Abstract
The history of life seems to be characterized by three large-scale trends in complexity: (1) the rise in complexity in the sense of hierarchy, in other words, an increase in the number of levels of organization within organisms; (2) the increase in complexity in the sense of differentiation, that is, a rise in the number of different part types at the level just below the whole; and (3) a downward trend, the loss of differentiation at the lowest levels in organisms, a kind of complexity drain within the parts. Here, I describe the three trends, outlining the evidence for each and arguing that they are connected with each other, that together they constitute an evolutionary syndrome, one that has recurred a number times over the history of life. Finally, in the last section, I offer an argument connecting the third trend to the reduction at lower levels of organization in “autonomy”, or from a different perspective, to an increase in what might be called the “machinification” of the lower levels.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Three of the large-scale trends in the history of life seem to be connected with each other. The first is the rise in the number of levels of organization within organisms, that is, a rise in complexity in the sense of hierarchy. The second is the increase in complexity in the sense of differentiation, in the number of different part types within a level. The third trend is also in complexity, but it is a downward trend, a loss of differentiation at the lowest levels in organisms, within its parts. It is a decomplexification, so to speak. In what follows, I describe the trends, outlining the evidence for each and arguing that together they constitute a kind of evolutionary syndrome, one that has recurred a number times in evolution. Finally, in the last section, I offer a vague, hand-waving, and improbable-sounding argument that connects the third trend to the evolutionary reduction at lower levels of organization in what is being called “autonomy”, or from a different perspective, to an increase in what might be called the “machinification” of those lower levels.
The story begins with hierarchy, but first a foreword. There is in evolutionary biology and philosophy of biology today a more or less standard way to think about hierarchy, a standard set of questions that we pose about the transitions from individuals to collective. The focus of these questions is fitness. In the language that has grown up around this issue, the fitness of a collective—a society or a colony—depends on the component individuals behaving altruistically to some degree, in other words, on their sacrificing some of their individual fitness for the good of the whole. And the central scientific question has become: under what conditions and how frequently is altruism of this sort expected to evolve.
But there is another tradition, pursued in parallel by a smaller group of biologists and philosophers, a tradition with its roots in Herbert Simon’s mid twentieth century essay “The Architecture of Complexity” (Simon 1962). Central thinkers in this tradition include Wimsatt (1974, 1976, 1994, 2007) and Salthe (1985, 2009, 2012). The focus of this work has been on structure, rather than fitness. What sorts of structural changes are required for new higher levels, collectives, to form? And what are the structural consequences of new levels once they have formed? What has been the historical pattern of change in hierarchical structure, and what forces have encouraged or limited the trend? This essay falls within this second research program. And the proposal here is that there have been two other major structural trends accompanying the rise of hierarchy in the history of life and that all three are connected with each other.
Trend 1: Increasing Hierarchy
Hierarchy is levels of nestedness, levels of parts within wholes, and Trend 1 is the addition of successive levels over time (Pettersson 1996; Valentine and May 1996; McShea 2001). The eukaryotic cell arose as an association of bacteria and therefore is one hierarchical level above a bacterium. A multicellular individual is one level above a eukaryotic cell. A colony or society is a level above a multicellular individual. We have rough dates for the first occurrences of these transitions in hierarchy (McShea 2001). The first bacterium can be dated at about 3.5 billion years ago, the first eukaryotic cell at 2.0 billion years, the first multicellular individual at 600 million years, and the first colonial organism at 480 million years ago. Figure 1 shows the trend trajectory (See McShea 2001; McShea and Changizi 2003).
Trend 1: the rise in hierarchy from the origin of life to the present. See text for discussion (Data from McShea 2001)
Some footnotes are needed. First, this is a trend in complexity but complexity only in one narrow sense, hierarchy (McShea 2001) or what Sterelny (1999) has called vertical complexity. In each transition, a new higher-level object formed, one consisting of a set of lower-level objects. Transitions in hierarchy are like the packing of a dozen eggs into a carton, the packing of a number of cartons into a larger box, and so on. And the trend is a rise in complexity only in this structural sense. There is no implication that higher-level objects are better adapted or more sophisticated.
