Abstract
Background
Limited data exist regarding efficacy and dosing of low-molecular-weight heparins, including enoxaparin, for morbidly obese patients. Prophylactic doses of 30 to 60 mg every 12 h have been described in bariatric surgery patients with appropriate anti-Xa levels reported between 0.18 and 0.6 units/mL.
Methods
Fifty-two laparoscopic gastric bypass or banding patients were enrolled. Patients were divided into two groups by the dose of enoxaparin that was given: Group 1—enoxaparin 30 mg every 12 hours—and Group 2—enoxaparin 40 mg every 12 h. Anti-Xa levels were obtained 4 h after the first and third doses. Levels between 0.18–0.44 units/mL were considered appropriate.
Results
There were 19 patients (74% female, mean body mass index [BMI] 48.4 kg/m2) in Group 1 and 33 patients (82% female, mean BMI 48.5 kg/m2) in Group 2. In Group 1, anti-Xa levels were 0.06 and 0.08 units/mL after the first and third doses, respectively. In Group 2, anti-Xa levels were 0.14 and 0.15 units/mL after first and third doses, respectively (p = NS). There was a statistically significant difference in anti-Xa levels between Group 1 first dose and Group 2 first dose (p < 0.05) and between Group 1 third dose and Group 2 third dose (p < 0.05). Percentage of appropriate anti-Xa levels at first dose differed 0% vs. 30.8% (Group 1 vs. Group 2; p = 0.01) and at third dose 9.1% vs. 41.7% (Group 1 vs. Group 2; p = 0.155).
Conclusion
When prophylactic dose enoxaparin of 30 mg every 12 h was changed to 40 mg every 12 h in bariatric surgery patients, anti-Xa levels significantly increased with prophylactic dose enoxaparin in bariatric surgery patients. The percentage of appropriate levels also increased; however, more than half of the patients receiving 40 mg every 12 hours failed to reach therapeutic levels. No levels were supratherapeutic. Dosage of 40 mg every 12 h may not be sufficient for bariatric surgery patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Currently, there is limited evidence to guide the use of prophylactic enoxaparin, especially in morbidly obese patients. One survey of the American Society for Bariatric Surgery members demonstrated that most (86%) felt that bariatric surgery patients were at high risk for a venous thromboembolic event, and most (95%) utilized routine prophylaxis [1]. The American College of Chest Physicians recommends to consider monitoring anti-Xa levels in patients weighing more than 150 kg or with a body mass index (BMI) greater than 50 [2]. However, this recommendation is limited to patients receiving enoxaparin for treatment, not prophylaxis. Aventis, the manufacturer of Lovenox® (enoxaparin), gives no specific recommendations in the package insert for use in the morbidly obese population. There is also a lack of consensus regarding the appropriate range of anti-Xa levels for prophylaxis. Micromedex suggests a range of 0.2–0.6 units/mL, whereas Lexi-Comp defines an appropriate range of anything less than 0.45 units/mL [3, 4]. The objective of the current study was to characterize anti-Xa levels in bariatric surgery patients receiving prophylactic enoxaparin.
Materials and Methods
Inclusion criteria included any patient undergoing laparoscopic banding or laparoscopic gastric bypass surgery. The study occurred between October 2005 and April 2006 at the Bariatric Center of Excellence of the Regional Medical Center at Memphis. There were two groups of patients. Group 1 (between October 2005 and December 2005) received enoxaparin 30 mg every 12 h. Group 2 (between December 2005 and April 2006) received enoxaprin 40 mg every 12 h. Patients received the first dose at 11:00 p.m. on the day of surgery. One surgeon (AKM) gave no preoperative dose; whereas one (DST) gave a dose 0 (30 mg or 40 mg) before induction of anesthesia. Some patients did receive a preoperative dose. Similar techniques utilized for laparoscopic gastric bypass have been previously described [5, 6]. All patients received pneumatic compression devices before induction of anesthesia. In addition, early ambulation (day of surgery) was encouraged for all patients.
The study was approved and conducted in accordance with the guidelines established by the University of Tennessee Health Science Center Institutional Review Board. Because all measurements from this study were performed as part of the clinical care of the patient and confidentiality procedures for the patient were maintained, the requirement for informed consent was waived. Anti-Xa levels were drawn 4 h after the first and third doses of enoxaparin. These levels were assessed at the study institution using the Stago assay. Nurses documented administration time on the medication administration record. All anti-Xa levels drawn earlier than 3 h postdose or later than 5 h postdose were excluded. Patients undergoing laparoscopic banding typically only had anti-Xa levels drawn after the first dose of enoxaparin as their usual 24-h admission resulted in discharge before the third dose.
In the statistical analysis, two-tailed paired and unpaired t tests were used to compare anti-Xa levels within each dosing regimen (first- versus third-dose levels) and between the two dosing regimens (30 mg versus 40 mg). In addition, Chi-square and Fisher’s exact tests were used to compare the percentage of appropriate anti-Xa levels (0.18–0.44 units/mL) within each dosing regimens and then between regimens. Statistical significance was defined by p values less than 0.05. Statistical analyses were performed using Statview 5.0.1, SAS Institute, California.
