Abstract
Background
Perioperative management of chronically anti-coagulated patients undergoing bariatric surgery requires a balance of managing hemorrhagic and thromboembolic risks. The aim of this study is to evaluate the incidence of hemorrhagic complications and their management in chronically anticoagulated (CAT) patients undergoing bariatric surgery.
Methods
A retrospective review of CAT patients undergoing bariatric surgery at an academic center from 2008 to 2015 was studied.
Results
A total of 153 patients on CAT underwent surgery [Roux-en-Y gastric bypass (n = 79), sleeve gastrectomy (n = 63), and adjustable gastric banding (n = 11)] during the study period: 85 patients (55%) were females; median age was 56 years (interquartile range [IQR] 49–64), and median BMI was 49 kg/m2 (IQR 43–56). The most common indications for CAT were venous thromboembolism (n = 87) and atrial fibrillation (n = 83). Median duration of procedure and estimated intraoperative blood loss was 150 min (IQR 118–177) and 50 ml (IQR 25–75), respectively. Thirty-day postoperative complications were reported in 33 patients (21.6%) including postoperative bleeding (n = 19), anastomotic leak (n = 3), and pulmonary embolism (n = 1). Nineteen patients (12%) with early postoperative bleeding were further categorized to intra-abdominal (n = 10), intraluminal (n = 6), and at the port site or abdominal wall (n = 3). All-cause readmissions within 30 days of surgery occurred in 19 patients (12%). There was no 30-day mortality.
Conclusion
In our experience, patients who require chronic anticoagulation medication are higher than average risk for postoperative complications and all-cause readmission rates. Careful surgical technique and close attention to postoperative anticoagulation protocols are essential to decrease perioperative risk in this high-risk cohort.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Perioperative management of patients with obesity requiring chronic anticoagulation therapy (CAT) is challenging, with respect to maintaining a fine balance between the increased risks of thromboembolic versus hemorrhagic complications [1]. CAT is a known risk factor for postoperative bleeding and thromboembolic events in patients undergoing elective surgical procedures [2,3,4,5]. Thus, perioperative management of this high-risk group should be tailored to patient characteristics and clinical setting including comorbidities, indication for the CAT, elective vs. emergent procedure being performed, need for perioperative bridging and therapeutic timing of the anticoagulants, risk of venous thromboembolism (VTE) and hemorrhage, and consequences of bleeding and the available expertise to control postoperative bleeding.
In one of the meta-analyses on the early postoperative outcomes of chronically anticoagulated patients with periprocedural bridging, the overall risk of VTE and major bleeding was 1 and 4.2%, respectively [6]. Increasingly, a large number of obese patients requiring CAT are opting for bariatric surgery [7]. CAT patients undergoing bariatric surgery may experience impaired absorption of anticoagulation medications postoperatively. Contributory factors include alteration in the anatomy of gastrointestinal tract, weight loss, and lack of oral intake. This may eventually result in difficult achievement of therapeutic levels postoperatively [8,9,10].
Current literature on early postoperative outcomes of patients on CAT undergoing bariatric surgery is limited to small retrospective series, with variable results on postoperative hemorrhagic events [1, 7, 10,11,12]. This study aims to evaluate the incidence of early postoperative morbidity in chronically anti-coagulated patients undergoing primary laparoscopic bariatric surgery at our institution. The primary end point of the study was defined as incidence of early postoperative (≤ 30 day) hemorrhagic and thromboembolic complications in patients on CAT. Secondary endpoints included reoperations, readmissions, major, and minor complications.
Materials and Methods
Study Cohort
After approval by our Institutional Review Board, a retrospective review of a prospectively maintained database was completed to identify patients with CAT that had undergone bariatric surgery from February 2008 to August 2015 at our institution. All patients with CAT who had undergone primary laparoscopic bariatric procedures including Roux-en-Y gastric bypass (RYGB), sleeve gastrectomy (SG), and adjustable gastric banding (AGB) were included. Patients without CAT and those in whom no data was available were excluded.
