Abstract
Background
Weight regain following bariatric surgery is not uncommon. Safe, effective weight loss treatment up to 1 year has been reported with the closed-loop gastric electrical stimulation (CLGES) system. Continuous recording of eating and activity behavior by onboard sensors is one of the novel features of this closed-loop electrical stimulation therapy, and may provide improved long-term weight maintenance by enhancing aftercare.
Methods
Four centers participating in a 12-month prospective multicenter randomized study monitored all implanted participants (n = 47) up to 24 months after laparoscopic implantation of a CLGES system. Weight loss, safety, quality of life (QOL), and cardiac risk factors were analyzed.
Results
Weight regain was limited in the 35 (74%) participants remaining enrolled at 24 months. Mean percent total body weight loss (%TBWL) changed by only 1.5% between 12 and 24 months, reported at 14.8% (95% CI 12.3 to 17.3) and 13.3% (95% CI 10.7 to 15.8), respectively. The only serious device-/procedure-related adverse events were two elective system replacements due to lead failure in the first 12 months, while improvements in QOL and cardiovascular risk factors were stable thru 24 months.
Conclusion
During the 24 month follow-up, CLGES was shown to limit weight regain with strong safety outcomes, including no serious adverse events in the second year. We hypothesize that CLGES and objective sensor-based behavior data combined to produce behavior change. The study supports CLGES as a safe obesity treatment with potential for long-term health benefits.
Trial registration
ClinicalTrials.gov identifier: NCT01448785
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Although bariatric surgery has been shown to be an effective treatment for weight loss and diabetes management, many patients do not wish to undergo a surgical procedure, despite meeting BMI and comorbidity qualifications. A recent paper surveyed potential candidates for bariatric surgery and found that the most common reason for not being interested in surgery was fear of complications from surgery [1]. This finding emphasizes the importance of new options for obesity treatment that are safer and have less negative side effects than current bariatric surgery, but provide clinically significant and longer-term weight loss than lifestyle modifications alone and pharmaceutical options. Gastric electrical stimulation (GES) is a reversible surgical option that offers support for long-lasting behavioral changes and is less invasive than conventional bariatric surgery options. Previously published data on weight loss with GES for the treatment of obesity are encouraging and do not preclude the possibility of successful treatment with second generation devices [2]. VBLOC Maestro® (Enteromedics) is a commercially available system which directly applies electrical stimulus to the abdominal branches of the vagus intending to block neural transmission, and recently reported significantly better weight loss at 18 months than a sham control with intensive lifestyle therapy [3]. The accepted success criteria for conventional bariatric therapy is > 50% EWL based on American Society of Metabolic & Bariatric Surgery (ASMBS) Medical Guidelines [4], but the FDA approval of the VBLOC Maestro® system supports that lesser efficacy is acceptable if the system produces significantly less safety risk, and produces less adverse events such as nausea, vomiting, GERD, and micronutrient deficiencies. The AMBS guidelines also suggest that success should “probably be related to factors other than mere weight loss, such as improvement or resolution of comorbidities, decreased mortality, enhanced quality of life, and positive psychosocial changes [4]. The closed-loop gastric electrical stimulation (CLGES) abiliti® system is a second generation system, with an onboard food intake sensor that detects food intake and triggers the electrical stimulation therapy, so that it is delivered when most effective, and avoids potential desensitization from continuous therapy delivery.
In addition, the CLGES system’s food sensor and three-axis accelerometer-based activity sensor collect objective data on the patients eating and exercise behavior 24 h/day. The data can be used at follow-ups and uploaded at home to an internet site. The objective sensor data allows the patient to self-monitor, which has been shown to be very effective in behavior modification, and can also be used by the clinician at follow-up to support a program of lifestyle modification [5].
In this study, we present 24 month weight loss maintenance and safety with CLGES from four centers during a safety monitoring period following a 12-month randomized multicenter trial comparing CLGES to laparoscopic adjustable gastric band [6]. In addition, changes in cardiovascular risk factors and quality of life were analyzed over the 24 month period.
