Abstract
Background
The mechanisms by which increased body weight influence bone mass density (BMD) are still unknown. The aim of our study was to analyze the relationship between anthropometric and body composition variables, insulin growth factor-I (IGF-I), adiponectin and soluble tumor necrosis factor-α receptors (sTNFR) 1 and 2 with BMD in two cohorts of morbid obese patients, before and after bypass surgery.
Methods
The first cohort included 25 women aged 48 ± 7.6 years studied before bypass surgery. The second included 41 women aged 46 ± 9.2 years, 12 months after surgery. We studied anthropometric variables obtained from whole body DEXA composition analysis. Serum IGF-I, intact serum parathyroid hormone, 25-hydroxivitamin D3, plasma adiponectin concentrations, sTNFR1, sTNFR2 concentrations were measured.
Results
In the first cohort, the BMI was 44.5 ± 3.6 kg/m2, parathyroid hormone, IGF-I, and adiponectin concentrations were lower, and sTNFR1 concentrations were higher than in the second cohort. In the multiple regression analysis, BMD remained significantly associated with body fat percentage (β −0.154, p = 0.01), lean mass (β 0.057, p = 0.016) and phosphate concentration (β 0.225, p = 0.05). In the second cohort, BMI was 31 ± 5.1 kg/m2. In the multiple regression analysis, BMD remained significantly associated with lean mass (β 0.006, p = 0.03).
Conclusion
The inverse correlation found between body fat and BMD in the first cohort indicates morbid obesity increases the risk of osteoporosis and we found a positive correlation with lean and fat mass before bariatric surgery and with lean mass after bypass surgery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity has been found to protect against osteoporosis, but the mechanisms by which increased body weight influence bone mineral density (DMO) are still unknown [1, 2]. The bone protective effects of obesity may involve increased weight bearing [3], increased aromatization of androgen to estrogen in adipose tissue [4], lowered levels of sex-hormone-binding globulin or a direct increased bone formation induced by high circulating levels of insulin [5] and insulin growth factor-I (IGF-I) [6], the growth factor more prevalent in bone [6, 7]. Recently, it has been speculated that the association of BMD with fat mass might be mediated by hormonal factors secreted by adipocytes including leptin and adiponectin [2, 8–10]. Moreover, immunological mediators, such as adipocytokines including TNF-α, interleukine-18 which are increased in obesity have recently been shown to influence bone metabolism [11, 12]. In this sense, early studies found that systemic leptin treatment of ob/ob mice stimulated bone formation and bone growth [13]. Likewise, it has been suggested that adiponectin might be implicated in the modulation of bone metabolism due to its marked structural similarity to RANK-L and osteoprotegerin, two proteins involved in the regulation of osteoclastogenesis. Also, adiponectin has been shown to activate as well as inhibit NF-κB, a transcription factor which is critical for osteoclastogenesis [14, 15]. Few studies have examined the association between serum adiponectin and BMD in humans, and although in one study an inverse association was found [16], this result was not confirmed in another study [17]; however none of these studies included patients with morbid obesity.
Furthermore, tumor necrosis factor-α (TNF-α) enhances bone resorption via stimulating osteoclast development and activity as well as bone formation. A genetic polymorphism of the TNFRSF1B gene which encodes 75 Kd TNF-α receptor has been associated with BMD, and treatment with anti-TNF-α antibody exerts beneficial effect on bone metabolism in patients with rheumatoid arthritis [18].
The aim of our study was to analyse the relationship among anthropometric and body composition variables, parathyroid hormone (PTH), 25-hydroxivitamin D3 (25(OH) D3), IGF-I, adiponectin and soluble TNF-α receptors (sTNFR) with BMD in two cohorts of morbid obese patients, before and after bypass surgery.