Results
Patients in both phases of the study were well matched in terms of age, gender, weight, and body mass index (BMI) as seen in Table 1. Lab values during hospitalization, including serum creatinine, hematocrit, and platelets, were also similar.
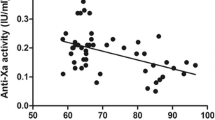
In Group 1, 19 first-dose and 11 third-dose anti-Xa levels were included. In Group 2, 26 first-dose and 12 third-dose anti-Xa levels were included. There was not a statistically significant difference between the first- and third-dose levels within either Group 1 or Group 2. However, when comparing the 30-mg regimen to the 40-mg regimen, a statistically significant increase was seen between the first-dose levels and between the third-dose levels (p < 0.05). Results are summarized in Fig. 1.
The percentage of appropriate anti-Xa levels was analyzed between the two dosing regimens as well. In Group 1, no patients had therapeutic levels after the first dose. In Group 2, almost one third of patients had a therapeutic level after the first dose. This difference was statistically significant (p = 0.01). For third-dose anti-Xa levels, a similar margin of increase was seen from 30 mg to 40 mg, but this difference was not statistically significant (p = 0.115) probably because of our sample size. Figure 2 illustrates these results.
We compared patients whose dose was appropriate versus those whose dose was too low. There were no differences in BMI (51.2 vs. 54.0; p = NS).
Discussion
Recently, Borkgren-Okonek et al. [7] studied the effects of prophylactic enoxaparin in 68 gastric bypass patients. Patients received a single dose of heparin 5,000 units subcutaneously 2 h before surgery. All patients received enoxaparin beginning 12 h postsurgery, but doses were stratified based on BMI. Patients with BMI less than or equal to 50 kg/m2 received enoxaparin 40 mg every 12 h, and patients with BMI greater than 50 kg/m2 were begun on enoxaparin 60 mg every 12 h. Anti-Xa levels were drawn 4 h after the first and third doses. In this study, the defined appropriate range for anti-Xa levels was 0.18–0.44 units/mL.
A significant increase in anti-Xa levels was seen between the first and third doses within each dosing group. For the 40-mg group, anti-Xa levels averaged 0.15 units/mL after the first dose and 0.22 units/mL after the third dose (p < 0.001). For the 60-mg group, anti-Xa levels averaged 0.16 units/mL after the first dose and 0.27 units/mL after the third dose (p < 0.001). When comparing the 40-mg group to the 60-mg group, no statistically significant difference was seen in the percentage of therapeutic anti-Xa levels (69% versus 72%).
Scholten et al. [8] studied the effects of enoxaparin 30 mg every 12 h versus 40 mg every 12 h in two successive groups of bariatric surgery patients. Their patients also received early ambulation, compression stockings, and pneumatic compression. There was a statistically significant difference in DVT complications between the treatment groups (5.4% in the 30-mg group versus 0.6% in the 40-mg group; p < 0.01). Rates of hemorrhage were equal. They concluded that using a prophylactic enoxaparin regimen of 40 mg every 12 h in bariatric surgery patients provided better protection than enoxaparin 30 mg every 12 h without increasing the risk of bleeding. Anti-Xa levels were not drawn to correlate outcomes.
For the current study, we expected to see an increase in the anti-Xa levels after the enoxaparin dose increased to 40 mg every 12 h, but we were surprised by low levels overall in both groups. With the 30-mg regimen, only 9% of patients reached the therapeutic range after the third dose. This 9% represents only one patient whose anti-Xa level was 0.19 units/mL, just 1/100th above the base of the therapeutic range. Based on the anti-Xa levels seen in our Group 1 patients, we feel that a prophylactic dosing regimen of enoxaparin 30 mg every 12 h was inadequate for the prevention of thromboembolism in bariatric surgery patients. For this reason, 40 mg every 12 h has now been utilized as the therapy of choice for our bariatric patients. However, almost 60% of patients in Group 2 were below the therapeutic range after the third dose.
There are several limitations to our study. First, the basis of this study was a purely pharmacokinetic one in which we sought to correlate anti-Xa levels with doses. Because there is not a firm consensus on prophylactic range, it is not known how these anti-Xa levels correspond with bleeding rates or occurrence of thromboembolism. We also did not measure risk factors or outcomes of bleeding and thromboembolism in our patients during hospitalization or during follow-up clinic visits. In addition, our sample size was relatively small, which could bias our results. The two different time periods (although they were one after the other) and the differences in dosing regimens between the two surgeons serve as other possible limitations. We decided not to base our dosing on BMI. Others utilized BMI-based dosing [7, 9]. For example, Paige et al. [9] based their dosage on BMI. Their study found no correlation between BMI and bleeding incidence. The last major limitation was that this was not a randomized study that looked at clinical outcomes. As thromboembolitic events and hemorrhage after LRYGB are relatively uncommon events, this would require an extremely large sample size.
