Abstract
A long-standing goal of speciation research is to describe how reproductive isolating barriers develop, when they arise along the ‘speciation continuum’, and to measure the strength with which they restrict gene flow. Drosophila arizonae and D. mojavensis are a recently diverged sister species pair distributed from the southwestern United States through southern Mexico. While incipient speciation in D. mojavensis has been studied for decades, relatively little attention has been directed toward D. arizonae, despite the fact that previous studies have revealed evidence for significant genetic differentiation among populations separated by geographic barriers. Here, we examine the potential for both pre- and post-mating reproductive isolation in D. arizonae from geographically isolated parts of North America. We find evidence for strong premating isolation between flies from northern mainland Mexico and southern mainland Mexico, but no evidence for postmating isolation in any cross. This study highlights the utility of the D. arizonae system for further investigation into the early evolution of premating isolation, and reinforces the potential of the D. arizonae/D. mojavensis system as a whole for studying the evolution of reproductive isolation at a range of divergence times.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Speciation progresses through an accumulation of reproductive barriers, which act to restrict gene flow between sexually reproducing populations (Dobzhansky 1937; Mayr 1942; Coyne and Orr 2004). Reproductive isolating barriers can act either before or after mating occurs. Premating isolation between populations results in reduced copulations that can include ecological, behavioral, or mechanical factors (Coyne and Orr 2004). Postmating isolation is further subdivided into barriers that act either after mating but before fertilization (postmating-prezygotic) or after fertilization (postzygotic). Postmating-prezygotic (PMPZ) barriers are often cryptic, involving interactions between sperm or seminal fluid and the internal environment of the female reproductive tract (Coyne and Orr 2004; Markow 1997; Sweigart 2010). Postzygotic barriers are more obvious to detect, often resulting in sterility or lethality in offspring (Orr 1995) or ecologically unfit intermediate phenotypes (Schluter 2001).
Speciation research attempts to understand how reproductive isolation evolves, the rate and order in which barriers develop, and the relative strength of barriers acting between diverging lineages. These matters have been addressed by studying different points along the ‘speciation continuum,’ ranging from populations in the initial stages of divergence to those where reproductive isolation is complete (Seehausen et al. 2014; Shaw and Mullen 2014). Examining populations in the early stages of divergence provides a clear view of early evolving reproductive barriers although it is not possible to determine whether the speciation process will go to completion (Coyne and Orr 2004). Studying species further along the continuum provides an advantage in knowing that the process of speciation is complete or nearly complete. It is difficult, however, to distinguish between barriers that first contributed to the onset of reproductive isolation and other barriers that may have accumulated over time since divergence (Coyne and Orr 2004; Demuth and Wade 2007). Furthermore, hybridization experiments between species across a range of divergence times show that hybrid vigor, outbreeding depression and partial reproductive compatibility develop at variable rates both within and among taxonomic groups (Edmands 2002). While important insights have emerged from individual case studies at different levels of divergence (Palumbi and Metz 1991; Edmands 2016; Jiggins et al. 2001; Boughman 2001; Ramsey et al. 2003; Sánchez-Guillén et al. 2012) systems in which a series of closely related taxa span a range of divergence times offer a particularly promising opportunity to integrate inferences regarding the evolution of reproductive isolation at different timescales (Seehausen et al. 2014; Shaw and Mullen 2014).
Cactophilic species of Drosophila endemic to deserts of North and South America have been the focus of studies on the evolution of reproductive isolation for decades. In particular, the sister species D. arizonae and D. mojavensis have served as a classic model in studies of speciation (Markow and Hocutt 1998; Etges 2014) in part because the system as a whole allows for the study of reproductive isolating barriers at different timescales. Interspecific crosses between D. mojavensis and D. arizonae exhibit premating isolation, PMPZ isolation, and postzygotic isolation, though the relative strength of most barriers depends on both the direction of the cross and the source population of males and females (Markow and Hocutt 1998; Wasserman and Koepfer 1977; Massie and Markow 2005; Kelleher and Markow 2007). On the other end of the continuum, more recently diverged D. mojavensis subspecies exhibit considerable genetic and ecological divergence, with evidence for modest reproductive isolation in some crosses (Markow and Hocutt 1998; Etges 2014; Markow 1991).