Second, the trend is only in the maximum. The highest level of hierarchy present on Earth rose with each transition. And a rising maximum does not by itself indicate the existence of any upward tendency. Maxima are expected to rise in evolutionary systems whenever diversity increases, even if reversals were common, even if for example multicellular organisms frequently reverted to single-celled existence. In the terminology that has grown up around the study of trends in evolution, we might say that a trend in the maximum might have occurred passively, without any driving forces such as natural selection (McShea 1994, 1996; Gould 1996).
Third, this series of transitions is reminiscent of what Maynard Smith and Szathmáry (1995) have called the “major transitions” in evolution. However, they are not the same. For one thing, the transitions in hierarchy are defined consistently, all of them meeting a single common set of structural criteria (McShea 2001). No such consistent set of criteria exists for the major transitions (McShea and Simpson 2011). Also, the origin of human societies counts as a major transition, but on a hierarchy scale it does not register as special. We are multicellular individuals and our evolutionary transition to sociality was not the first, nor is the level of sociality we have achieved especially impressive. Many species—notably the bryozoan colonies that arose 480 million years ago—arose earlier and are more intensely social, more committed to sociality, than we are.
Finally, the trend in Fig. 1 does not take into account what might be called “degrees of hierarchy”, or the degree of “individuation” of the entities at each level. The first multicellular organism was likely just a collection of identical cells attached to each other (in the lineage leading to animals, perhaps something like a choanoflagellate). Highly individuated multicellular organisms like us came later. Or to make the same point at the next level up: corals and bryozoans are both colonies of multicellular individuals, but bryozoans are more individuated at the colony level than are corals. That is, bryozoans have some degree of differentiation among the multicellular units, the zooids, and even the colony equivalent of tissues and organs, both virtually absent in corals. In other words, a bryozoan colony is more of full-blown individual than a coral colony. Figure 1 shows first occurrences only of highly individuated organisms at each level. (For further discussion of the concept of individuation, see Boardman and Cheetham 1973. For a higher-resolution graph showing first occurrences of intermediate levels of individuation, see McShea 2001, McShea and Changizi 2003.)
Trend 2: Increasing Complexity
A second trend is the rise in number of part types or degree of differentiation among parts. A carton with a dozen eggs, 11 of them white and 1 of them brown, has two part types (ignoring the carton) and therefore has more part types than a carton of all whites or all browns. This is complexity too but in the sense of differentiation, more precisely, differentiation within a level. When variation is discrete, the measure is number of part types. When continuous, it is degree of differentiation among parts. This usage of the word complexity is increasingly becoming standard in biology (e.g., Doolittle 2012; Finnigan et al. 2012). It is has also been called horizontal complexity (Sterelny 1999) or “pure complexity”, to distinguish it from the colloquial usage (McShea and Brandon 2010).
The clearest instance of this trend is the rise in number of cell types over the past 540 million years within the animals, the metazoans. Valentine et al. (1994) counted cell types in a number of modern metazoans and plotted the numbers against the times of origin of the larger groups of which the moderns are a part. The graph is reproduced here in Fig. 2. Consider just the first data point, the sponges. A modern sponge has about 10 cell types and its larger group—the sponges generally—originated in the early Cambrian, about 540 million years ago (more likely a bit earlier, we now believe). In constructing the graph, the assumption was that the first sponges had about the same number of cell types as modern sponges. Other points are plotted using the same assumption. Overall, the graph shows a rise in the maximum number of cell types from sponges to humans. That is, if we think of cells as the parts of multicellular organisms, it shows a rise in the maximum number of part types at the cell level .
