. 2005;29:826–9.
Kellner M, Yassouridis A, Manz B, et al. Corticotropin-releasing hormone inhibits melatonin secretion in healthy volunteers: a potential link to low-melatonin syndrome in depression? Neuroendocrinology. 1997;65:284–90.
Srinivasan V. Melatonin secretion after surgery. Lancet. 2001; 17; 357(9255):557–8.
Massion AO, Teas J, Herbert JR. Meditation Melatonin and Cancer. In: Shaffi M, Shaffi S, eds. Melatonin in psychiatric and neoplastic disorders. Washington DC: American Psychiatric Press, 1998: 261–94.
Claustrat A, Brun J, Chazot G. The basic physiology and pathophysiology of melatonin. Sleep Med Rev. 2005;9:11–24.
Kärkelä J, Vakkuri O, Kaukinen S, et al. The influence of anaesthesia and surgery on the circadian rhythm of melatonin. Acta Anaesthesiol Scand. 2002;46:30–6.
Hansen MV, Madsen MT, Andersen LT, et al. Effect of melatonin on cognitive function and sleep in relation to breast cancer surgery: a randomized, double-blind, placebo-controlled trial. Int J Breast Cancer. 2014;27
Acknowledgements
We would like to thank Prof. Dr. Hakan Parlakpınar for his assistance with the study.
Funding
The financial support for the project number 2016/49 was supplied by the Inonu University Scientific Research Project Center.
Author information
Authors and Affiliations
Contributions
Neslihan Altunkaya conduct of the study, data collection. Attestation: Dr. Altunkaya approved. Mehmet Ali Erdogan study design, manuscript preparation. Attestation: Dr. Mehmet Ali Erdogan approved. Ulku Ozgul conduct of the study, data collection. Attestation: Dr. Ulku approved. Mukadder Sanli Data collection. Attestation: Dr. Sanli approved. Muharrem Ucar data collection, conduct of the study. Attestation: Dr. Ucar approved the final manuscript. Onural Ozhan analysis of samples. Attestation: Mr. Ozhan approved the final manuscript. Fatih Sumer data collection. Attestation: Dr. Sumer approved. Selim Erdogan analysis of samples. Attestation: Dr. Erdogan approved. Mahmut Durmuş manuscript preparation. Attestation: Dr. Durmus approved the final manuscript.
Corresponding author
Ethics declarations
Informed Consent
Informed consent was obtained from all individual participants included in the study. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
IRB: Malatya Clinical Research Ethics Committee, Inonu University, Malatya, Turkey inu.dhek@inonu.edu.tr
Rights and permissions
About this article
Cite this article
Altunkaya, N., Erdogan, M.A., Ozgul, U. et al. Changes in Melatonin, Cortisol, and Body Temperature, and the Relationship Between Endogenous Melatonin Levels and Analgesia Consumption in Patients Undergoing Bariatric Surgery. OBES SURG 28, 3186–3192 (2018). https://doi.org/10.1007/s11695-018-3313-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-018-3313-x