Definition of Chronic Anticoagulation Therapy
CAT was arbitrarily defined as the use of oral anticoagulants for at least 1 month before the bariatric surgery. Treatment was monitored according to the International normalization ratio (INR) for the oral anticoagulants, and therapeutic range for these patients was in between 2 and 3 [13] according to their co-morbidities.
Perioperative Management of Anticoagulation Therapies
All patients underwent a preoperative consultation with bariatric physician and anesthesiologist, during which perioperative management of anticoagulation was planned. Indications for bridging anticoagulation therapies included the following: mechanical heart valve, venous thromboembolism (VTE) (recent, recurrent, or with thrombophilia), or high-risk conditions for stroke in patients with atrial fibrillation (CHA2DS2VASc score more than 5). For the bridging, CAT was stopped approximately 5 days before surgery and LMWH or UH was initiated 2 days after the last dose of CAT; the choice of one or the other was mainly based on the bariatric physician’s recommendation. The last preoperative dose was administered 24 h before the surgery. Patients received perioperative pharmacoprophylaxis for VTE. Postoperative bridging was initiated on day 2 or day 3 after surgery, once the hemostasis was achieved until INR was therapeutic. After surgical procedure and upon discharge from the hospital, these patients are followed up by their primary care physicians or the INR clinic.
Data Collection and Statistical Analysis
Study data was collected and managed using REDCap (Research Electronic Data Capture) hosted at our institution and customized for this study [14]. Collected data included baseline patient demographics; charlson comorbidity index [15]; CHA2DS2VASc score (congestive heart failure, hypertension, age, diabetes mellitus, history of stroke or transient ischemic attack, vascular disease, and gender) [16]; weight-related comorbidities (diabetes mellitus, hypertension, hyperlipidemia, reflux disease, sleep apnea), indications for CAT (prior VTE, atrial fibrillation, mechanical/prosthetic heart valve, and thrombophillic conditions), and past history of abdominal procedures.
Furthermore, type of anticoagulant, the use of therapeutic and bridge anticoagulant, and its timing with respect to the bariatric procedure (preoperative, intraoperative, and postoperative) were collected. Perioperative variables included preoperative laboratory data (level of hemoglobin, platelet count, partial thromboplastin time, prothrombin time, and INR), American Society of Anesthesiologist (ASA) score, and concurrent procedures performed, duration, type (RYGB, SG, and AGB), and technique (laparoscopic, open, or laparoscopic converted to open). In addition, intraoperative complications (significant bleeding > 250 ml, need for intraoperative transfusions, and conversion to open surgery) and length of hospital stay were also noted.
Postoperative data constituted early postoperative complications (< 30 days). Postoperative complications were categorized as bleeding complications, major complications, and minor complications according to the ASMBS outcomes reporting standards [17]. Bleeding complications were further categorized depending on the source of bleeding (intra-abdominal, intraluminal, or port-site) and the management performed (need for reoperation and transfusion and stopping anticoagulation or conservative management). Major complications included venous thromboembolism requiring administration of anticoagulant/intervention, anastomotic leak requiring reoperation/percutaneous drainage/stent placement/management with parenteral nutrition and nil per oral (NPO), bowel obstruction requiring reoperation, trocar site hernia requiring reoperation, myocardial infarction, renal failure requiring dialysis, respiratory failure requiring intubation, chronic nausea/vomiting requiring parenteral nutrition, surgical site infection requiring debridement/washout and small bowel stenosis, and stricture or obstruction requiring reoperation. Minor complications included poor oral intake requiring intravenous hydration/enteral access, marginal ulcer diagnosed on upper endoscopy, anastomotic stricture requiring dilation, pneumonia requiring antibiotics, port site seroma, anemia managed with transfusion, acute renal failure managed with intravenous hydration, postoperative ileus managed conservatively, trocar site infection managed with drainage and local wound care, urinary tract infection requiring antibiotics and negative re-exploration performed to rule out leak, or unexplained tachycardia. Continuous variables were summarized by medians with interquartile range (IQR) and categorical variables by counts and percentages.