Research Design and Methods
Participants
The study design and the patient inclusion/exclusion criteria have been described previously [6]. The study was a randomized, multicenter trial, with the primary endpoints pertaining to the 12-month safety and efficacy of CLGES versus the laparoscopic adjustable gastric band. All patients signed an informed consent that specified a monitoring period with bimonthly visits for the CLGES group for another 2 years following the 12-month study period. The protocol was approved by the ethics committees at each of the nine European centers that participated in the study. The main inclusion criteria for participants was a BMI > 40 kg/m2 or a BMI between 35 and 40 kg/m2 with an existing comorbidity. Participants with Hba1c ≥ 7.0 without insulin treatment were excluded in order to limit the potential for non-device-related medical issues. Participants had to have a > 5-year history of obesity and no previous bariatric surgery. The study is registered at ClinicalTrials.gov (identifier NCT01448785).
Treatment and Follow-up
Following a pre-screening based on the Three-Factor Eating Questionnaire (TFEQ) [7], the participants underwent medical screening, and those that met the criteria were randomized 2:1 to CLGES or LAGB therapy. The screening and treatment of both groups was described in detail previously [6], but for the purposes of this report, the treatment of the CLGES group will be briefly described.
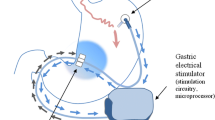
Those randomized to the CLGES group underwent an endoscopic gastric stimulation sensitivity test, where electrical stimulation is delivered through an endoscopically placed electrode, and gastric symptoms are reported on a visual scale by the patient. If the symptoms induced by the GES met the pre-specified criteria then the participant proceeded to system implantation. The abiliti system is laparoscopically implanted, and the components include a lead and a subcutaneously placed implantable pulse generator (IPG). Proximally placed on the lead is a stimulation electrode which is sutured to the anterior lessor curvature at the “goose foot” where the nerve of Latarjet terminates in three main branches. At the distal end of the lead is the transgastric sensor probe which is placed through the gastric wall in the body of the stomach. Once the system is activated and the sensor probe detects food intake, the electrical stimulation therapy is triggered based on a control algorithm run by the microprocessor housed in the IPG. There is also a three-axis accelerometer in the IPG used to record physical activity 24 h/day. The IPG is implanted in a subcutaneous pocket in the lower left quadrant of the abdomen, and the lead is tunneled to the subcutaneous pocket and attached to the connectors on the IPG header.
Two weeks after implant, the participants returned to the clinic for programming and activation of their CLGES therapy. A second sensitivity test is performed with the implanted system, where the therapy parameters are adjusted and the patient records their symptoms on a visual scale. Based on this testing, the therapy is programmed so that moderate stimulation is delivered on detection of food intake during allowed predetermined meal and snack times, and stronger stimulation meant to stop food intake is delivered during periods outside these allowed times. The average duration of therapy received by the trial participants was approximately 3 h/day, dependent on number of food intakes detected and programmed therapy duration. At each follow-up, the therapy parameters could be adjusted depending on patient feedback and weight loss progress. Participants received diet and exercise counseling at the time of discharge from the hospital and at each visit, according to standard post-surgical protocols for bariatric patients. In the CLGES group, the dietary and exercise counseling was supplemented by the sensor-based records of the patient’s eating and activity behavior downloaded from the device.
Data Collection and Analysis
Follow-up visits occurred at 2 weeks, monthly until 6 months, and bimonthly thereafter to 12 months. After the final study visit at 12 months, regular bimonthly follow-up visits were requested for the CLGES group to maintain stimulation therapy until battery depletion or for 24 additional months, whichever came first. This 24-month period was specified in the protocol as a monitoring period, and during these visits device assessment, adjustment of therapy parameters, and download of sensor data record was done, as well as medical evaluation which included vitals and weight recording, review and record of changes in concomitant medication, and review and record of any adverse events. Laboratory assessments and impact of weight on quality of life-Lite (IWQOL-Lite) [8] questionnaire were administered at baseline, 6 and 12 months, and also at 18 and 24 months. Subgroups with comorbidities at baseline were defined and analyzed separately to determine if improvement or resolution of comorbidity occurred. Diabetes subgroup was defined as HbA1c ≥ 6% [9] and dyslipidemia subgroups were defined as total cholesterol > 200 mg/dl, HDL cholesterol < 40 mg/dl in men and < 50 mg/dl in women, LDL cholesterol > 130 mg/dl or triglyceride > 150 mg/dl [10].