Subjects and Methods
Cohorts for Analysis
We studied two cohorts of morbid obese patients that were recruited from the Endocrinology Service of the Hospital Universitario de Bellvitge (Barcelona, Spain). The first cohort included a group of 25 white women aged 48 ± 7.6 years with a body mass index (BMI) of 44.5 ± 3.6 kg/m2 studied before the bypass. The second cohort included a group of 41 white women aged 46 ± 9.2 years with a BMI of 31 ± 5.1 kg/m2 after gastric bypass; before the surgery, the BMI of the patients was 45.1 ± 4.1 kg/m2. Gastric bypass operation was undergone according to a modification of the method described by Capella and Capella [19]. In brief, a small gastric upper pouch (20 ml) was created along the lesser curvature of the stomach. The stomach was stapled, and the pouch was anastomosed to a loop of jejunum with a hand-sewn anastomosis. The opening between pouch and jejunum was 12 mm in diameter with a Roux-en-Y reconstruction. Preoperative anthropometric measurements were made, and blood samples were collected prior to surgery in the first group, and anthropometric measurements and blood samples were determined 12 months after surgery in the second group. In all cases, primary exclusion criteria were any acute major cardiovascular event in the previous 6 months; recent or ongoing infection; a history of cancer disease; treatment with anti-inflammatory drugs or insulin. Patients with Cushing’s syndrome, thyroid dysfunction or other major endocrine disorders were also ruled out. The subjects with overt eating disorders, major psychiatric disease, excessive alcohol consumption (more than 60 g/day), and are heavy smokers (more than 12 cigarettes/day) were also excluded in accordance with our institutional bariatric surgery guidelines. After gastric bypass, all patients were administered 1,200 mg of calcium and a multivitamin pill once daily. Patients receiving medical treatment for diabetes mellitus or dyslipemia before surgery discontinued all hypoglycemic agents and hypolipemic drugs at least 1 month before basal determinations. All subjects had signed a written consent to the study, which was approved by the research ethics board of our hospital.
For normal values, data of previous of epidemiological studies in our population [20–22] were considered and expressed in Table 1.
Anthropometric Measurements
Height and weight were measured with the patient standing in light clothes and without shoes. BMI was calculated as body weight divided by height squared (kg/m2). Waist to hip ratio was calculated as the ratio of waist and hip circumferences. Obesity was classified according to the World Health Organization criteria [23].
Total fat mass and total lean mass were obtained from whole body DEXA body composition analysis. Fat mass (kg), lean tissue mass (kg) and BMD (gram per square centimeter of the entire skeleton) were evaluated by total body scanning employing dual-energy X-ray absorptiometry (DXA; LUNAR DXA-IQ DEXA, Madison, WI, USA). All scans were performed in slow scan mode and analyzed using Lunar smart scan version 4.6 c with the slowest scan mode.
Analytical Methods
Blood samples were drawn from each subject before breakfast between 8 a.m. and 9 a.m., after an overnight rest. All samples were stored at −80°C until analytical measurements were performed. Intact serum PTH was measured by two-site immunoradiometric assay (Diagnostic System Laboratories, Webster, TX, USA); intra- and interassay coefficient of variation (CV) were 10% and 11%, respectively. 25(OH) D3 concentrations were determined using a radioimmunoassay (DiaSorin, Stillwater, MN, USA). Serum total IGF-I concentrations after acid-ethanol extraction were measured by immunoradiometric assay (Nichols, San Juan de Capistrano, CA, USA); intra-assay and an inter-assay CV were 5.2% and 9.4%, respectively. Plasma adiponectin concentrations were measured using standard radioimmunoassay kits (Linco Research, MS, USA). For human adiponectin, the kit has a sensitivity of 0.001 μg/ml in a 100-μl sample size, and range of 0.001–0.2 μg/ml. All samples were diluted 1/500. The intra- and interassay CV were 8% and 12%, respectively. sTNFR1 and sTNFR2 were determined by solid phase enzymoimmunoassay with amplified reactivity (Bio Source Europe S.A., Fleunes, Belgium). The limit of detection was 0.5 ng/ml for sTNFR1 and 0.1 ng/ml for sTNFR2, and the intra- and interassay CV were 7% and 9%, respectively.
Statistical Analysis
Descriptive statistics are presented as mean ± standard deviation for variables with a normal distribution and as median (75th percentile) for variables with a non-Gaussian distribution. Student’s t test and analysis of variance (ANOVA) were used to compare normal distributed variables and the Mann–Whitney U test and the Kruskal–Wallis test were used to compare non-normal distributed variables. Relationships among variables were tested by Spearman’s correlation coefficient. Univariate and multivariate linear regression analyses were performed to identify independent factors affecting BMD and to estimate the final predictors of its variability. A value of p < 0.05 was considered to be statistically significant. All statistical analyses were performed using the Statistical Package for Social Sciences (SPSS/Windows version 12, SPSS, Chicago, IL, USA).
