Abstract
Background
A meta-analysis regarding bone loss after bariatric surgery, designed to compare surgical and nonsurgical groups, has not yet been performed. Therefore, we performed a meta-analysis to compare the differences between bariatric surgical groups and nonoperated controls with regard to bone mineral density.
Methods
In March 2015, we performed a review of the literature using PubMed, EMBASE, and the Cochrane Library. The search focused on retrospective and prospective studies, including but not limited to randomized studies published in English.
Results
Among 1299 studies that were initially screened, ten met the selection criteria. For all types of bariatric surgery, bone density at the femoral neck was lower in the surgical group than in the nonsurgical control group (mean difference [MD] −0.05 g/cm2; 95 % confidence interval [CI], −0.07 to −0.02; p = 0.001); no difference in bone density was found between the two groups at the lumbar spine (MD −0.01 g/cm2; 95 % CI −0.07 to 0.05; p = 0.661). The analysis of Roux-en-Y gastric bypass showed similar results.
Conclusion
Bone density at the femoral neck decreased after bariatric surgery, compared to that in nonsurgical controls, whereas bone density at the lumbar spine did not show a difference between groups. Further larger scale studies with comparative nonsurgical controls are warranted to overcome the heterogeneity among studies in this analysis and to add evidence of possible bone loss subsequent to bariatric surgical procedures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bariatric surgical procedures, including Roux-en-Y gastric bypass (RYGB) and adjustable gastric banding (AGB), increased from 40,000 in 1998 to 468,609 in 2013 worldwide, in line with the growth of laparoscopic surgery [1]. Bariatric surgery is effective, particularly for morbidly obese patient groups (body mass index [BMI] ≥40 kg/m2, or 35 kg/m2 with other obesity-related complications), exerting long-term sustained effects on weight loss in 62 % of gastric bypass patients and 47 % of gastric banding patients [2]. Bariatric surgery is reported to improve diabetes mellitus, dyslipidemia, hypertension, and sleep apnea in greater than 60 % of patients [2, 3].
Despite the positive effects of bariatric surgery, concerns have been raised over potential adverse effects on the skeletal system, e.g., increased bone loss and fragility [4]. This may be explained by decreased calcium intake and absorption, increased parathyroid hormone (PTH) due to lower vitamin D absorption, decreased estrogen level in women, decreased plasma leptin and ghrelin, and increased concentration of adiponectin [5–7]. Long-term complications after gastrectomy include osteoporosis and osteomalacia. Osteomalacia was estimated to occur in 2.5 % of patients following gastric bypass in the USA [8]. Therefore, the development of secondary bone loss after bariatric surgery and resultant increase in skeletal fragility is an important issue that warrants further research, given the substantial growth of bariatric surgical procedures.
Bone density measurement using dual-energy X-ray absorptiometry is a clinically proven surrogate marker applicable to measuring bone strength and fracture risks [9, 10]. Despite a few limitations in bone strength measurement [11], dual-energy X-ray absorptiometry has been widely used in studies on the postoperative bone strength of patients undergoing bariatric surgical procedures. However, systematic reviews or relevant meta-analyses on this issue are rare, and bone mineral density (BMD) change after surgery differs according to body region and the type of surgery [12–15]. A meta-analysis on BMD after bariatric surgery showed that bone density decreased after mixed surgical procedures but not in restrictive surgeries [14]. Another review on this issue showed that BMD at the spine and radius was greater in postsurgical patients compared to an obese population, according to cross-sectional and retrospective research [12]. Moreover, a meta-analysis regarding bone loss after bariatric surgery, designed to compare surgical and nonsurgical groups, has not yet been performed. Therefore, the relationship between bariatric surgery and BMD is still inconclusive. The aim of this meta-analysis is to evaluate the difference in bone mineral density between those who had bariatric surgery and nonoperated controls among studies with morbidly obese patients.
Materials and Methods
Data Source and Keywords
Two researchers independently searched MEDLINE (PubMed), EMBASE, and the Cochrane library database in March 2015, using selected keywords “bariatric surgery or gastric bypass or gastric sleeve or sleeve gastrectomy or gastric banding,” AND “bone or bone density or fracture or osteoporosis,” for English-based thesis titles and abstracts, and performed an initial screening. For EMBASE, we used quoted keywords to maintain a specific word sequence (“bariatric surgery”/exp OR “bariatric surgery” OR “gastric bypass”/exp OR “gastric bypass” OR “gastric sleeve”/exp OR “gastric sleeve” OR “sleeve gastrectomy”/exp OR “sleeve gastrectomy” OR “gastric banding”/exp OR “gastric banding”) AND (“bone”/exp OR “bone” OR “bone density”/exp OR “bone density” OR “fracture”/exp OR “fracture” OR “osteoporosis”/exp OR “osteoporosis”). The researchers aggregated the initially screened articles and collected original texts on the internet or at medical libraries.