Results
Demographics
There were 3882 patients who underwent primary laparoscopic bariatric surgery during the study period. We identified 153 patients (3.9%) on CAT among them. Eighty-five (56%) patients were female with median age of 56 years (IQR 49–64) and the median preoperative BMI of 49 kg/m2 (IQR 43–56). Associated pertinent comorbidities included hypertension (n = 130, 85%), sleep apnea (n = 122, 80%), dyslipidemia (n = 107, 70%), diabetes mellitus (n = 90, 59%), gastroesophageal reflux disease (n = 66, 43%), chronic obstructive pulmonary disease (n = 15, 10%), and renal insufficiency (n = 15, 10%). Among the study cohort, 83 patients (54%) had a previous history of abdominal surgery.
Indications for chronic anticoagulation were VTE (n = 87), atrial fibrillation (n = 83), prosthetic heart valve (n = 4), coagulation disorder including factor V Leiden deficiency (n = 8), protein C deficiency (n = 6), protein S deficiency (n = 3), anti-phospholipid syndrome (n = 2), and prothrombin gene mutation (n = 1). One hundred seventeen patients (76%) had a single indication for CAT while 36 patients (24%) had more than one indication for CAT. Median CHA2DS2VASc score was 4 (IQR 3–5). Eighty-five percent patients had a CHA2DS2VASc score ≥ 3. The demographic data is summarized in Table 1.
Perioperative Details
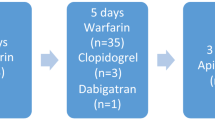
Preoperative therapeutic anticoagulants included the following: warfarin (n = 128, 85%), LMWH (n = 5, 3%), dabigatran (n = 9, 5%), apixaban (1, 1%), and rivaroxaban (n = 10, 6%). Duration of discontinuation of therapeutic anticoagulation before index surgery was 5 days (IQR 5–6), and it was resumed a median of 2 days postoperatively (IQR 1–3). Postoperatively, two patients were not resumed on therapeutic anticoagulation as both the patients no longer required long-term oral anticoagulants. Bridging dose of anticoagulants was used in 74 patients (48%) with either LMWH (n = 50), UH (n = 14), LMWH and UH together (n = 5), or fondaparinux (n = 5). Most patients (94%) received perioperative pharmcothromboprophlaxis. Median preoperative hemoglobin and INR were 13.6 mg/dl (IQR 12.4–14.5) and 1.2 (IQR 1.1–1.5), respectively. Data on perioperative use of anticoagulants is summarized in Table 2.
Bariatric procedures performed included RYGB (n = 79), SG (n = 63), and AGB (n = 11). Median duration of procedure and estimated intraoperative blood loss was 150 min (IQR 118–177) and 50 ml (IQR 25–75), respectively. Significant intraoperative bleeding or intraoperative transfusions were reported in six patients. The median postoperative length of stay was 4 days (IQR 3–5). Pertinent perioperative data is summarized in Table 3.
Thirty-Day Postoperative Details
Early postoperative complications (< 30 days) were observed in 33 patients (21.6%) including postoperative bleeding (n = 19), anastomotic leak (n = 3), pulmonary embolism (n = 1), organ space surgical site infection (n = 1), poor oral intake requiring intravenous (IV) hydration or enteral access (n = 3), marginal ulcer (n = 3), anastomotic stricture (n = 1), pneumonia requiring antibiotics (n = 1), port site seroma (n = 1), anemia managed with transfusion (n = 1), and acute renal failure managed with IV hydration (n = 1). Overall 30-day composite complications in patients with single and multiple indications for CAT were 24% (n = 27/117) and 17% (n = 6/36), respectively.
Nineteen patients (12%) had early postoperative bleeding (< 30 days) were further categorized based on the site of bleeding as follows: intra-abdominal (n = 10), intraluminal (n = 6), and at the port site or abdominal wall (n = 3). Thirteen patients with postoperative bleeding were managed conservatively with transfusion of blood products, and six patients required surgical intervention and blood product transfusions. Four out of 19 patients developed bleeding complications as a result of supratherapeutic INR postoperatively. The incidence of bleeding complications was further categorized according to indications for CAT [single indication for CAT, 14% (16/117); multiple indications for CAT, 8% (3/36)], type of CAT [patients on warfarin, 14% (18/128) and patients on DOAC, 4% (1/25)] and perioperative bridging [patients with bridging, 16% (12/74) and 9% (7/79)], respectively.