Historically, D. arizonae has been studied mainly in relation to its sister species, yet it shares many of the attributes that initially made D. mojavensis an attractive study model for incipient speciation. Drosophila arizonae is distributed from southern Arizona USA, down through mainland Mexico, all the way south into Guatemala (Markow and Hocutt 1998). More recent collections have also identified populations throughout the Baja Peninsula as well as southern California, USA that are presumed to have resulted from relatively recent colonization events (Reed et al. 2007). Previous studies using mitochondrial and nuclear markers reveal evidence for population structure along this expansive range. Most notably, combining information across these studies reveals strong consensus that southern populations are highly differentiated from all other populations (Machado et al. 2007; Reed et al. 2007; Matzkin 2008), but there is some disagreement about the phylogenetic placement of this group relative to other D. arizonae. Inferences from multiple nuclear markers placed the monophyletic southern clade as nested within the larger D. arizonae clade (Machado et al. 2007; Reed et al. 2007), while a phylogeny based on mitochondrial cytochrome oxidase I grouped the southern clade as sister to D. mojavensis, thus making D. arizonae paraphyletic (Reed et al. 2007). Both nuclear and mitochondrial markers also support significant genetic differentiation between the California population and other locations including Baja and northern Mexico/Arizona, though not to the extent observed for southern populations (Machado et al. 2007; Reed et al. 2007). Additionally, there appears to be differentiation between some, but not all, populations from Baja and northern Mexico/Arizona (Machado et al. 2007; Reed et al. 2007). The observed population structure likely results, at least in part, from physical barriers such as the Sierra Madre Occidental, Trans-Mexican Volcano Belt and the Sea of Cortez that isolate populations from different regions. Ecological information across much of D. arizonae’s range is scant, but in northern Mexico its primary host plant is cina cactus (Stenocereus alamosensis), although it has also been collected from other columnar and prickly pear hosts in this region (Fellows and Heed 1972). Little is known about host plant associations in other areas, but cina cactus is not present over much of D. arizonae’s current range, suggesting the potential for ecological divergence in host plant use (Ruiz et al. 1990). This potential is further underscored by the fact that the population from southern California was found associated with citrus, indicating that D. arizonae is capable of utilizing diverse resources.
The high degree of genetic differentiation and ecological divergence among isolated D. arizonae populations suggests that the potential for reproductive isolation among these populations should be examined. While past studies investigating premating reproductive isolation between northern and southern mainland D. arizonae populations using no choice and male choice experiments found no evidence for isolation (Wasserman and Koepfer 1977; Baker 1947), a more recent study utilizing an ecologically realistic multiple-choice test (Massie 2006) found significant premating isolation between flies from these two locations. This latter study, however, was performed using only a single strain from each location, precluding any strong inferences. Furthermore, none of these studies included the more recently discovered populations from southern California and the Baja peninsula, and the potential for postmating isolation has yet to be examined for any cross.
Here, we assess the potential for both premating and postmating isolation among D. arizonae from northern mainland Mexico, southern mainland Mexico, the Baja peninsula, and southern California. In addition to providing evidence for the early evolution of reproductive isolating barriers in this system, our findings expand the utility of the D. mojavensis/D. arizonae system as a model for studying the process of divergence across the ‘speciation continuum.’
Methods
Stocks and Husbandry
We obtained D. arizonae stocks used for the experiments from personal collections, the Drosophila Species Stock Center, and from donations by Dr. Luciano Matzkin. Stocks represented four main geographic regions: southern Mexico, southern California, USA, Baja California, Mexico, and northern Mexico (Fig. 1). Given that the majority of stocks had been maintained as isofemale strains in the laboratory for varying amounts of time prior to experimentation, we merged stocks within regions to create more genetically diverse mass populations several generations before the experiments described below. The Sonora, Mexico population (heretofore “northern”) included 16 isofemale lines collected near San Carlos, Sonora, Mexico in 2006. The southern California population (heretofore “Riverside”) was comprised of five isofemale lines collected from Riverside, CA in 2002. The Baja population (heretofore “Baja’) included a line started from a multi-female collection from San Quintín (Drosophila stock center id: 15081-1271.29; collected in 2008) and four lines collected nearby from El Rosario in 2003. Flies from the southern region (heretofore “southern”) included lines from multiple localities that formed a monophyletic group in a previous phylogeographic study: one line founded from a multifemale collection from Tuxtla Guitierrez, Mexico (Drosophila Stock Center id: 15081-1271.14; collected in 1987), two isofemale lines from San Luis Potosi, Mexico (Drosophila Stock Center id: 15081-1271.31 and 15081-1271.32; collected in 2005), and two lines founded from multi-female collections from Hidalgo, Mexico (Drosophila Stock Center id: 15081-1271.17; collected in 1987, and 15081-1271.07). Given that these stocks were originally collected from different localities within the southern region, we first confirmed that there was no reproductive isolation among them before merging into the mass population. Each population was maintained at large size throughout the experiment in multiple half-pint milk bottles with Carolina Instant Drosophila media. Larval and adult densities were monitored to ensure they were roughly equivalent, and all environmental conditions were kept constant (Casares et al. 2005). For both premating and postmating isolation experiments, virgin flies were collected daily and sorted under light CO2 anesthesia. Virgin males and females were kept separately in groups of approximately 15 flies for 9–13 days to ensure they reached reproductive maturity prior to experimentation.