All adverse events (AE) were recorded, and the site investigator determined the type and severity of each adverse event and the relation to the device or procedure. The device- or procedure-related AE are reported here. The rating criteria were similar to those used in LAGB pre-market approval studies [11, 12]. They were classified as (a) mild when easily tolerated, possibly requiring the prescription of a new pharmaceutical or nutritional advice, (b) moderate when they interfered with usual activities requiring an unscheduled visit or an adjustment of the stimulation, and (c) severe when they required a re-hospitalization or a surgical or endoscopic intervention.
All data, collected at each investigative site by dedicated staff designated by the sponsor, were entered in a computer database for later analysis by the site investigators and the sponsor’s clinical department. A field clinical engineer assigned to the study sites assisted the investigative staff throughout the study.
Statistics
Descriptive statistics for all efficacy and safety endpoints include the number of observations, means ± standard deviations and ranges for continuous variables, crude event rates, and rates per patient-year for recurrent events, counts, and percentages for categorical variables and two-sided 95% confidence intervals (CI). Excess weight was calculated as the difference between the weight at the time of device implant and the ideal body weight corresponding to a BMI of 25 kg/m2. The effect of CLGES therapy on comorbidities, and quality of life was analyzed comparing baseline to 6, 12, 18, and 24 month averages with one-way ANOVA tests. A P value < 0.05 was considered significant. The SAS statistical package, version 9.3 (SAS Institute Inc., Cary, NC) and Minitab® 17.1.0 (Minitab Inc., State College, PA) were used for these analyses.
Results
Study Population
A CONSORT diagram through 24 months for the two Italian and two Spanish centers reporting is shown in Fig. 1, including reasons for attrition prior to 24 months. The primary endpoints for comparison of CLGES and LAGB were complete at 12 months. Following the end of the study at 12 months the CLGES group entered a 24-month safety monitoring period in order to obtain long-term safety data in this new therapy system, while the LAGB patients were completed. There is no difference in the baseline mean age, weight, and BMI of the 76 women and 30 men included in the total CLGES group at baseline (39 ± 11 years, 121 ± 21 kg, and 42 ± 5 kg/m2) and that of the 38 women and 9 men in the Spanish and Italian cohort reporting here (38.3 ± 12 years, 115 ± 19 kg, and 42 ± 6 kg/m2).
Thirty-five (74%) participants remained enrolled in the study at 24 months, and 32 (91%) of those enrolled attended the 24 (± 2)-month visit analysis.
Weight Loss
An important measure of weight loss, percent total body weight loss (%TBWL) was 14.8% (95% CI 12.3–17.3) at 12 months, and remained clinically significant at 24 months averaging 13.3% (95% CI 10.7–15.8) (Fig. 2). The percentage change in TBWL between 12 and 24 months was 1.5%, meeting the published definition for weight maintenance of < 3% [13]. The %EWL of the population averaged 40.8% (95% CI 33.9 to 47.7) at 12 months, with some weight regain at 24 months (average %EWL 34.3, 95% CI 27.3 to 41.3). The percent of participants achieving > 20% EWL at 24 months was 76.4 (56.5–86.4%). The distribution of weight loss for the study population is shown in Fig. 3, where each participant still enrolled in the study at 24 months is represented by a solid bar and patients withdrawn by 24 months by hatched bars, indicating their last measured %EWL. There were four patients that were withdrawn prior to 24 months based on dissatisfaction with weight loss and/or a loss of the feeling of satiety formerly provided by the CLGES. In order to confirm that these withdrawals did not produce a bias in the 24-month results, including their weight loss at the time of withdrawal (20.1, 56.1, 34.9, and 36.4%) in the analysis increases the average %EWL at 24 months to 35.6%. The %EWL using intent-to-treat including all 47 implanted participants with last measurement carried forward imputation yields an average %EWL of 31.4% (95% CI 24.8 to 38.0).
Individual excess weight loss at 24 month where all 47 implanted participants are represented by a bar. The solid bars represent participants remaining in the study at 24 months, and hatched bars represent the last excess weight loss measured for patients who withdrew prior to 24 months (n = 12 patients as specified in CONSORT diagram in Fig. 1)
Safety
Twenty participants (20/47, 43%) experienced a device-/procedure-related adverse event during the 24 months following implant (Table 1), for a total of 34 events, 94% being mild or moderate. Ten participants experienced more than one adverse event. The events that occurred in > 10% of patients were back/shoulder pain which is thought to be due to the gastric neural stimulation leading to referral of pain to the back and shoulder, and painful stimulation, which were both treated with stimulation parameter adjustment. The most common AE, mild or moderate “back and shoulder pain”, had early onset (5.4 ± 3.3 months) and resolved within (3.2 ± 6.5 months).