Trend 2: the rise in number of cell types in animals over Phanerozoic Eon. See text for explanation (Redrawn from Valentine et al. 1994)
We do not have similar documentation for an increasing trend in cell types in other multicellular groups, like plants and fungi. But the existence of such a trend in these groups is likely. Notice that horizontal complexity is always specific to a particular level, and so this trend tells us nothing about horizontal complexity at other levels. For example, it tells us nothing about what happens to the number of part types within cells—their nuclei, mitochondria, and such. In principle it is possible that as number of cell types rises, the number of part types within cells falls. (And in fact, that is exactly what seems to have happened. See Trend 3.)
What about the level of colonies or societies? At the colony level, the largest parts are bigger, more inclusive units than cells. They are the multicellular individuals that make up the colony. The parts of an ant are its cells, but the parts of an ant colony are the individual ants themselves. And therefore the horizontal complexity of a colony (measured at the level of individuals) is the number of types of individual. For a social insect colony that might be the number of castes or for a colonial marine invertebrate colony, the number of zooid types. Graphs analogous to Fig. 2 do not exist for colonial organisms, but there is plenty of circumstantial evidence for a trend. Coral colonies without any differentiation among individual coral polyps (zooids) pre-date bryozoans with multiple zooid types. Undifferentiated colonies are probably ancestral in all of the major social insect groups. Down at the level of bacteria, monomorphic filaments consisting of identical bacterial cells predate differentiated colonies with two cell types. And so on.
In any case, the enumeration of examples and even formal treatments like Valentine et al. are almost unnecessary, because there is an obvious and powerful logic that underlies the evolution of horizontal complexity generally. Organisms contain enormous redundancy, identical parts many times repeated. This is true at all levels of organization. A simple sponge contains many cells of the same type. A simple social insect colony contains many identical individuals. A simple marine invertebrate colony contains many zooids of the same type. And these parts are expected to differentiate spontaneously, simply on account of the accumulation of heritable accidents, mutations. A colony with only one zooid type is likely—with the accumulation of heritable accidents—to give rise to a colony in which the zooids differ from each other. This accumulation can be opposed by selection, and indeed is often massively opposed, because most spontaneous differentiation is expected to be unfavorable. And when this happens, parts and individuals can remain identical. But the tendency to differentiate persists, in all lineages at all times, awaiting the advent of advantageous or even merely neutral variants. The point is that monomorphy is in principle an unstable condition, tending spontaneously to “decay” into differentiation, that is, into horizontal complexity. Interestingly, the directional instability is still present, even after the first differentiated variants have arisen. Parts that are only somewhat different from each other will still tend to accumulate accidents, which in turn will tend to make them ever more different from each other. Identical twins become ever more different from each other as they age.
The underlying principle here has been called the zero-force evolutionary law or ZFEL (McShea and Brandon 2010). The ZFEL says that in any evolutionary system, complexity in the sense of differentiation will—in the absence of forces and constraints—increase on average, and further that even when forces and constraints are present, there will be a “tendency” for complexity to increase. This tendency, a kind of upward push, may or may not produce an actual trend. If sufficiently opposed by selection, it will not. But when selection against complexity is weak or absent, it will (McShea and Brandon 2010; Fleming and McShea 2013). Accidents will accumulate, with the result that horizontal complexity will rise.
Notice that while random variation can produce the ZFEL, there is no suggestion here that the resulting complex structures in organisms are random, nonfunctional, neutral, or even disadvantageous. The ZFEL does not deny selection. Indeed, the ZFEL can operate even if all change is selection driven. This is a critical and sometimes misunderstood point. Two initially identical parts in an organism, each subject to different and independent selection pressures, say the front and back teeth in a tooth row, will tend to become different from each other. For example, selection might transform a tooth at the front into an incisor, adapted for chopping, while a different and independent selection pressure transforms a tooth at the back into a molar, adapted for crushing. The teeth are not changing randomly, but they are changing randomly with respect to each other. And as a result, the complexity of the tooth row increases. That is the ZFEL. And the only requirement is that the two teeth evolve to some degree independently.