Results
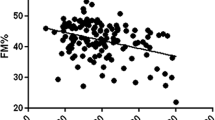
In the first cohort of 25 preoperative women participating in our study, BMI was 44.5 ± 3.6 kg/m2 and anthropometric, clinical, and metabolic characteristics of these subjects are summarized in Table 1. They showed higher BMI, fat mass, lean mass, body fat, lower IGF-I and adiponectin and higher sTNFR2 than in the second cohort. In the univariate analysis, BMD negatively correlated with age (r = −0.402, p = 0.046) and body fat percentage (r = −0.471, p = 0.017) and showed a positive correlation with lean mass (r = 0.45, p = 0.024) and phosphate concentration (r = 0.497, p = 0.043); no significant correlation was found among IGF-I, serum adiponectin, and sTNFR1 and sTNFR2 with BMD (Table 2). To examine independent predictors of preoperative BMD, we performed a multiple regression analysis with BMD as a dependent variable and age, BMI, fat mass, lean mass, IGF-I, adiponectin, sTNFR1 and sTNFR2, calcium, and phosphate as independent variables. BMD remained significantly associated with body fat percentage (β −0.154, p = 0.01), lean mass (β 0.057, p = 0.016) and phosphate concentration (β 0.225, p = 0.05)—R 2 for the model = 0.86.
In the second cohort of 41 women, BMI was 31 ±5.1 kg/m2, and their anthropometric, clinical, and metabolic characteristics are presented in Table 1. In the univariate analysis, BMD correlated positively with lean mass (r = 0.318, p = 0.043), phosphate concentration (r = 0.342, p = 0.044), and IGF-I (r = 0.467, p = 0.019). No correlation was found between BMD and plasma adiponectin, sTNFR1, and sTNFR2 (Table 3). Multiple regression analysis was performed with BMD as a dependent variable and age, fat mass, lean mass, fasting serum adiponectin, IGF-I, sTNFR1 and sTNFR2, calcium, and phosphates as independent variables. BMD remained significantly associated with lean mass (β 0.006, p = 0.03), R 2 for the model = 0.26. In this cohort, PTH correlated negatively with 25(OH) D3 concentrations (r = −0.443, p = 0.014) and calcium (r = −0.483, p = 0.003) and positively with weight (r = 0.362, p = 0.030), BMI (r = 0.368, p = 0.032), and lean mass (r = 0.350, p = 0.036).
Discussion
In morbidly obese women, lean mass was associated with BMD both before and after bariatric surgery, and IGF-I, adipocytokines, and circulating proinflammatory cytokines were not associated with changes in BMD in this obese women population; but as the study was not experimental, it could not elucidate what the determinant of BMD was.
IGF-I is a key growth factor highly present in bone and has been implicated in osteogenesis with action on renal tubules, resulting in induction of 1,25(OH) D3 production. Some studies support that growth hormone (GH) may modulate vitamin D metabolism mainly through IGF-I than by the PTH regulatory pathway, suggesting that GH/IGF system is important for skeletal integrity [21, 22], explaining in part the close relationship between IGF-I and the vitamin D system [22]. In our study, we have found low IGF-I concentrations, mainly in patients before gastric bypass. This observation is in agreement with previous reports in which obesity has been associated with normal or low total IGF-I [17].
Obese adults have complex regulatory mechanisms between 25(OH) D3 and PTH not well understood. Obesity has been associated with low 25(OH) D3 and high intact PTH with a wide variability in 1,25(OH) D3 concentrations according to race and age [24]. Vitamin D deficiency has been reported up to 80% of cases in morbid subjects [25] and after bypass surgery, postmenopausal women show evidence of secondary hyperparathyroidism, elevated bone resorption and patterns of bone loss [31], which is highest in the first year after gastric bypass [26]. In our both studied cohorts, we have observed lower 25(OH) D3 concentrations than in healthy nonobese controls with a negative correlation between PTH after bariatric surgery, suggesting a compensatory secondary hyperparathyroidism. Despite these variations observed in vitamin D, homeostasis in morbid women, no association with BMD was detected in our population. We acknowledge the limitations of our study as consequence than in bariatric surgery, calcium absorption is a great source of variability, pointed out by the low value of R 2 of the regression model outlined in these patients.
Adipocytokines has been proposed as new players in bone metabolism. Adiponectin is one of the most abundant circulating proteins; it is considered an anti-inflammatory cytokine mainly expressed by adipose tissue and is structurally similar to TNF-α, RANK-L and osteoprotegerin with a broad range of biological actions. Some studies suggest that adiponectin carries signals from adipose tissue to muscle, liver, and even to bone [27] with a stronger association with BMD than leptin [28, 29]. However, few studies have examined the association between adiponectin and BMD. The preliminary results suggest that adiponectin is not a determinant for BMD either in healthy men [29] or in perimenopausal women [17]. These observations are in agreement with our findings in morbid obese women, in which no association was observed before and after weight loss. The limited data in this sense make us to be cautious because there are mechanisms at molecular level that can justify the participation of these proteins in bone metabolism.