There were two severe adverse events (SAE) that occurred between implant and 24 months were two elective surgical device replacements in the first 12 months, due to lead failure. No complications resulted from the revisions.
The events that occurred between 12 and 24 months were 13 mild and 1 moderate adverse event (41% of the 24 month total). Nine of these events (64%) were patient complaints related to stimulation delivered with no intake due to compromised leads, the average onset month being 14.7 ± 4.6 months. The other events included back/shoulder pain rated as moderate (1) and mild (1), nausea/vomiting (1), heartburn acidity (1), and a pocket hematoma which resolved without treatment.
There were seven elective device explants following study withdrawal prior to 24 months due to autoimmune disease (1), pregnancy (2), and patient dissatisfaction with weight loss (4). These elective explants following withdrawal were not counted as adverse events. In the case of the four due to dissatisfaction with the therapy, the explant was performed with a conversion to laparoscopic sleeve gastrectomy.
Changes in Cardiovascular Risk Factors and Quality of Life
The changes in cardiovascular risk factors and quality of life were analyzed through 24 months.
In the participants (n = 10) who had pre-diabetes or diabetes diagnosed at baseline, the HbA1c levels were lower at all follow-ups through 24 months. The average % Hba1c was reduced from a baseline level of 6.2 to 5.8% at 24 months, though the statistical significance was lost (Fig. 4). For dyslipidemia, in the subgroup of patients who were diagnosed at baseline with lipid levels meeting higher risk thresholds, there were significant improvements at the follow-up visits through 24 months, except in triglyceride reduction (Fig. 5).
Trends in concentrations of blood lipids measured at baseline, month 6, month 12, month 18, and month 24 in the participants who had clinically high measurements at baseline. Chart A shows trend in total cholesterol (n = 12 with > 150 mg/dl at baseline). Chart B shows trend in triglycerides (i = 12 with > 150 mg/dl at baseline). Chart C shows trend in high-density lipoproteins (HDL) (n = 17 including men with HDL < 40 mg/dl and women with HDL < 50 mg/dl at baseline). Chart D shows trend in low-density lipoproteins (LDL) (n = 12 with LDL > 130 mg/dl at baseline) (*p<.05)
Participants had stable improvement in quality of life based on QOL-lite score. The total QOL-Lite score improved by 50% from 52 (95% CI 44 to 61) at baseline, to 78 (95% CI 72 to 84), 77 (95% CI 71 to 83), and 81 (95% CI 74 to 87) at 12, 18, and 24 months, respectively.
Discussion
Participants with CLGES therapy maintained weight loss through 24 months. The system also continued to be very safe, with no additional SAE reported following the two elective device replacements prior to 12 months. Only 43% (95%CI 28.4 to 56.7) of subjects experienced an adverse event between baseline and 24 months, providing evidence that the CLGES system is significantly safer at this 24-month endpoint than the gastric band with a reported 78.7% (95%CI 73.5 to 84.0) experiencing an AE by 24 months [14], including a 2.2% rate of revision. In the 24 months safety reporting for vBLOC, 375 events were reported over 24 months [15], resulting in an average of 1.5 events per patient-year, also a significantly higher rate of adverse events than experienced with the abiliti system [6]. Though the weight loss achieved was only moderate relative to other bariatric procedures, the mean 13.3% (95% CI 10.7 to 15.8) TBWL at 24 months follow-up is clinically significant [16], and may be balanced with the decreased safety risk. Based on the above factors along with low side effects and lack of dietary restrictions with this therapy, the most appropriate patients may be those with one or more of the following characteristics: BMI < 50, higher surgical risk, less willing to have invasive or aggressive procedures, and interested in a reversible procedure where they have more control of their treatment.
We hypothesize the greater weight loss efficacy with the CLGES system compared to that reported with other GES systems is due to multiple factors, including the closed-loop aspect of the system which avoids continuous therapy delivery, the individually tailored therapy parameters, and the sensor-based reporting of eating and activity behavior.