One patient developed pulmonary embolism on postoperative day (POD) 12 after SG and was readmitted. She was started on IV heparin drip and discharged after obtaining therapeutic INR. Two patients had more than one early complication after RYGB. Both the patients had initial postoperative bleeding complications that were managed with transfusion of blood products and stopping anticoagulants. One of the patients had surgical evacuation of clots and subsequently developed intra-abdominal abscess that was drained percutaneously. The other patient had poor oral intake due to marginal ulcer that was treated with proton-pump inhibitors for 6 months.
All-cause readmissions within 30 days of surgery occurred in 19 patients (12%). Discharge diagnosis for readmissions in the early postoperative period included postoperative bleeding (n = 9), VTE (n = 1), anastomotic leak (n = 2), poor oral intake requiring IV hydration/endoscopic intervention (n = 3), marginal ulcer (n = 2), anastomotic stricture (n = 1), and acute renal failure managed with IV hydration (n = 1). Early postoperative outcomes (< 30 days) are summarized in Table 4.
Discussion
This study aimed to evaluate the prevalence of early postoperative morbidity in chronically anti-coagulated patients undergoing primary laparoscopic bariatric surgery at our institution. The primary findings of this study are that through early postoperative period: (1) the prevalence of intraoperative and postoperative hemorrhagic complications was relatively higher and was noted in six patients (4%) and 19 patients (12%), respectively; (2) one patient (0.6%) developed pulmonary embolism. Additionally, these patients also experienced higher composite morbidity (21.6%) and 30-day readmissions (12.4%) when compared to national averages for non-CAT patients undergoing bariatric surgery [18].
Periprocedural management of patients on CAT requires a fine balance to prevent thrombotic and hemorrhagic complications [1]. These patients constitute a high-risk cohort, due to their associated comorbidities. This was reflected by a higher median age adjusted Charlson comorbidity index of 3 in our population as well. The higher risks for thrombotic and hemorrhagic events in the early postoperative period in the CAT patients can be broadly divided into patient-related and surgical risk factors. Patient characteristics including morbid obesity, history of multiple or severe thrombophilia, recent history (< 3 months) of VTE, recent (< 6 months) stroke or TIA, mechanical heart valve, CHADS2 score > 4, need for interruption of anticoagulation, and duration of procedure impart a higher thromboembolic risk [5, 13]. Previous history of bleeding especially with invasive procedures, administration of anticoagulants with concomitant anti-platelet agents and non-steroidal anti-inflammatory drugs, and the type of invasive procedure performed confers increased bleeding risks during the early postoperative period [19,20,21,22,23]. In addition, altered gastrointestinal anatomy in patients undergoing bariatric surgery may influence pharmacokinetics of anticoagulants. Reduced caloric intake, decreased and unavailable absorptive surfaces, alkaline pH in the gastric pouch, and increased transit time in RYGB are some of the factors responsible for decreased absorption of anticoagulants [8,9,10].
One patient (0.65%) in our study developed pulmonary embolism in the early postoperative period. This is similar to what has been previously reported for non-CAT patients undergoing bariatric surgery and might have been a result of our institutional practice for strict and extensive thromboprophylaxis in high-risk patients [24]. Six patients (3.9%) required intraoperative transfusion of blood. Of these, two patients further developed hemorrhagic complication in the early postoperative period. Hemorrhagic complications were noted in 19 patients (12.4%) in the early postoperative period. Majority of these were intraabdominal (52.6%) followed by intra-luminal (31.6%) and port-site bleeding (15.8%). Six patients (31.6%) with postoperative bleeding required surgical intervention while the rest were managed conservatively by either stopping the anticoagulants or blood transfusion. With respect to index surgeries performed, the rate of bleeding complications was 15.2% (n = 12) for RYGB, 8.0% (n = 5) for SG, and 18.2% (n = 2) for LAGB. Both the LAGB patients had port site/abdominal wall hematoma secondary to supratherapeutic INR. Overall, the incidence of hemorrhagic complications in our cohort was similar to what has been previously reported in studies including CAT patients undergoing bariatric surgery. The incidence of early postoperative hemorrhage in these studies ranged from 14 to 28% for RYGB procedures and 6.7% for SG patients [1, 7, 10, 12]. Overall, the incidence of hemorrhagic complications in our cohort was similar to what has been previously reported in studies including CAT patients undergoing bariatric surgery. In our experience, patients who require CAT medication are higher than average risk for postoperative bleeding complications after bariatric surgery. Therefore, careful surgical technique and tissue handling are essential during the surgery. Additionally, close attention to postoperative anticoagulation protocols is required to minimize the risk of bleeding.