For the pro-inflammatory cytokines, endogenous TNF-α prevents the attainment of maximum achievable peak BMD in vivo but in vitro TNF-α suppresses tumor growth factor-β through induction of NF-κB. In mice models deficient in TNF-α or its receptor sTNFR1 shows an increase in peak bone mass, resulting from elevated bone formation. These effects were mimicked by over expression of NF-κB and prevented after NF-κB suppression [18]. On the other hand, in human studies, patients treated with infliximad, a TNF-α blocker for rheumatoid arthritis treatment, had preservation of BMD in the lumbar spine and femoral neck compared to those treated with methotrexate showing that with TNF-α blockers, BMD increased while bone resorption seems decreased [18, 30].
Moderately obese women generally have increased BMD and reduced risk of fractures, but the effects of extreme obesity on BMD have not been well examined and remain controversial. The inverse correlation found in our study between body fat and BMD in the first cohort suggest that extreme obesity may increase the risk of osteoporosis as have been demonstrated either in mice or in postmenopausal women [31, 32]. However, data in humans are contradictory about the relationship between lean and fat mass and BMD [29, 33, 34]. In the sample studied, there are both obese patients and type 2 diabetes and also post-diabetic patients following gastric bypass; this fact could also have an influence in the synthesis of adipocytokines.
Our study does not support the existence of a direct link between adipose tissue and bone metabolism, but it is associated with greater loss of BMD, and we are unaware to suggest the mechanisms involved in this downregulation. Probably, in patients with morbid obesity, the regulation of BMD on the basis of the different axis studied may work in a different way than in non-morbid obesity, and in this extreme situation, the systems studied might be switched off, but they will make up when the patient achieve the normal, overweight, or less obese.
References
Tremollieres FA, Pouilles JM, Ribot C. Vertebral postmenopausal bone loss is reduced in overweight women: a longitudinal study in 155 early postmenopausic women. J Clin Endocrinol Metab. 1993;77:683–6.
Morberg CM, Tetens I, Black E, et al. Leptin and bone mineral density: a cross-sectional study in obese and non obese men. J Clin Endocrinol Metab. 2003;88:5795–800.
Trayhurn P, Beattie JH. Physiological role of adipose tissue: white adipose tissue as an endocrine and secretory organ. Proc Nutr Soc. 2001;60:329–39.
Kleerekoper M, Nelson DA, Peterson EL, et al. Body composition and gonadal steroids in older white and black women. J Clin Endocrinol Metab. 1994;79:775–9.
Reid D, Evans M, Cooper G, et al. Circulating insulin levels are related to bone density in normal postmenopausal women. Am J Physiol. 1993;265:293–7.
Gómez JM. Sex and age differences in serum leptin, insulin-like growth factor-I components and sex-hormone binding globulin in a healthy population. Prot Pept Lett. 2007;14:708–11.
Gómez JM, Maravall FJ, Gómez N, et al. The IGF-I system component concentration that decrease with ageing are lower in obesity in relationship to body mass index and body fat. Growth Horm & IGF Res. 2004;14:91–6.
Vendrell J, Broch M, Vilarrasa N, et al. Resistin, adiponectin, ghrelin, leptin and proinflammatory cytokines: relationships in obesity. Obes Surg. 2004;12:962–71.
Vilarrasa N, Vendrell J, Maravall J, et al. Distribution and determinants of adiponectin, resistin and ghrelin in randomly selected healthy population. Relationship with IGF-I system. Clin Endocrinol (Oxf). 2005;63:329–35.
Díez JJ, Iglesias P. The role of the novel adipocyte-derived hormone adiponectin in human disease. Eur J Endocrinol. 2003;148:293–300.
Hotamisligil GS, Arner P, Caro JF, et al. Increased adipose tissue expression of tumor necrosis alpha in human obesity and insulin resistance. J Clin Invest. 1995;95:2409–15.
Wood IS, Wang B, Jenkins JR, Trayhurn P. The pro-inflammatory cytokine IL-18 is expressed in human adipose tissue and strongly upregulated by TNF alpha in human adipocytes. Biochem Biophys Res Commun. 2005;337:422–9.