Trend 3: The Complexity Drain
At the same time that complexity is increasing, a deeper current flows in the opposite direction. The parts themselves are getting simpler. As the cells of multicellular organisms are becoming more and more different from each other, the cells are losing part types (McShea 2002). As colonies are becoming more complex, as the individuals that constitute them are getting more different from each other, the individuals themselves are losing internal complexity. Their complexity is being (partly) drained.
The most obvious reason is natural selection for streamlining, or in other words, for economy (McShea 2002). Consider the origin of multicellularity. In a free-living single-celled species, a protist, every cell must be omnicompetent. Every cell must be able to perform all functions necessary for survival and reproduction. The same is true in the early stages of multicellularity, the first step in the origin of a new level (Trend 1), in which the multicellular individual consists of multiple identical cells. Each cell must be able to feed, protect itself, metabolize, reproduce, and so on. However, as differentiation proceeds (Trend 2), that changes. As cells diversify into distinct types, they become specialized for particular functions, perhaps one type specialized for reproduction, another for defense, another for feeding, and so on. From the perspective of each cell, specialization is possible because certain functions are being taken over by the other cells, in other words, by the whole. As this process proceeds, selection favors a stripping down of specialized cells, a stripping down of their insides, a loss of redundant functionality, in the interest of economy. In extreme cases, certain cells in highly individuated multicellular organisms perform just a single function and lose all but a few of their parts, all but a few of their organelles and other internal structures. Human blood cells, for example, have lost all of their internal structures. They are about as stripped down, as simple, as possible (McShea 2002). (To be clear, if the parts of a multicellular organism are cells, Trend 3 is not a loss of cell types but rather a loss of parts within cells, of what might be called subparts.)
I will discuss the evidence for this shortly, but first an apparent problem needs to be addressed. The cells of multicellular individuals are reasonably discrete entities, the various types relatively easy to distinguish and count. One level up, the parts of colonies are reasonably discrete multicellular individuals, again with types easy to distinguish and count. In both cases, the parts are objects—bounded, separate, and distinct from other objects. But what about the parts of cells? Some organelles, such as chloroplasts and mitochondria, are object-like. But for other structures, such as junctions between cells, it is not so clear. Junctions are invested in and continuous with the cell membrane, raising the question of whether they constitute a part type distinct from the membrane. Within cells, many structures have this kind of continuity.
The problem is solvable in principle, and in many cases in practice. To solve it in principle, we can define parts as sets of elements that interact strongly with each other and less strongly with other elements outside the set (McShea and Venit 2001). Parts are entities that are relatively integrated internally and relatively isolated externally. The molecules in the cork in a wine bottle are tightly bonded to each other and weakly bonded to the molecules of the glass bottle that surround them. So the cork is a part. The label on the bottle is a part. So is the glass bottle itself. These are all parts of the entire unit consisting of the bottle, the cork, and the label together. This is an easy case, because it involves solid objects. But parts can be non-solid. At a cocktail party, a set consisting of me and the three other people I am talking to at some moment constitute a part, that is, a part of the party. The four of us are, at that moment, interacting strongly with each other and much less with others outside our group. It does not matter that 10 minutes later we might all be talking to other people. Parts can be temporary.
In biological systems, patterns of integration and isolation are sometimes hard to discern. A thigh bone is clearly a part of the organism but what about the rounded head on the thigh bone, at the point where it joins the hips. That rounded head has a name—the caput femoris—but having a name does not make it a distinct part. What matters is whether the bony elements that make up the caput femoris are more tightly connected to each other than they are to bony elements that connect them to the thigh bone. And that in turn depends on the pattern of molecular bonding in that part of the bone, on the response of those bonds to stress when the bone is stressed, etc. In other words, even given this relatively clear understanding of parts, demarcating them in organisms is often difficult in practice.