The weight loss results are also better than the 12 and 27 month excess weight loss of 28.7 and 27.5%, respectively, obtained in an early feasibility trial of the CLGES abiliti® system involving three German centers [17]. One possible reason that the patients lost more weight in the current study is because the field personnel were trained in the use of the sensor data at the clinical follow-up. Retrospective analysis of the use of the programmer during follow-up sessions provides quantitative evidence that when the patient and their clinician viewed the activity and exercise data that was downloaded from the IPG at each follow-up, it had a positive effect on their weight loss. This analysis was possible because the programmer stores digital files that record when each window tab was accessed, therefore the number of times the sensor data window was viewed could be determined. The analysis showed that the more frequently the clinician viewed the sensor data with the patients, and made it part of their counseling sessions, the better the weight loss was at that center. There was a positive correlation between percent weight loss and average number of sensor data views when analyzed by center. Multiple regression analysis showed that the relationship between frequency of sensor data views and outcome was independent of the frequency of visit attendance. The powerful effect of being observed on behavior has been reported [18], and also the value of self-monitoring for behavior change [5], supporting that this aspect of the system could have a real effect on patient outcome. In addition, Busetto et al. [19] analyzed the behavior changes as measured by the onboard sensors that occurred during the first year following system implantation in this population. They concluded that there was significant improvement in eating and activity seen in the participants, and hypothesize that the feedback of the sensor-based data may have produced the changes.
Limitations
Studies are needed to understand how to optimize both the stimulation parameters and location in order to most effectively modulate the vagal satiety signaling [20] in the clinical setting, both acutely and with extended therapy. The implantable stimulator used in this study was early generation technology and some battery depletion began to occur around 24 months. It is expected that the next generation system would have an extended battery life due to technological improvements and optimized programming.
Importantly long-term studies with an optimized system are needed to determine sustainability of weight loss and verify safety with an implant of extended duration.
Conclusion
The performance of this system is encouraging given the clinical benefits and the safety and reversibility of the procedure and implanted system. The results warrant further study, particularly in the long term, and point to the utility of further work to optimize GES systems for the treatment of obesity.
References
Fung M, Wharton S, Macpherson A, et al. Receptivity to bariatric surgery in qualified patients. J Obes. 2016;2016:6.
Abell TL, Chen J, Emmanuel A, et al. Neurostimulation of the gastrointestinal tract: review of recent developments. Neuromodulation. 2015;18(3):221–227; discussion 227. https://doi.org/10.1111/ner.12260.
Shikora SA, Wolfe BM, Apovian CM, et al. Sustained weight loss with vagal nerve blockade but not with sham: 18-month results of the ReCharge trial. J Obes. 2015;2015:365604.
Mechanick JI, Kushner RF, Sugerman HJ, et al. American Association of Clinical Endocrinologists, the Obesity Society, and American Society for Metabolic & bariatric surgery medical guidelines for clinical practice for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient. Surg Obes Relat Dis. 2008;4(5 Suppl):S109–84. https://doi.org/10.1016/j.soard.2008.08.009.
Burke LE, Wang J, Sevick MA: Self-monitoring in weight loss: a systematic review of the literature. J Am Diet Assoc 2011, 111(1):92–102, 1, DOI: https://doi.org/10.1016/j.jada.2010.10.008.
Horbach T, Meyer G, Morales-Conde S, Alarcón del Agua I, Favretti F, Anselmino M, Rovera G, Dargent J, Stroh C, Susewind M et al: Multicenter randomized study of closed-loop gastric electrical stimulation versus laparoscopic adjustable gastric band for the treatment of obesity. Int J Obes 2016, 40 (12):1891–1898, DOI: https://doi.org/10.1038/ijo.2016.159.
Stunkard A, Messick S. Eating inventory: manual. San Antonio, TX: Pearson Education, Inc.; 1988.
Kolotkin RL, Norquist JM, Crosby RD, et al. One-year health-related quality of life outcomes in weight loss trial participants: comparison of three measures. Health Qual Life Outcomes. 2009;7(1):53. https://doi.org/10.1186/1477-7525-7-53.
Gillett MJ: International expert committee report on the role of the A1c assay in the diagnosis of diabetes: Diabetes Care 2009; 32(7): 1327-1334. The Clinical biochemist Reviews 2009, 30(4):197–200.