Map showing geographic origin of Drosophila arizonae lines used to analyze pre- and postmating reproductive isolation. Multiple lines were merged from each geographic area to create mass populations prior to experimentation. Gray triangles represent mountain ranges that may serve as geographic barriers between D. arizonae populations. Localities: 1 Riverside, CA, 2 San Quintín, Baja California, 3 El Rosario, Baja California, 4 San Carlos, Sonora, 5 San Luis Potosi, 6 Hidalgo, and 7 Tuxtla Guitierrez, Chiapas
Premating Isolation Experiments
We used a multiple-choice experimental design to test for premating isolation. At least 12 h before mating, 16 females and 16 males from two focal regions were randomly assigned to fresh media containing blue, red, green, or no food dye. Food dye can be observed in the abdomen under a microscope, and was used to differentiate flies from different populations. Previous studies have shown this type of coloring to have no effect on mating preference (Wu et al. 1995; Jennings and Etges 2010). All mating trials were in the morning, which is when flies typically mate. For each trial, we placed 60 flies (15 of each sex from two populations) in a mating chamber constructed from an 8″ × 1″ petri dish with holes drilled to allow access with an aspirator. We removed copulating pairs from the mating chamber with an aspirator, waiting a minimum of 30 s to ensure they were not pseudocopulating (flies typically copulate for ~ 3 min). Following suggested guidelines for multiple-choice mating experiments, we terminated each trial after 30 min or until half of all possible matings occurred (15) (Casares et al. 2005; Gilbert and Starmer 1985). After each trial, source populations of copulating males and females were determined by assessing abdomen color. Ten to 11 replicate trials were performed for each cross combination.
We used the program JMATING (Carvajal-Rodriguez and Rolan-Alvarez 2006) to analyze the mating data. Although our study design involved multiple replicates of each mating experiment, this structure is not explicitly accounted for in the JMATING analyses. Thus, to examine whether replicates for each experiment could be pooled prior to analysis, we tested whether there was heterogeneity of odds ratios among replicates using Breslow–Day tests with Tarone’s adjustment. We found no evidence for heterogeneity among replicates in any of the experiments (P > 0.05), and therefore pooled across replicates for the JMATING analyses. These analyses calculated the index of sexual isolation I PSI , which ranges between −1 and 1, with zero indicating random mating and one indicating complete reproductive isolation. We also used the isolation asymmetry index (IA PSI ) to determine the symmetry of mating combinations for the interpopulation reciprocal crosses; an IA PSI of 1 indicates symmetrical matings and equal preference for males/females of any given combination while any deviation from 1 indicates asymmetry. Significance of isolation indices was tested using bootstrapping (10,000 replicates). Given that we performed six experiments in total we used the Holm–Sidak step-down procedure (Holm 1979) to account for multiple testing.