However, if we allow ourselves some assumptions, the difficulties can be reduced. We can use certain physical properties as signs that a boundary between parts is likely present, as indicators of probable parts. Physical separation is one such property. Objects that are physically separate from other objects—like the eggs in a carton—are likely well integrated internally and isolated externally and are therefore probable parts. In a eukaryotic cell, this includes mitochondria, chloroplasts, and the nucleus. Such parts are what I call “flee floating”. Then, objects that are physically in contact with others but differ from them in composition (as the cork differs from the bottle) are probably distinct parts, on the assumption that changes in composition correspond to reductions in connectedness. And in that case, an intercellular junction would count as a part of a cell, even though it is in contact with the cell membrane. These I call “compositional parts”. Finally, entities that are distinct in shape from their surrounds, as the head of the thigh bones differs from the rest of the bone, are probable parts on the assumption that changes in shape correspond to changes in the pattern of connectedness. On this assumption, a pseudopod of an Amoeba counts as a part of the organism, because it differs in shape—however temporarily—from the rest of the cell membrane. These are “shape parts”.
So here is the claim of Trend 3. As multicellular organisms become more complex, as they acquire multiple cell types, the complexity of the cells themselves decreases. The cells lose part types. The evidence for this trend, shown in Table 1, is a series of comparisons between cells in multicellular organisms, such as animals (metazoans) and plants, on the one hand, and free-living eukaryotic cells, or protists, on the other. The top left of Table 1 compares average number of part types within cells in metazoans (e.g., human melanocytes, molluscan sensory cells, sponge myocytes) with average number of part types within protistan cells (e.g., paramecium, red algal cells). The figure shows cell-part counts for three increasingly inclusive categories of parts (F = free-floating, FC = free-floating + compositional, FCS = free-floating + compositional + shape) plus a fourth category that includes structures whose status as parts is uncertain, questionable parts (FCS?). In all cases, the single-celled protists had more part types, on average. The same goes for a comparison between metazoan cells and a likely sister group—arguably, a near ancestor of the metazoans—the choanoflagellates (Table 1, top right). Choanoflagellate cells are solitary or live in undifferentiated colonies. And finally, comparisons between land plant cells and protistan cells (bottom left) and between land plants and their sister group, the chlorophytes (bottom right), yield essentially the same results: cells in multicellular organisms are simpler, on average. (See McShea 2002 for further explanation of methods and results.)
The absolute value of the numbers in Table 1 may be somewhat puzzling in that they are quite low. For example, the average for protists ranges from 1.69 to 5.54 [depending on the number of categories of parts included (F vs. FCS?)]. Surely, one might wonder, protists have more parts than that. In fact, they do. The reason for the low numbers has to do with the way the data were collected. Counts of parts were based on pictures (electron micrographs) and descriptions of cells in the primary literature, and many part types are not reported in that literature (i.e., they are absent from the photos and not mentioned in the descriptions). To accommodate this, the assumption was made that certain parts, like mitochondria and various membrane-bound vesicles, were present unless their absence was specifically mentioned in the description. In other words, every cell was assumed to have a certain “standard set” of parts, and all part counts for cells are totals over and above the standard set (see McShea 2002 for a list). So the protistan total of 5.54 is actually average number of part types in addition to the standard set.
Table 1 is about the transition to multicellularity. But Trend 3 likely occurs in hierarchical transitions at all levels, including the transition from solitary multicellular individual to society or colony. The evidence here is more anecdotal and requires some assumptions but is compelling nonetheless, I think. Among the social insects, the larger, more individuated colonies with greater numbers of castes have smaller individuals than smaller less-individuated colonies (Anderson and McShea 2001). Also, in the more-individuated colonies, individuals in each caste are more specialized, typically with smaller behavioral repertoires (C. Anderson, personal communication) and therefore arguably have fewer brain structures—parts—that produce behaviors. Some even have fewer organs, for example female worker individuals lacking ovaries. The evidence is more direct in bryozoan colonies. In colonies with greater morphological differentiation among zooids, the average number of anatomical part types per zooid is lower (McShea and Venit 2001).