Selection Criteria of Relevant Studies
We selected articles that met the selection criteria (i.e., retrospective and prospective studies, including but not limited to randomized studies designed to compare bariatric surgical and nonsurgical groups) to be included in the meta-analysis. The control group included individuals who were not operated on and were compared to those who underwent bariatric surgery. We only selected articles written in English. We tried to obtain the complete data for analysis by contacting the authors of articles with insufficient or missing data. Selected variables were extracted from the studies: study design, type of surgery, time point after surgery, number of participants, gender, initial BMI (kg/m2) or weight (kg), BMI or weight at the time of BMD, the changes in weight or BMI, and BMD (g/cm2) at the femoral neck and lumbar spine.
Statistical Analysis
All statistical analyses were calculated using STATA 12.0 (Stata Corp, College Station, TX, USA). The presence of publication bias was evaluated by using Egger’s test for the characteristics of BMD data. Heterogeneity of the included studies was estimated by using Higgins I2[I 2 = (Q − df)/Q × 100]. Either a fixed effects or random effects model was used for analysis. The fixed effects model was used when I 2 < 50 % and the random effects model when I 2 ≥ 50 %. A forest plot was used to present the point estimates of effects with CIs and the pooled estimates with CIs observed in individual studies. Mean difference of BMI or BMD with 95 % CI was calculated. Weighted mean difference (the difference between two means weighted by the precision of the study) was used, considering that the scales were the same among the individual studies included herein. Subgroup analysis was performed with reference to different types of bariatric surgery, body region (e.g., lumbar and femoral), BMI of the surgical group and controls at the time of BMD, and time point after surgery.
Results
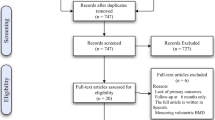
The literature review identified 1299 titles from PubMed (n = 455), EMBASE (n = 828), and the Cochrane Library (n = 16). A total of 1055 titles were selected, with 244 overlapping titles excluded. A further 1010 titles that failed to meet the selection criteria (retrospective and prospective studies, including randomized studies, designed to compare bariatric surgical vs. nonsurgical groups) were excluded, and 45 articles were included in the final review. Two articles that were not written in English, 5 that failed to provide sufficient data, 27 that lacked control groups, and 1 that lacked bone density variables were excluded. A total of ten articles were included in the final analysis (Fig. 1). Table 1 outlines the articles included in the final analysis. In sum, 241 patients undergoing bariatric surgical procedures and 261 subjects in the nonsurgical control group were analyzed. The time lapse after bariatric surgery ranged from 9.8 months to 10 years. No publication bias was observed, with p for bias =0.926.
Analysis without differentiation of the types of surgical procedures found no difference in BMI between surgical and nonsurgical groups (mean difference [MD] −3.62 kg/m2; 95 % CI, −7.67 to 0.43; p = 0.080) (Supplementary Fig. 1). Bone density in the femoral neck was lower in the surgical group than in the nonsurgical control group (MD −0.05 g/cm2; 95 % CI, −0.07 to −0.02; p = 0.001). In studies of whether BMI of the surgical group at the time of BMD was less than that of the nonsurgical controls, the bone density at the femoral neck was lower in the surgery group than that in controls (MD −0.10 g/cm2; 95 % CI, −0.15 to −0.06; p < 0.001). However, in studies of whether BMI of the surgical group at the time of BMD was equal to or higher than that in the nonsurgical controls, the BMD of surgical and nonsurgical groups did not differ (MD −0.02 g/cm2; 95 % CI, −0.05 to 0.01; p = 0.238) (Fig. 2). No difference in lumbar spine bone density was found between the two groups (MD −0.01 g/cm2; 95 % CI, −0.07 to 0.05; p = 0.661); the result was the same when we divided the studies according to the difference in BMI at the time of BMD for the two groups (Fig. 3).