Postmating Isolation Experiments
We conducted postmating isolation experiments separately from the premating experiments. Single-pair matings were staged in the morning and included all possible mating combinations between populations (six total experiments). We placed females individually into vials containing two males, and copulations were observed. Following copulation, individual females were moved into new vials, and were then transferred to fresh food daily for a total of 8 days. The total amount of progeny surviving to adulthood was recorded for each female. The most powerful method of analysis for our postmating dataset would be a factorial ANOVA, as this would account for main effect differences of female and male source populations, and the interaction would test for the potential presence of postmating isolation. However, we were unable to transform the data to achieve normality of residuals (an assumption of the ANOVA procedure), due to the presence of excess zeros in each dataset. As a solution, we split the dataset and conducted two separate tests: (1) we first used a Fisher’s exact test to examine whether there were differences among crosses in the proportion of matings that failed to produce any progeny, and (2) for matings that did produce progeny, we used a factorial ANOVA to examine mean progeny production (square root transformation normalized the residuals on this dataset). If the interaction term was significant we conducted all pairwise post hoc tests to further dissect the nature of the interaction using Tukey’s method to correct for multiple testing. A case where crosses between regions (A × B) produce significantly less offspring than their corresponding pure crosses (A × A and B × B) would suggest the presence of either postmating-prezygotic barriers (e.g. reduced oviposition or failed fertilization) or hybrid inviability, though additional studies would be necessary to differentiate between these possibilities. We favored the factorial ANOVA approach over an alternative one-way non-parametric approach because the latter would compare crosses individually without accounting for potential regional differences in male fertility or female fecundity.
To test for the possibility of hybrid sterility in crosses between geographic regions, we assessed whether F1 offspring of each cross were capable of producing F2 progeny. Specifically, pairs of F1 virgin male and female flies were placed together in individual vials and the proportion of vials producing any F2 progeny was counted. This was done separately for each cross (12 crosses between regions and 4 pure crosses; N = 30–34 vials for each cross) and the proportion of vials producing progeny for all crosses was compared using a Chi square test. With this design, additional tests would be necessary to differentiate between male or female sterility as a cause of any reduction in the proportion of vials producing F2 offspring for a particular cross, but equivalent F2 production among within and between region crosses would indicate that hybrids do not show elevated levels of complete sterility (we did not quantify offspring production so the potential for subfertility was not examined).
Results
Premating Isolation Experiments
Total deviations from random mating for all cross combinations are given in Table 1. We found significant premating isolation between southern and northern crosses (I PSI 0.3961, p = 0.0004; Table 1), while no other combinations deviated from random mating. The IA PSI index revealed a major reduction in copulations between southern females and northern males, compared to the number of copulations between northern females and southern males (IA PSI = 0.640, p = 0.002; Table 1).
Postmating Isolation Experiments
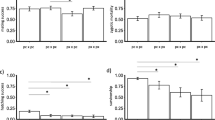
After correcting for multiple testing, comparisons of the proportion of matings that failed to produce progeny revealed differences among crosses in only one experiment (Fig. 2). More specifically, both crosses between Baja and Riverside exhibited higher failure rates where no offspring were produced (17 and 18 %) than the pure Baja cross (0 %) (Fig. 2). After correcting for multiple testing, results from factorial ANOVAs comparing progeny production revealed a significant female × male interaction in the Baja/Riverside experiment and the southern/Riverside experiment. In the former case, crosses between Baja females and Riverside males produced more progeny than pure Baja crosses, while crosses between Riverside females and Baja males produced more progeny than either pure cross (Fig. 3). Similarly, in the southern/Riverside experiment, crosses between regions resulted in higher progeny production than the pure southern cross (Fig. 3). Three other experiments revealed a significant female effect, with southern females producing more progeny than Baja females, northern females more than southern females, and Riverside females producing more than northern females. We found no evidence consistent with elevated rates of F1 male or female sterility in offspring of crosses between regions. The proportion of F1 crosses that produced progeny was uniformly high (0.88–1), and did not differ among crosses (χ2 = 13.54; p = 0.561).
Proportion of crosses that produced progeny for all possible cross combinations. Significance was assessed using Fisher’s exact test. In cases where the test was significant after adjusting for multiple testing (Holm–Sidak step-down), group means under different letters were significantly different. S southern, B Baja, N northern, R Riverside, CA
Mean progeny production for all possible cross combinations. Error bars represent 95 % CIs. In cases where there was a significant male × female interaction, individual crosses grouped under different letters were significantly different (post hoc comparisons with Tukey’s adjustment; α = 0.05). S southern, B Baja, N northern, R Riverside, CA
Discussion
We found a significantly high degree of premating isolation between geographically isolated southern and northern D. arizonae. These results agree with, and strengthen, previous evidence for premating isolation between single strains derived from Peralta, AZ and Hidalgo, Mexico (Massie 2006). This pattern also is consistent with previous studies showing that southern D. arizonae show the most genetic differentiation from all other D. arizonae localities (Machado et al. 2007; Reed et al. 2007; Matzkin 2008). While we did not detect statistically significant premating isolation between southern D. arizonae and those from Baja or Riverside, these crosses had the second and third highest isolation indices respectively (Table 1). Although there is some evidence to suggest genetic subdivision among flies from northern Mexico, the Baja Peninsula, and southern California (Machado et al. 2007; Reed et al. 2007; Matzkin 2008), we found no evidence that this genetic divergence is accompanied by the evolution of premating isolation.