Because bariatric surgery patients are at risk for thromboembolism after bariatric surgery, it is important to determine the optimal prophylactic doses of low-molecular-weight heparins without increasing risk of bleeding. Based on the results of our study, enoxaparin 40 mg every 12 h is more appropriate therapy than 30 mg every 12 h in this patient population. However, considering that only about 40% of patients achieved therapeutic levels at steady state, a dose greater than 40 mg may be needed.
Prophylaxis for venous thromboembolism (VTE) is still controversial in patients undergoing laparoscopic Roux-en-Y gastric bypass. Two groups reported an approximate 1% VTE rate utilizing subcutaneous heparin [10, 11]. Another group reported a 3.5% VTE rate utilizing subcutaneous enoxaparin [12]. Still, another group suggests no pharmacologic anticoagulant based on their protocol and results of a 0.26% VTE rate [13]. Hamad and Choban undertook a study examining five various enoxaparin dosing regimens in five different centers [14]. The center that gave enoxaparin only on discharge, not during hospitalization, had the highest rate of VTE. Another study examined two doses of nadroparin and demonstrated no statistical differences in anti-Xa levels [15]. Interestingly, however, the lower doses of nadroparin had lower corresponding anti-Xa levels. In addition, the mean anti-Xa levels were all at 0.18 units/ml or lower.
More studies need to be conducted to evaluate the most appropriate dose while correlating anti-Xa levels to inpatient and outpatient occurrences of thromboembolism and bleeding.
References
Wu EC, Barba CA. Current practices in the prophylaxis of venous thromboembolism in bariatric surgery. Obes Surg 2000;10(1):7–13; discussion 14.
Hirsh J, Guyatt G, Albers GW, et al. The seventh ACCP conference on antithrombotic and thrombolytic therapy. Chest 2004 Sep;126:172S–3S.
Enoxaparin. DRUGDEXTM System. Greenwood Village, CO: Thomson Micromedex, 2006 Feb. http://www.thomsonhc.com.
Lexi-Comp, Inc. Lexi-Complete. 2006 Feb.
Madan AK, Frantzides CT. Triple-stapling technique for jejunojejunostomy in laparoscopic gastric bypass. Arch Surg 2003;138(9):1029–32.
DeMaria EJ, Sugerman HJ, Kellum JM, et al. Results of 281 consecutive total laparoscopic Roux-en-Y gastric bypasses to treat morbid obesity. Ann Surg 2002;235(5):640–7.
Borkgren-Okonek MJ, Hart RW, Pantano JA, et al. Stratified enoxaparin dosing achieves prophylactic anti-factor Xa concentrations in gastric bypass surgery. Surg Obes Relat Dis 2005;1(3):226.
Scholten DJ, Hoedema RM, Scholten SW. A comparison of two different prophylactic dose regimens of low molecular weight enoxaparin in bariatric surgery. Obes Surg 2002;12:19–24.
Paige JT, Gouda BP, Gaitor-Stampley V, et al. No correlation between anti-factor Xa levels, low-molecular-weight heparin, and bleeding after gastric bypass. Surg Obes Relat Dis 2007;3(4):469–75.
Miller MT, Rovito PF. An approach to venous thromboembolism prophylaxis in laparoscopic Roux-en-Y gastric bypass surgery. Obes Surg 2004 Jun–Jul;14(6):731–7.
Prystowsky JB, Morasch MD, Eskandari MK, et al. Prospective analysis of the incidence of deep venous thrombosis in bariatric surgery patients. Surgery 2005 Oct;138(4):759–63; discussion 763–765.
Gonzalez R, Haines K, Nelson LG, et al. Predictive factors of thromboembolic events in patients undergoing Roux-en-Y gastric bypass. Surg Obes Relat Dis 2006 Jan–Feb;2(1):30–5; discussion 35–36.
Gonzalez QH, Tishler DS, Plata-Munoz JJ, et al. Incidence of clinically evident deep venous thrombosis after laparoscopic Roux-en-Y gastric bypass. Surg Endosc 2004 Jul;18(7):1082–4.
Hamad GG, Choban PS. Enoxaparin for thromboprophylaxis in morbidly obese patients undergoing bariatric surgery: findings of the prophylaxis against VTE outcomes in bariatric surgery patients receiving enoxaparin (PROBE) study. Obes Surg 2005 Nov–Dec;15(10):1368–74.
Kalfarentzos F, Stavropoulou F, Yarmenitis S, et al. Prophylaxis of venous thromboembolism using two different doses of low-molecular-weight heparin (nadroparin) in bariatric surgery: a prospective randomized trial. Obes Surg 2001 Dec;11(6):670–6.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rowan, B.O., Kuhl, D.A., Lee, M.D. et al. Anti-Xa Levels in Bariatric Surgery Patients Receiving Prophylactic Enoxaparin. OBES SURG 18, 162–166 (2008). https://doi.org/10.1007/s11695-007-9381-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-007-9381-y