Patients requiring CAT who undergo bariatric surgery are a high-risk cohort whose overall morbidity in the early postoperative period was expectedly higher (21.6%) when compared to non-CAT bariatric population [25]. This finding was similar to the composite morbidity reported in earlier studies in bariatric patients on CAT [1, 7, 10, 12]. Additionally, 30-day readmission rates (12.4%) were comparatively higher than the reported national average of 5% [18]. More than 50% of the readmissions were related to postoperative bleeding in our study. Despite a higher risk, the incidence of bleeding related 30-day readmissions was comparatively lower to those reported by Bechtel et al. (5.9 vs 16%) [7].
To our knowledge, this study has the largest series of CAT patients who underwent bariatric surgery reported in literature to date. The study limitations include retrospective nature, use of single academic institution, assessment of only early postoperative outcomes, and absence of a matched control group to compare outcomes with.
Conclusion
In our experience, patients who require chronic anticoagulation medication are higher than average risk for postoperative bleeding complications after bariatric surgery. Careful surgical technique and close attention to postoperative anticoagulation protocols are required to decrease risk. Additionally, all-cause readmission rate for these patients (12%) is higher than the 5% national average. Since, these patients are high risk for readmission, targeting them for additional resources after discharge to prevent readmissions may be warranted.
References
Mourelo R, Kaidar-Person O, Fajnwaks P, et al. Hemorrhagic and thromboembolic complications after bariatric surgery in patients receiving chronic anticoagulation therapy. Obes Surg. 2008;18(2):167–70. https://doi.org/10.1007/s11695-007-9290-0.
Iqbal CW, Cima RR, Pemberton JH. Bleeding and thromboembolic outcomes for patients on oral anticoagulation undergoing elective colon and rectal abdominal operations. J Gastrointest Surg. 2011;15(11):2016–22. https://doi.org/10.1007/s11605-011-1611-x.
Yoshida T, Kitano S, Matsumoto T, et al. Laparoscopic cholecystectomy in patients undergoing anticoagulant therapy. Surg Today. 1998;28(3):308–12. https://doi.org/10.1007/s005950050128.
McLemore EC, Harold KL, Cha SS, et al. The safety of open inguinal herniorraphy in patients on chronic warfarin therapy. Am J Surg. 2006;192(6):860–4. https://doi.org/10.1016/j.amjsurg.2006.08.058.
Spyropoulos AC, Al-Badri A, Sherwood MW, et al. Periprocedural management of patients receiving a vitamin K antagonist or a direct oral anticoagulant requiring an elective procedure or surgery. J Thromb Haemost. 2016;14(5):875–85. https://doi.org/10.1111/jth.13305.
Siegal D, Yudin J, Kaatz S, et al. Periprocedural heparin bridging in patients receiving vitamin K antagonists: systematic review and meta-analysis of bleeding and thromboembolic rates. Circulation. 2012;126(13):1630–9. https://doi.org/10.1161/CIRCULATIONAHA.112.105221.
Bechtel P, Boorse R, Rovito P, et al. Warfarin users prone to coagulopathy in first 30 days after hospital discharge from gastric bypass. Obes Surg. 2013;23(10):1515–9. https://doi.org/10.1007/s11695-013-0972-5.
Martin KA, Lee CR, Farrell TM, et al. Oral anticoagulant use after bariatric surgery: a literature review and clinical guidance. Am J Med. 2017;130(5):1–8. https://doi.org/10.1016/j.amjmed.2016.12.033.