Consistent with our findings in D. arizonae, early evolution of premating isolation is also observed in some crosses between D. mojavensis subspecies. Specifically, crosses involving D. mojavensis sonorensis females and D. mojavensis baja males exhibit significant premating isolation (Wasserman and Koepfer 1977; Markow 1991). The mechanistic basis of this isolation remains unresolved, but could be influenced by differences in courtship behaviors and contact pheromones (e.g. cuticular hydrocarbons), as reported in other recently diverged Drosophila pairs (Etges 2014; Chung et al. 2014; Shirangi et al. 2009; Nanda and Singh 2012). Exploring these possibilities in D. arizonae is an important avenue for future research due to the strength of premating isolation observed between southern and northern populations. Moreover, a better understanding of D. arizonae’s ecology is needed, particularly in understudied southern portions of its range. Ecological factors such as larval rearing substrates and temperature are known to influence cuticular hydrocarbons profiles in D. mojavensis (Etges 2014), and thus may play an important role in mating interactions between D. arizonae populations.
The pattern of premating isolation between southern and northern D. arizonae is asymmetrical, with fewer copulations observed between southern females and northern males than the reciprocal pairing. This corroborates the previous study (Massie 2006), which also found fewer copulations between southern females and northern males. Asymmetrical premating isolation is commonly observed in other groups of recently diverged Drosophila (Yukilevich and True 2008; Yukilevich 2012; Arthur and Dyer 2015; Robertson 1988, Ehrman and Wasserman 1987), including D. mojavensis subspecies (Wasserman and Koepfer 1977; Markow 1991). In crosses between D. mojavensis sonorensis and D. mojavensis baja asymmetrical premating isolation has been hypothesized to result from reproductive character displacement driven by historical reinforcement in Sonora, where D. mojavensis sonorensis females have been selected to be more discriminating due to the presence of D. arizonae (Wasserman and Koepfer 1977). In our study, however, northern D. arizonae females, which are sympatric with D. mojavensis, mated readily with males from all of the other populations. These results suggest that the presence of D. mojavensis does not fundamentally alter D. arizonae female mate discrimination. On the other hand, copulations between southern females, which are allopatric with D. mojavensis, and northern males were rare. Since we did not quantitatively analyze specific elements of courtship, it is not clear whether this isolation reflects patterns of female mate discrimination, or whether males contribute more directly to the reduction in interpopulation copulations. Little is known about Drosophila community structure in the southern portions of D. arizonae’s range, but it is possible that the presence of other closely related sympatric species, such as D. navojoa, could influence mating interactions. In fact, previous studies have suggested that the presence of D. navojoa alters D. mojavensis mate discrimination in areas where these species overlap (Markow and Maveety 1985). Nevertheless, it is interesting to note that even if unexplored community dynamics do influence patterns of mate discrimination, the effects do not appear to be universal, in that we did not detect significant isolation in other crosses involving southern populations (though there was a trend toward higher isolation). The same is also true in D. mojavensis, where premating isolation is not detected in crosses involving D. mojavensis sonorensis and other subspecies subspecies (Wasserman and Koepfer 1977; Markow 1991). Altogether, these results suggest that complex processes in different populations likely drive the evolution of mating interactions, which results in somewhat diverse outcomes in crosses between diverging populations.
We acknowledge the caveat that our conclusions about levels of premating isolation are based on experiments with stocks that had been maintained in the laboratory for several years prior to experimentation. While we believe that some caution is warranted in interpreting these results, the similarity between our results and those reported by Massie (2006), both in terms of the strength of isolation and the pattern of asymmetry (southern females discriminating strongly against northern males), strengthens the case that these findings reflect real biological differences rather than laboratory artifacts. This is made even more convincing by the fact that these experiments were performed by different laboratories using different northern and southern D. arizonae strains.