Jellinger PS, Smith DA, Mehta AE, et al. American Association of Clinical Endocrinologists’ guidelines for management of dyslipidemia and prevention of atherosclerosis. Endocr Pract. 2012;18(2):269–93. https://doi.org/10.4158/EP.18.2.269.
Allergan: Summary of safety and effectiveness, LAP-BAND® Adjustable Banding system In.; 2010.
Ethicon Endo-Surgery I: Summary of safety and effectiveness data, REALIZE adjustable gastric band. In.; 2007.
Stevens J, Truesdale KP, McClain JE, et al. The definition of weight maintenance. Int J Obes. 2006;30(3):391–9. https://doi.org/10.1038/sj.ijo.0803175.
Cunneen SA, Brathwaite CE, Joyce C, et al. Clinical outcomes of the REALIZE adjustable gastric band-C at 2 years in a United States population. Surg Obes Relat Dis. 2013;9(6):885–93. https://doi.org/10.1016/j.soard.2013.02.009.
Apovian CM, Shah SN, Wolfe BM, et al. Two-year outcomes of vagal nerve blocking (vBloc) for the treatment of obesity in the ReCharge trial. Obes Surg. 2017;27(1):169–76. https://doi.org/10.1007/s11695-016-2325-7.
Wing RR, Lang W, Wadden TA, et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011;34(7):1481–6. https://doi.org/10.2337/dc10-2415.
Horbach T, Thalheimer A, Seyfried F, et al. Abiliti closed-loop gastric electrical stimulation system for treatment of obesity: clinical results with a 27-month follow-up. Obes Surg. 2015;25(10):1779–87. https://doi.org/10.1007/s11695-015-1620-z.
McCarney R, Warner J, Iliffe S, et al. The Hawthorne effect: a randomised, controlled trial. BMC Med Res Methodol. 2007;7(1):30. https://doi.org/10.1186/1471-2288-7-30.
Busetto L, Torres AJ, Morales-Conde S, et al. Impact of the feedback provided by a gastric electrical stimulation system on eating behavior and physical activity levels. Obesity (Silver Spring). 2017;25(3):514–21. https://doi.org/10.1002/oby.21760.
Guo X, Li Y, Yao S, et al. Parameter selection and stimulating effects of an adjustable gastric electrical stimulator in dogs. Obes Surg. 2014;24(1):78–84. https://doi.org/10.1007/s11695-013-1037-5.
Acknowledgements
We would like to acknowledge the following investigators and site personnel who participated in the study: Investigators are as follows: Spain—Salvador Morales Conde, MD, Isaías Alarcón del Agua MD, Hospital Virgen del Rocio, Sevilla; Antonio José Torres, MD, Andrés Sanchez Pernaute, MD, Miguel A. Rubio, MD, Complutense University of Madrid Hospital Clinico “San Carlos”, Madrid; Antonio José Torres, Fernando Lapuente, Felipe Acedo, Antonio Picardo, Hospital Universitario Madrid Monteprincipe, Madrid. Italy—Franco Favretti, MD, Vicenza Regional Hospital, Vicenza; Giuseppe M. Rovera MD, San Luca Torino, Torino; Marco Anselmino MD, University Hospital Pisa, Pisa.
We would also like to thank sponsor personnel Javier Sanchez and Ksenija Mlinar for the support with device programming and data collection.
Funding
The study was financially supported by IntraPace Inc., San Jose, CA, USA.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Informed consent was obtained from all individual participants included in the study.
The study was conducted in accordance with Good Clinical Practice and consistent with the Declaration of Helsinki.
Conflict of Interest
Authors 1, 2, 4, 5, 6, 7, and 8 have nothing to disclose. Author 3 reports personal fees from IntraPace Inc., during the conduct of the study; and personal fees from Novo Nordisk, outside the submitted work. Authors 9 and 10 report personal fees from IntraPace Inc., during the conduct of the study.
Rights and permissions
About this article
Cite this article
Morales-Conde, S., Alarcón del Agua, I., Busetto, L. et al. Implanted Closed-Loop Gastric Electrical Stimulation (CLGES) System with Sensor-Based Feedback Safely Limits Weight Regain at 24 Months. OBES SURG 28, 1766–1774 (2018). https://doi.org/10.1007/s11695-017-3093-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-017-3093-8