The same simplification seems to have occurred at the lowest hierarchical levels, in the transition from solitary bacterium to eukaryotic cell. Again the evidence is anecdotal but strongly suggestive of Trend 3. The mitochondria in eukaryotic cells contain fewer genes than the free-living eubacteria from which they evolved. Some of those genes are known to have been transferred in the course of evolution to the nucleus. Or in present terms, the ability to perform certain functions was transferred from lower-level individuals (mitochondria) to the whole (the eukaryotic cell), and assuming number of functions is correlated with number of parts, the number of parts in those individuals can be expected to have declined.
Interestingly, a similar sort of transformation has been proposed recently in bacterial communities, putatively explained by what is being called the Black Queen hypothesis (Morris et al. 2012). The hypothesis arose from the observation that a loss of genes and of the ability to produce certain critical metabolic substances occurs in some bacterial species in open-ocean communities. This can occur when the metabolic substances are “leaky”, meaning that they leak out of the species that produce them and thereby become available to the entire bacterial community. The hypothesis proposes that selection favors loss in other species of the ability to produce these substances, in those species that can then live off the leakage and survive without incurring the cost of production. The Black Queen hypothesis invokes only selection acting on individuals, but nothing in the logic of the argument prohibits the involvement of selection at the level of bacterial associations—a bacterial superorganism—as well. Those bacterial associations that partition the costs of metabolism among specialists might have an advantage over other communities that do not—simply on account of the presumed advantages of the division of labor—leading to a reduction in functional capability and presumably a loss of part types in the specialists. To the extent that this is the case, this would be an example of Trend 3: selection on a whole favoring the reduction in complexity within the parts as they specialize.
Finally, a note on cross-level analysis: hierarchical explanation generally requires a minimum of three levels, a focal level plus the next level up and the next level down (Salthe 2012). The next level up is what might be called the context, while the next level down is where mechanism resides. The story of Trend 3 implicitly invokes three levels. The focal level is the cell level, the context is the multicellular individual within which the cell evolves (the next level up), and the mechanism of the trend is the loss of parts within cells (the next level down). Now, notice how the story changes when we shift the focus to the level of the multicellular individual. The next level up is now the ecological context within which the individual evolves, and the next level down is the individual’s cells. And what we see here is the increase in complexity of the individual, driven (perhaps) by selection at the next level up for division of labor, and explained mechanistically by the increase in cell types at the next level down. This is Trend 2. The point is that the two trends could be different aspects of same process, viewed at two different hierarchical levels. At least, in principle they could be. It could also be that they have independent causes. I turn to the problem of causes in the next section.
An Evolutionary Syndrome
One well-known story about the evolution of complexity is nonsense. The story says that complexity in some broad sense increases in evolution. Early organisms were single cells, and they were simple. Single cells persist in the modern world but now we also have organisms with sophisticated eyes, brains, and immune systems. And the sophistication of these structures is what makes these modern organisms complex. The story is entertaining but from a scientific perspective it is nonsense, because no one has the slightest idea what complexity in the sense of sophistication means. The same goes for complexity in the sense of “advancement” or “excellence” or any number of other terms that seek to capture what is so special about certain modern organisms, what is so special about us. At present, there is no way to objectively assess whether a human is more complex than a bacterium in any of these senses. We are certainly bigger. And hierarchically deeper, as discussed earlier. We have more genes. We compose music and play tennis while they do not. Interestingly, it seems we also have a greater energy density, or energy usage per mass (Chaisson 2010). But no trend has been demonstrated in sophistication, advancement, or excellence. Complexity in this broad, colloquial sense is simply not an objective, measurable variable.
The problem of defining and measuring broad-sense complexity—or colloquial complexity—has been with biology for over a century. Only a few biologists have addressed it directly. It hovers in the background, moving to the foreground occasionally in popular books on evolution and in questions raised by students and by the public (Ruse 1996). My own view is that it is time for biology to concede that broad-sense complexity is not a scientific concept and that therefore questions about it are poorly posed, not answerable even in principle. And if we are going to use the word complexity—and it is arguable that we should not, given its long and unproductive history—we need to define it narrowly, in a way that allows us to apply it scientifically, to assess objectively whether one organism is more complex than another.