Combining evidence from comparisons of reproductive failure, progeny production, and fertility of F1 offspring reveals little evidence of postmating isolation in any cross. Although crosses between Baja and Riverside exhibited higher failure rates than the pure Baja cross, these results are counterbalanced by the fact that successful crosses between flies from these regions actually produced more progeny than the pure Baja cross. Differences in failure rates may actually be due to unusually low failure rate of the pure Baja cross in that particular experiment (0 %), as failure rates for this cross in the two other experiments involving Baja were lower and more on par with the Baja/Riverside pure crosses. Taken together, the data do not support postmating isolation between flies from Baja and Riverside. We did not measure F2 hybrid fertility or quantify F1 hybrid offspring production relative to parental reproductive output. Sterile F2 hybrids would implicate postmating isolating barriers, as would a reduction in F1 hybrid reproductive output. Although we failed to detect reduced progeny production or hybrid sterility/inviability in this study, additional experiments focusing on these and other forms of postmating isolation (e.g. conspecific sperm precedence) might reveal evidence for more cryptic incompatibilities in crosses between regions.
Synthesizing information from studies of geographically isolated D. arizonae and D. mojavensis populations, it is clear that premating isolation evolves rapidly, having been detected among divergent populations of each species. In both cases, isolation is asymmetrical and depends on the specific population combination used in the cross, such that males or females that are isolated from individuals of one population may readily mate with individuals from another population (Markow and Hocutt 1998; Wasserman and Koepfer 1977; Massie and Markow 2005). Thus, whatever population-specific selective pressures or random processes drive the evolution of premating isolation, the effects are idiosyncratic. The dynamic nature of the evolution of premating isolation is also evident at longer timescales. Given its early evolution among recently diverged populations within each species, one might predict that strong premating isolation would characterize all crosses between D. mojavensis and D. arizonae. However, while premating isolation is relatively high for crosses between northern D. arizonae females and all D. mojavensis subspecies, in the reciprocal crosses isolation ranges from strong to virtually absent depending on the D. mojavensis subspecies used in the cross (Markow and Hocutt 1998; Wasserman and Koepfer 1977; Massie and Markow 2005). Altogether, these results suggest that premating isolation has the capacity to evolve rapidly, but also may be highly labile over evolutionary time. This is not necessarily surprising given that this form of isolation typically involves a strong behavioral component, and behavioral traits are known to be particularly labile (Blomberg et al. 2003).
References
Arthur, N. J., & Dyer, K. A. (2015). Asymmetrical sexual isolation but no postmating isolation between the closely related species Drosophila suboccidentalis and Drosophila occidentalis. BMC Evolutionary Biology, 15, 38. doi:10.1186/s12862-015-0328-y.
Baker, W. K. (1947). A study of isolating mechanisms found in Drosophila arizonensis and D. mojavensis. University of Texas Publications, 4720, 126–136.
Blomberg, S. P., Garland, T., & Ives, A. R. (2003). Testing for phylogenetic signal in comparative data: Behavioral traits are more labile. Evolution; International Journal of Organic Evolution, 57(4), 717–745. doi:10.1111/j.0014-3820.2003.tb00285.x.
Boughman, J. W. (2001). Divergent sexual selection enhances reproductive isolation in sticklebacks. Nature, 411(6840), 944–948. doi:10.1038/35082064.
Carvajal-Rodriguez, A., & Rolan-Alvarez, E. (2006). JMATING: A software for the analysis of sexual selection and sexual isolation effects from mating frequency data. BMC Evolutionary Biology, 6(1), 40. doi:10.1186/1471-2148-6-40.
Casares, P., Piñeiro, R., & Carracedo, M. C. (2005). Is premating isolation in Drosophila overestimated due to uncontrolled factors? Journal of Genetics, 84(3), 259–264. doi:10.1007/BF02715796.
Chung, H., Loehlin, D. W., Dufour, H. D., Vaccarro, K., Millar, J. G., & Carroll, S. B. (2014). A single gene affects both ecological divergence and mate choice in Drosophila. Science (New York, N.Y.), 343(6175), 1148–1151. doi:10.1126/science.1249998.
Coyne, J., & Orr, H. A. (2004). Speciation. Sunderland, MA: Sinauer Associates.
Demuth, J. P., & Wade, M. J. (2007). Population differentiation in the beetle Tribolium castaneum. II. Haldane’s rule and incipient speciation. Evolution, 61(3), 694–699. doi:10.1111/j.1558-5646.2007.00049.x.