Irwin AN, McCool KH, Delate T, et al. Assessment of warfarin dosing requirements after bariatric surgery in patients requiring long-term warfarin therapy. Pharmacotherapy. 2013;33(11):1175–83. https://doi.org/10.1002/phar.1307.
Schullo-Feulner AM, Stoecker Z, Brown GA, et al. Warfarin dosing after bariatric surgery: a retrospective study of 10 patients previously stable on chronic warfarin therapy. Clin Obes. 2014;4(2):108–15. https://doi.org/10.1111/cob.12046.
Borbély Y, Juilland O, Altmeier J, et al. Perioperative outcome of laparoscopic sleeve gastrectomy for high-risk patients. Surg Obes Relat Dis. 2017;13(2):155–60. https://doi.org/10.1016/j.soard.2016.08.492.
Gerin O, Rebibo L, Dhahri A, et al. The safety of laparoscopic sleeve gastrectomy in patients receiving chronic anticoagulation therapy: a case-matched study. Obes Surg. 2015;25(9):1686–92. https://doi.org/10.1007/s11695-015-1590-1.
Douketis JD, Spyropoulos AC, Spencer FA, et al. Perioperative management of antithrombotic therapy. Chest. 2012;141(2):e326S–50S. https://doi.org/10.1378/chest.11-2298.
Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–81. https://doi.org/10.1016/j.jbi.2008.08.010.
Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83. https://doi.org/10.1016/0021-9681(87)90171-8.
Lip GYH, Nieuwlaat R, Pisters R, et al. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137(2):263–72. https://doi.org/10.1378/chest.09-1584.
Brethauer SA, Kim J, Chaar M El, Papasavas P, Eisenberg D, Rogers A, Ballem N, Kligman M, Kothari S (2015) Standardized outcomes reporting in metabolic and bariatric surgery. https://doi.org/10.1016/j.soard.2015.02.003
Berger ER, Huffman KM, Fraker T, et al. Prevalence and risk factors for bariatric surgery readmissions. Ann Surg. 2016;1(1):122–31. https://doi.org/10.1097/SLA.0000000000002079.
Tafur AJ, McBane R, Wysokinski WE, et al. Predictors of major bleeding in peri-procedural anticoagulation management. J Thromb Haemost. 2012;10(2):261–7. https://doi.org/10.1111/j.1538-7836.2011.04572.x.
Capodanno D, Angiolillo DJ. Management of antiplatelet therapy in patients with coronary artery disease requiring cardiac and noncardiac surgery. Circulation. 2013;128(25):2785–98. https://doi.org/10.1161/CIRCULATIONAHA.113.003675.
Schafer AI. Effects of nonsteroidal antiinflammatory drugs on platelet function and systemic hemostasis. J Clin Pharmacol. 1995;35(3):209–19. https://doi.org/10.1002/j.1552-4604.1995.tb04050.x.
Weibert RT. Oral anticoagulant therapy in patients undergoing dental surgery. Clin Pharm. 1992;11(10):857–64.
Technology Assessment Status Evaluation—update: endoscopic band ligation. November, 1996. ASGE. American society for gastrointestinal endoscopy. Gastrointest Endosc. 1998;47:573–5.
Aminian A, Andalib A, Khorgami Z, et al. Who should get extended thromboprophylaxis after bariatric surgery?: a risk assessment tool to guide indications for post-discharge pharmacoprophylaxis. Ann Surg. 2017;265(1):143–50. https://doi.org/10.1097/SLA.0000000000001686.
Schauer PR, Mingrone G, Ikramuddin S, et al. Clinical outcomes of metabolic surgery: efficacy of glycemic control, weight loss, and remission of diabetes. Diabetes Care. 2016;39(6):902–11. https://doi.org/10.2337/dc16-0382.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval Statement
For this type of study, formal consent is not required.
Informed Consent Statement
This study does not require informed consent.
Rights and permissions
About this article
Cite this article
Sharma, G., Hanipah, Z.N., Aminian, A. et al. Bariatric Surgery in Patients on Chronic Anticoagulation Therapy. OBES SURG 28, 2225–2232 (2018). https://doi.org/10.1007/s11695-018-3120-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-018-3120-4