Here, two narrow structural definitions are invoked, both widely accepted in biology. One is based on hierarchy: complexity as number of levels of nestedness, or vertical complexity. And the other is based on differentiation: complexity as number of part types at a given level, or horizontal complexity. Both are measureable in any number of particular cases, as we have seen. And using them, we are now in a position to say something meaningful about complexity in the history of life. Here is what seems to be going on, summarized in Fig. 3. First, organisms are arising with an ever greater numbers of levels of nestedness, greater vertical complexity. Bacteria associated to form the eukaryotic cell, one level up from bacteria. Eukaryotic cells associated to form multicellular individuals, two levels up. And multicellulars formed colonies, three levels up. Hierarchically shallow organisms persist in the modern world, but hierarchically deeper ones have arisen. There has been a trend in the maximum (Trend 1).
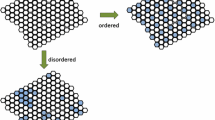
A schematic representation of the three trends, which together constitute an evolutionary syndrome (see text). The figure shows the transformation of a free-living lower-level entity (left: medium-sized circle) into a poorly individuated higher-level entity (middle: an undifferentiated collective consisting of seven identical lower-level entities), and then into a highly individuated higher-level entity (right: large circle with seven former lower-level entities as parts). This is Trend 1, an increase in hierarchy. At the same time, the number of types of medium-sized circle increased from 1 to 4. (In the middle there is only one part type—the seven identical lower-level entities are one type—while on the right, there are four. Starting from the 9 o’clock position, medium-sized circles 1, 2 and 5 are one type, 3 and 4 are a second type, 6 is a third, and the one in the center is a fourth.) In other words, there was an increase in horizontal complexity. This is Trend 2, an increase in number of part types. Finally, notice that the free-living lower-level entity on the left has four subpart types, the groups of tiny circles within it, with the four types distinguished by the four shades of gray. On the right, each of the seven parts of the higher-level entity has fewer (clockwise from the 9 o’clock position, 2, 2, 0, 0, 2, 3, and then 1 for the part in the middle). This is Trend 3, the loss of subparts within the parts, the complexity drain
Second, that trend in hierarchy seems to have been accompanied by a trend in differentiation at the level just below the whole (Trend 2). As new higher levels have arisen, their parts have been more differentiated, and the organisms themselves therefore more complex in a horizontal sense. In multicellular individuals, number of cell types has risen. In colonies, number of types of individual or zooid has risen. (An interesting but unaddressed question is whether the parts—the organelles—of free-living eukaryotic cells, protists, have become more differentiated over time.) As for hierarchy, this trend appears as a rise in the maximum, in the case of animals as a rise in the maximum number cell types.
Third, as the first two trends have proceeded, there has been a hollowing out of the parts themselves. The former bacteria that anciently associated to form the eukaryotic cell have lost parts and become simpler. The cells of multicellular organisms have become simpler. And the individuals in colonies have lost parts—organs, tissues, cell types, and even behaviors (and presumably the neural structures that generate them)—becoming horizontally simpler in the process (Trend 3).
Causes and Trend Mechanisms
The causes of these trends are partly known and partly not. Consider Trend 1. It seems almost inevitable that body size rises with hierarchy, raising the possibility that this trend is driven by selection for large size. [I say “almost inevitable” because another known trend is the accompanying decrease in size of the lower-level units, working against the size increase of the whole (Anderson and McShea 2001). Still, the net result is usually increase.] On the other hand, if selection is the driving force, it is a weak driving force, or else the path is obstructed by serious constraints. The origin of a eukaryotic-cell-grade colony of bacteria required over a billion years (starting the clock at the origin of life) and has not happened again since, so far as we know. (Of course, the study of bacterial biofilms is in its infancy, and may yet uncover some candidates.) Multicellularity arose many times within the eukaryotes, as did coloniality among the multicellulars, but there have been reversals as well, that is, returns to solitary living. And the substantial number of known reversals raises the possibility that while selection may favor increase sometimes, it might favor decreases just as often, leaving no net advantage to hierarchy. And in fact, one study of this problem (Marcot and McShea 2007) found exactly that, equal numbers of increases and decreases in hierarchy. If that is right, then the trend in the maximum is not driven, but is instead the result of passive spread of a diversifying system away from a bacterial lower boundary (McShea 1994; Gould 1996).