The analysis of RYGB found BMI was lower in the surgical group than in the nonsurgical group (MD −5.80 kg/m2; 95 % CI, −10.80 to −0.79; p = 0.023) (Supplementary Fig. 2). The femoral neck bone density was lower in the surgical group than in the nonsurgical control group (MD −0.03 g/cm2; 95 % CI, −0.06 to −0.00; p = 0.045); however, there was no difference in BMD among the studies in which BMI of the surgery group was equal to or higher than that of controls (Fig. 4). Consistent with the analysis that did not consider different types of surgical procedures, no difference in lumbar spine bone density was found between the surgical and nonsurgical groups (MD −0.03 g/cm2; 95 % CI, −0.06 to 0.01; p = 0.149); the results were the same, regardless of the difference in BMI at the time of BMD (Fig. 5). In a subgroup analysis of studies at a time point two or more years after surgery, the MD in BMD between RYGB and controls was not significant, both at the femoral neck (MD −0.02 g/cm2; 95 % CI, −0.05 to 0.01; p = 0.235) (Supplementary Fig. 3) and lumbar spine (MD −0.04 g/cm2; 95 % CI, −0.08 to 0.00; p = 0.056) (Supplementary Fig. 4).
Discussion
Subjects who underwent bariatric surgery had a lower BMD than nonoperated controls at the femoral neck, but there was no difference in BMD at the lumbar spine between subjects and controls. The result was the same, both in studies of different types of bariatric surgery and those of RYGB alone. This is the first study to consider both the bariatric surgery group and nonoperated controls in regard to BMD in a meta-analysis.
Bariatric surgery may adversely affect bone. Weight loss after surgery induces mechanical unloading, which, in turn, affects the progress of bone loss, although some exceptions exist [16–18]. However, some studies showed little association between weight loss and bone loss, which warrant more research on the mechanisms underlying this phenomenon [19, 20]. Secondary hyperparathyroidism may occur after surgery due to the malabsorption of vitamin D and calcium [21]. However, there are some reports that BMD decreased significantly in the absence of changes in PTH or vitamin D levels [18, 22]. Some biomarkers, including leptin and adiponectin, may act as mediators between the surgery and the BMD changes. Given the findings of in vitro studies that leptin could positively affect bone density by increasing bone formation and decreasing resorption [23, 24], decreased leptin concentrations after bariatric surgery may affect bone loss due to the loss of body fat mass [25]. Serum adiponectin levels were inversely associated with BMD [26], and the increased level of adiponectin after bariatric surgery may be related to bone loss [5]. Yet, there are insufficient data on the actual mechanisms relating these biomarkers and bone density in relation to bariatric surgery.
There are several studies on the presence of postbariatric surgery bone loss measured by BMD changes. Recently, a meta-analysis of BMD after bariatric surgery reported that mixed surgery, such as RYGB, resulted in the reduction of BMD after 1 year, whereas the effect of restrictive surgery, such as gastric banding, on the BMD was not significant. That paper, however, did not have a nonsurgical comparator group to serve as a control for the effect of bariatric surgery itself.
We found that the decrease in BMD due to bariatric surgery is only significant at the femoral neck and not at the lumbar spine, regardless of the type of surgery. In a subgroup analysis of studies considering whether the BMI of the surgical group at the time of BMD was less than that of controls, the bone density at the femoral neck was lower in the surgery group, both for all types of surgery and RYGB alone. When we focused on studies in which BMI of the surgery group was equal to or higher than that of controls, BMD was not different at either the femoral neck or lumbar spine, regardless of the type of surgery. This finding suggests that the weight loss caused by bariatric surgery predominantly affects BMD at the femoral neck not the lumbar spine. Some studies of bariatric surgery and subsequent bone loss showed that surgery resulted in a decreased BMD at the hip and unchanged BMD at the lumbar spine [16, 17]. There was a strong association between the extent of weight loss and amount of bone loss at the hip, a weight-bearing site. On the contrary, a more cancellous lumbar spine may have been influenced by the anabolic effect of slightly increased PTH, rather than being affected by weight loss [17], which is consistent with our findings. When we analyzed studies with a time point after surgery ≥2 years, there was no difference in regard to femoral neck and lumbar BMD between RYGB and controls, which implies that weight loss has a greater effect on bone density than the duration after bariatric surgery. Weight loss eventually is accompanied by a change in body composition, which varies according to the type of bariatric surgery, and may act differently on the change in BMD of different body regions [27, 28]; however, the association between the change in body composition after bariatric surgery and that of BMD remains unclear.
This study has several limitations. First, there was heterogeneity among the studies included. Some studies did not separate BMD of men and women, and the samples included individuals of different ethnicity, women at various stages of menopause, and various surgical procedures. Some studies also lacked data for initial BMI, had very small numbers of participants, or had controls without identical follow-up periods. Most of this heterogeneity is thought to be due to the different types of surgery; therefore, we conducted subgroup analysis with RYGB alone to overcome this limitation, and the heterogeneity was reduced. Second, most of the study designs were retrospective, which could not rule out the possibility of variable quality of surgical technique, selection of participants according to the surgeon’s preference, or the subjects’ choice of treatment. Third, the duration after surgery ranged from 9.8 months to 10 years, which could decrease the homogeneity of the studies. However, we conducted a subgroup analysis of studies with of ≥2 years after RYGB, and the heterogeneity among studies was much reduced. Fourth, BMD is a surrogate marker for bone density, not an endpoint, such as a fracture, which must be considered before interpreting the results of the study. Finally, bias may occur if relevant studies are missed by restricting reports to those published in English or to only certain databases.