Hamrick MW, Pennington C, Newton D, et al. Leptin deficiency produces contrasting phenotypes in bones of the limb and spine. Bone. 2004;34:376–83.
Yamauchi T, Kamon J, Waki H, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7:941–6.
Straczkowski M, Kowalska I, Nikolajuk A, et al. Increased serum interleukin-18 is associated with hypoadiponectinemia in obesity, independently of insulin resistance. Intern J Obes. 2006;31:221–5.
Lenchik L, Register TC, Hsu F-C, et al. Adiponectin as a novel determinant of bone mineral density and visceral fat. Bone. 2003;33:646–51.
Oh KW, Lee WY, Rhee EJ, et al. The relationship between serum resistin, leptin, adiponectin, ghrelin levels and bone mineral density in middle-aged men. Clin Endocrinol (Oxf). 2005;63:131–8.
Sambrook P. Tumor necrosis factor blockade and the risk of osteoporosis: back to the future. Arthritis Res Ther. 2007;9:107.
Capella RF, Capella JF. Reducing early technical complications in gastric by-pass surgery. Obes Surg. 1997;7:149–57.
Molina A, Vendrell J, Gutiérrez C, et al. Insulin resistance, leptin and TNF-α system in obese morbid women after gastric bypass surgery. Obes Surg. 2003;13:615–21.
Gómez JM, Maravall FJ, Gómez N, et al. 25-(OH) D3 regulation in a healthy population randomly selected. Horm Metab Res. 2004;36:48–53.
Gómez JM. The role of insulin-like growth factor I components in the regulation of vitamin D. Curr Pharm Biothec. 2006;7:125–32.
World Health Organization. Obesity: preventing and managing the global epidemic. Report on a WHO consultation on obesity. WHO/NUT/NCD/98.1. Geneva: WHO, 3–5 June, 1997.
El-Kadre LJ, Rocha PR, de Almeida Tinoco AC, et al. Calcium metabolism in pre- and postmenopausal morbidly obese women at baseline and after laparoscopic Roux-en-Y gastric bypass. Obes Surg. 2005;14:1062–6.
Ybarra J, Sánchez-Hernández I, Gich T, et al. Unchanged hypovitaminosis D and secondary hyperparathyroidism in morbid obesity after bariatric surgery. Obes Surg. 2005;15:330–5.
Goode LR, Brolin RE, Chowdhury HA, et al. Bone and gastric bypass surgery: effects of dietary calcium and vitamin D. Obes Res. 2004;12:40–7.
Maeda K, Takahashi M, Funahashi T, et al. PPARgamma ligands increase expression and plasma concentrations of adiponectin, and adipose-derived protein. Diabetes. 2001;50:2094–9.
Vilarrasa N, Vendrell J, Sánchez-Santos R, et al. Effect of weight loss induced by gastric bypass on proinflammatory interleukin-18, soluble tumor necrosis factor-α receptors, C-reactive protein and adiponectin in morbidly obese patients. Clin Endocrinol (Oxf). 2007;67:679–86.
Kontogianni MD, Dafni UG, Routsias JG, et al. Blood leptin and adiponectin as possible mediators of the relation between fat mass and BMD in perimenopausal women. J Bone Miner Res. 2004;19:546–51.
Seriolo B, Paolino S, Sulli A, et al. Bone metabolism exchanges during anti-TNF-alpha therapy in patients with active rheumatoid arthritis. Ann N Y Acad Sci. 2006;1069:420–7.
Santos JL, Lera L, Pérez-Bravo F, et al. Adiposity and bone mineral density of Chilean elderly women in relation to toll-like receptor 4 gene polymorphisms. Ann Hum Biol. 2006;33:585–92.
Núñez NP, Carpenter CL, Perkins SN, et al. Extreme obesity reduces bone mineral density: complementary evidence from mice and women. Obesity. 2007;15:1980–7.
Edelstein SL, Barrett-Connor E. Relation between body size and bone mineral density in elderly men and women. Am J Epidemiol. 1993;138:160–9.
Reid I, Plank LD, Evans MC. Fat mass is an important determinant of whole body bone density in premenopausal women but not in men. J Clin Endocrinol Metab. 1992;75:779–82.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gómez, J.M., Vilarrasa, N., Masdevall, C. et al. Regulation of Bone Mineral Density in Morbidly Obese Women: A Cross-sectional Study in Two Cohorts Before and After Bypass Surgery. OBES SURG 19, 345–350 (2009). https://doi.org/10.1007/s11695-008-9529-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-008-9529-4