Dobzhansky, T. (1937). Genetics and the origin of species. New York: Columbia University Press.
Edmands, S. (2002). Does parental divergence predict reproductive compatibility? Trends in Ecology and Evolution, 17(11), 520–527. doi:10.1016/S0169-5347(02)02585-5.
Edmands, S. (2016). Heterosis and outbreeding depression in interpopulation crosses spanning a wide range of divergence. Evolution, 53(6), 1757–1768.
Ehrman, L., & Wasserman, M. (1987). The significance of asymmetrical sexual isolation. In M. K. Hecht, et al. (Eds.), Evolutionary biology (pp. 1–20). New York: Plenum Press.
Etges, W. J. (2014). No boundaries: Genomes, organisms, and ecological interactions responsible for divergence and reproductive isolation. The Journal of Heredity, 105(Suppl 1), 756–770. doi:10.1093/jhered/esu039.
Fellows, D. P. P., & Heed, W. B. B. (1972). Factors affecting host plant selection in desert-adapted cactiphilic drosophila. Ecology, 53(5), 850–858. doi:10.2307/1934300.
Gilbert, D. G. D., & Starmer, W. T. T. (1985). Statistics of sexual isolation. Evolution, 39(6), 1380–1383. doi:10.2307/2408793.
Holm, S. (1979). A simple sequentially rejective multiple test procedure. Scandinavian Journal of Statistics, 6(2), 65–70. doi:10.2307/4615733.
Jennings, J. H., & Etges, W. J. (2010). Species hybrids in the laboratory but not in nature: A reanalysis of premating isolation between Drosophila arizonae and D. mojavensis. Evolution, 64(2), 587–598. doi:10.1111/j.1558-5646.2009.00834.x.
Jiggins, C. D., Naisbit, R. E., Coe, R. L., & Mallet, J. (2001). Reproductive isolation caused by colour pattern mimicry. Nature, 411(MAY), 302–305. doi:10.1038/35077075.
Kelleher, E. S., & Markow, T. A. (2007). Reproductive tract interactions contribute to isolation in Drosophila. Fly, 1(February), 33–37.
Machado, C. A., Matzkin, L. M., Reed, L. K., & Markow, T. A. (2007). Multilocus nuclear sequences reveal intra- and interspecific relationships among chromosomally polymorphic species of cactophilic Drosophila. Molecular Ecology, 16(14), 3009–3024. doi:10.1111/j.1365-294X.2007.03325.x.
Markow, T. A. (1991). Sexual isolation among populations of Drosophila mojavensis. Evolution, 45, 1525–1529.
Markow, T. A. (1997). Assortative fertilization in Drosophila. Proceedings of the National Academy of Sciences of the United States of America, 94(15), 7756–7760. doi:10.1073/pnas.94.15.7756.
Markow, T. A., & Hocutt, G. D. (1998). Reproductive isolation in Sonoran desert Drosophila: Testing the limits of the rules. In S. H. Berlocher & D. J. Howard (Eds.), Endless forms: Species and speciation (pp. 234–244). Oxford: Oxford University Press.
Markow, T. A., & Maveety, N. (1985). More character displacement for reproductive isolation in the Mulleri complex. Drosophila Information Service, 61, 115.
Massie, K. (2006). Sexual isolation between Drosophila mojavensis and Drosophila arizonae. The University of Arizona. http://arizona.openrepository.com/arizona/handle/10150/193326. Accessed 13 November 2015.
Massie, K., & Markow, T. A. (2005). Sympatry, allopatry and sexual isolation between Drosophila mojavensis and D. arizonae. Hereditas, 142(2005), 51–55. doi:10.1111/j.1601-5223.2005.01911.x.
Matzkin, L. M. (2008). The molecular basis of host adaptation in cactophilic Drosophila: Molecular evolution of a glutathione S-transferase gene (GstD1) in Drosophila mojavensis. Genetics, 178(2), 1073–1083. doi:10.1534/genetics.107.083287.
Mayr, E. (1942). Systematics and the origin of species. Genome Biology,. doi:10.1073/pnas.0502030102.
Nanda, P., & Singh, B. N. (2012). Behavioural reproductive isolation and speciation in Drosophila. Journal of Biosciences, 37(2), 359–374. doi:10.1007/s12038-012-9193-7.
Orr, H. A. (1995). Population genetics of speciation: The evolution of hybrid incompatibilities. Genetics, 139, 1805–1813. doi:10.1534/genetics.107.081810.