In conclusion, BMD at the femoral neck decreased after bariatric surgery compared to that in nonsurgical controls, but BMD at the lumbar spine did not show a difference between the groups. Further larger scale studies with a prospective design and comparative nonsurgical controls are warranted to strengthen the conclusions of this meta-analysis.
References
Angrisani L, Santonicola A, Iovino P, et al. Bariatric Surgery Worldwide 2013. Obes Surg. 2015;25(11):2166–8.
Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292(14):1724–37.
Buchwald H, Estok R, Fahrbach K, et al. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med. 2009;122(3):248–56. e5.
Goldner WS, O’Dorisio TM, Dillon JS, et al. Severe metabolic bone disease as a long-term complication of obesity surgery. Obes Surg. 2002;12(5):685–92.
Carrasco F, Ruz M, Rojas P, et al. Changes in bone mineral density, body composition and adiponectin levels in morbidly obese patients after bariatric surgery. Obes Surg. 2009;19(1):41–6.
Shaker JL, Norton AJ, Woods MF, et al. Secondary hyperparathyroidism and osteopenia in women following gastric exclusion surgery for obesity. Osteoporos Int. 1991;1(3):177–81.
Ricci TA, Heymsfield SB, Pierson Jr RN, et al. Moderate energy restriction increases bone resorption in obese postmenopausal women. Am J Clin Nutr. 2001;73(2):347–52.
Parfitt AM, Podenphant J, Villanueva AR, et al. Metabolic bone disease with and without osteomalacia after intestinal bypass surgery: a bone histomorphometric study. Bone. 1985;6(4):211–20.
Ammann P, Rizzoli R. Bone strength and its determinants. Osteoporos Int. 2003;14 Suppl 3:S13–8.
Lewiecki EM. Bone densitometry and vertebral fracture assessment. Curr Osteoporos Rep. 2010;8(3):123–30.
Bolotin HH. DXA in vivo BMD methodology: an erroneous and misleading research and clinical gauge of bone mineral status, bone fragility, and bone remodelling. Bone. 2007;41(1):138–54.
Scibora LM, Ikramuddin S, Buchwald H, et al. Examining the link between bariatric surgery, bone loss, and osteoporosis: a review of bone density studies. Obes Surg. 2012;22(4):654–67.
Viegas M, Vasconcelos RS, Neves AP, et al. Bariatric surgery and bone metabolism: a systematic review. Arq Bras Endocrinol Metabol. 2010;54(2):158–63.
Rodriguez-Carmona Y, Lopez-Alavez FJ, Gonzalez-Garay AG, et al. Bone mineral density after bariatric surgery. A systematic review. Int J Surg. 2014;12(9):976–82.
Liu C, Wu D, Zhang JF, et al. Changes in bone metabolism in morbidly obese patients after bariatric surgery: a meta-analysis. Obes Surg. 2015.
Stein EM, Carrelli A, Young P, et al. Bariatric surgery results in cortical bone loss. J Clin Endocrinol Metab. 2013;98(2):541–9.
Fleischer J, Stein EM, Bessler M, et al. The decline in hip bone density after gastric bypass surgery is associated with extent of weight loss. J Clin Endocrinol Metab. 2008;93(10):3735–40.
Yu EW, Bouxsein ML, Roy AE, et al. Bone loss after bariatric surgery: discordant results between DXA and QCT bone density. J Bone Miner Res. 2014;29(3):542–50.
Abegg K, Gehring N, Wagner CA, et al. Roux-en-Y gastric bypass surgery reduces bone mineral density and induces metabolic acidosis in rats. Am J Physiol Regul Integr Comp Physiol. 2013;305(9):R999–R1009.
Yu EW, Bouxsein ML, Putman MS, et al. Two-year changes in bone density after Roux-en-Y gastric bypass surgery. J Clin Endocrinol Metab. 2015;100(4):1452–9. doi:10.1210/jc.2014-4341.
Slater GH, Ren CJ, Siegel N, et al. Serum fat-soluble vitamin deficiency and abnormal calcium metabolism after malabsorptive bariatric surgery. J Gastrointest Surg. 2004;8(1):48–55. discussion 4-5.
Stemmer K, Bielohuby M, Grayson BE, et al. Roux-en-Y gastric bypass surgery but not vertical sleeve gastrectomy decreases bone mass in male rats. Endocrinology. 2013;154(6):2015–24.
Cornish J, Callon KE, Bava U, et al. Leptin directly regulates bone cell function in vitro and reduces bone fragility in vivo. J Endocrinol. 2002;175(2):405–15.
Holloway WR, Collier FM, Aitken CJ, et al. Leptin inhibits osteoclast generation. J Bone Miner Res. 2002;17(2):200–9.
Terra X, Auguet T, Guiu-Jurado E, et al. Long-term changes in leptin, chemerin and ghrelin levels following different bariatric surgery procedures: Roux-en-Y gastric bypass and sleeve gastrectomy. Obes Surg. 2013;23(11):1790–8.
Biver E, Salliot C, Combescure C, et al. Influence of adipokines and ghrelin on bone mineral density and fracture risk: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2011;96(9):2703–13.
Olbers T, Bjorkman S, Lindroos A, et al. Body composition, dietary intake, and energy expenditure after laparoscopic Roux-en-Y gastric bypass and laparoscopic vertical banded gastroplasty: a randomized clinical trial. Ann Surg. 2006;244(5):715–22.
Chaston TB, Dixon JB, O’Brien PE. Changes in fat-free mass during significant weight loss: a systematic review. Int J Obes. 2007;31(5):743–50.
Ott MT, Fanti P, Malluche HH, et al. Biochemical evidence of metabolic bone disease in women following Roux-Y gastric bypass for morbid obesity. Obes Surg. 1992;2(4):341–8.
Guney E, Kisakol G, Ozgen G, et al. Effect of weight loss on bone metabolism: comparison of vertical banded gastroplasty and medical intervention. Obes Surg. 2003;13(3):383–8.
Goode LR, Brolin RE, Chowdhury HA, et al. Bone and gastric bypass surgery: effects of dietary calcium and vitamin D. Obes Res. 2004;12(1):40–7.
Von Mach MA, Stoeckli R, Bilz S, et al. Changes in bone mineral content after surgical treatment of morbid obesity. Metabolism. 2004;53(7):918–21.
Pereira FA, de Castro JA, dos Santos JE, et al. Impact of marked weight loss induced by bariatric surgery on bone mineral density and remodeling. Braz J Med Biol Res. 2007;40(4):509–17.
Valderas JP, Velasco S, Solari S, et al. Increase of bone resorption and the parathyroid hormone in postmenopausal women in the long-term after roux-en-y gastric bypass. Obes Surg. 2009;19(8):1132–8.
de Vasconcelos RS, Viegas M, Marques TF, et al. Factors associated with secondary hyperparathyroidism in premenopausal women undergoing Roux-en-y gastric bypass for the treatment of obesity. Arq Bras Endocrinol Metabol. 2010;54(2):233–8.
Hintze LJ, Cremon AS, Bevilaqua CA, et al. Factors associated with bone mineral density in women who underwent bariatric surgery. Acta Sci Health Sci. 2014;36(1):105–12.
Costa TL, Paganotto M, Radominski RB, et al. Calcium metabolism, vitamin D and bone mineral density after bariatric surgery. Osteoporosis Int. 2015;26(2):757–64.
Acknowledgments
We would like to acknowledge Dr. Yu and Dr. Keller for providing further data for their studies.
Conflict of Interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
For this type of study, formal consent is not required.
Additional information
Byung-Joon Ko and Seung Kwon Myung contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig 1
Mean difference in BMI between all types of surgery and controls (n=9, random effects model). (TIFF 555 kb)
Supplementary Fig 2
Mean difference in BMI between patients who underwent Roux-en-Y gastric bypass and controls (n=6, random effects model). (TIFF 513 kb)
Supplementary Fig 3
Mean difference in femoral neck BMD between patients who underwent Roux-en-Y gastric bypass and controls by time point after surgery (n=6, fixed effects model). (TIFF 649 kb)
Supplementary Fig 4
Mean difference in lumbar BMD between patients who underwent Roux-en-Y gastric bypass and controls by time point after surgery (n=7, fixed effects model). (TIFF 688 kb)
Rights and permissions
About this article
Cite this article
Ko, BJ., Myung, S.K., Cho, KH. et al. Relationship Between Bariatric Surgery and Bone Mineral Density: a Meta-analysis. OBES SURG 26, 1414–1421 (2016). https://doi.org/10.1007/s11695-015-1928-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-015-1928-8