Palumbi, S. R., & Metz, E. C. (1991). Strong reproductive isolation between closely related tropical sea urchins (genus Echinometra). Molecular Biology and Evolution, 8(2), 227–239.
Ramsey, J., Bradshaw, H., & Schemske, D. (2003). Components of reproductive isolation between the monkeyflowers Mimulus lewisii and M. cardinalis (Phrymaceae). Evolution, 57(7), 1520–1534. doi:10.2307/3448754.
Reed, L. K., Nyboer, M., & Markow, T. A. (2007). Evolutionary relationships of Drosophila mojavensis geographic host races and their sister species Drosophila arizonae. Molecular Ecology, 16(5), 1007–1022. doi:10.1111/j.1365-294X.2006.02941.x.
Robertson, H. M. (1988). Mating asymmetries and phylogeny in the Drosophila melanogaster Species Complex. University of Hawaii Press. http://scholarspace.manoa.hawaii.edu/handle/10125/1066. Accessed 13 November 2015.
Ruiz, A., Heed, W. B., & Wasserman, M. (1990). Evolution of the mojavensis cluster of cactophilic Drosophila with descriptions of two new species. The Journal of heredity, 81(1), 30–42.
Sánchez-Guillén, R. A., Wellenreuther, M., & Cordero-Rivera, A. (2012). Strong asymmetry in the relative strengths of prezygotic and postzygotic barriers between two damselfly sister species. Evolution, 66(3), 690–707. doi:10.1111/j.1558-5646.2011.01469.x.
Schluter, D. (2001). Ecology and the origin of species. Trends in Ecology and Evolution, 16(7), 372–380. doi:10.1016/S0169-5347(01)02198-X.
Seehausen, O., Butlin, R. K., Keller, I., et al. (2014). Genomics and the origin of species. Nature Reviews Genetics, 15, 176–192. doi:10.1038/nrg3644.
Shaw, K. L., & Mullen, S. P. (2014). Speciation continuum. The Journal of Heredity, 105(Suppl 1), 741–742. doi:10.1093/jhered/esu060.
Shirangi, T. R., Dufour, H. D., Williams, T. M., & Carroll, S. B. (2009). Rapid evolution of sex pheromone-producing enzyme expression in Drosophila. PLoS Biology, 7(8), e1000168. doi:10.1371/journal.pbio.1000168.
Sweigart, A. L. (2010). The genetics of postmating, prezygotic reproductive isolation between Drosophila virilis and D. americana. Genetics, 184(2), 401–410. doi:10.1534/genetics.109.111245.
Wasserman, M., & Koepfer, H. R. (1977). Character displacement for sexual isolation between Drosophila mojavensis and Drosophila arizonensis. Evolution, 31(4), 812–823.
Wu, C. I., Hollocher, H., Begun, D. J., Aquadro, C. F., Xu, Y., & Wu, M. L. (1995). Sexual isolation in Drosophila melanogaster: A possible case of incipient speciation. Proceedings of the National Academy of Sciences of the United States of America, 92(March), 2519–2523. doi:10.1073/pnas.92.7.2519.
Yukilevich, R. (2012). Asymmetrical patterns of speciation uniquely support reinforcement in Drosophila. Evolution; International Journal of Organic Evolution, 66(5), 1430–1446. doi:10.1111/j.1558-5646.2011.01534.x.
Yukilevich, R., & True, J. R. (2008). Incipient sexual isolation among cosmopolitan Drosophila melanogaster populations. Evolution; International Journal of Organic Evolution, 62(8), 2112–2121. doi:10.1111/j.1558-5646.2008.00427.x.
Acknowledgments
The authors would like to thank Kim Cox, Kim Hoang, Dominic Royster, Martha Bales, and Alexis Masceranas for assistance in the lab, and Daniel Matute for helpful comments on the manuscript. This work was supported by a University of Colorado Colorado Springs Council on Research and Creative Works award to J.M.B.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Joseph A. McGirr and Lena M. Johnson have contributed equally to this work.
Rights and permissions
About this article
Cite this article
McGirr, J.A., Johnson, L.M., Kelly, W. et al. Reproductive Isolation Among Drosophila arizonae from Geographically Isolated Regions of North America. Evol Biol 44, 82–90 (2017). https://doi.org/10.1007/s11692-016-9393-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11692-016-9393-4