For Trend 2, horizontal complexity, the problem of causes has been considered from a number of different angles. First, it has been argued that horizontal complexity should be favored by selection (Bonner 1998, 2003) on account of the advantages of division of labor. The argument is that a group of differentiated specialists can perform tasks more efficiently than a group of identical generalists. Also, with increases in body size, some division of labor becomes imperative. For example, small organisms can rely on diffusion to feed and breathe but large ones need a circulatory system, which in turn requires novel part types. Both arguments suggest an increase in complexity driven by selection.
On the other hand, Valentine et al. (1994) argue that the rise in number of cell types in animals had no driving force, that selection favored losses of cell types as often as it favored gains. And indeed, studies of horizontal complexity in parts larger than cells often find no drive toward gains (e.g., for vertebral columns, McShea 1993). If this argument and finding are general, if gains and losses are equally common, then the rise in the maximum for horizontal complexity is passive, the result of the passive spread of the group away from a complexity minimum (as perhaps for hierarchy) (McShea 1994). At present, little is known about the relative frequency of selection-driven increase and decrease in horizontal complexity. Cases of driven increase are known (e.g., in arthropod limb differentiation, Adamowicz et al. 2008), but so are cases of long-term driven decrease (Sidor 2001).
Both arguments sound reasonable, but they are incomplete in that we need in take into account the zero-force law, the spontaneous tendency for parts to differentiate. The ZFEL says that in the absence of selection and constraints, complexity in the sense of differentiation should rise in all lineages at all times. Obviously this is not what we see. Modern sponges are probably as simple as ancient ones. Complexity rises in some lineages, but also decreases, and simple forms persist. Therefore, some factor needs to be invoked to oppose the ZFEL. The obvious candidate is selection, massive and pervasive selection against complexity, or just against change, stabilizing selection. Another possibility is pervasive developmental constraint, resisting the ZFEL at almost every turn.
Looking again at the hypotheses above, we are left with two possibilities: (1) a driven trend with the ZFEL and selection-favoring-complexity both pushing complexity upward (combining Bonner’s view with the ZFEL), while stabilizing selection and constraints oppose them and cancel them out (leaving the Valentine et al. pattern, roughly equal numbers of increases and decreases); or (2) the ZFEL alone pushing complexity upward, while selection and constraints together oppose the ZFEL, overwhelming and cancelling it out (again leaving the Valentine et al. pattern).
For Trend 3, the loss of complexity within parts, I can think of only one plausible story at this point: loss driven by selection for streamlining. As parts become specialized, much of their internal machinery becomes nonfunctional and will tend to be removed by selection for economy. The story makes sense, but sadly there is at present no positive evidence for it.
The Syndrome
It is tempting to put together the three trends into a causal cascade, played out in time. The whole trajectory might begin with a rise in hierarchy, with the origin of a new level. Then, as a new level arises, the lower-level units differentiate, either on account of the ZFEL or the selective advantages of the division of labor or both. Finally, as differentiation proceeds, selection favors the stripping down of the lower-level units in the interest of economy. However natural this causal tale might sound, it is just that, a tale, and alternatives can be imagined. Indeed, how much more intellectually satisfying it would be to discover that all three trends occur together and share a common cause, some fourth factor still unknown. At present, in the absence of a demonstrable fourth factor, and in the absence of clear evidence, I am inclined to leave the question of causes unanswered. And so, while awaiting further evidence, it may be best simply to think of the three trends as a kind of syndrome, a combination of “symptoms” associated with the rise of hierarchy, perhaps with a single underlying—but still unknown—cause.
Autonomy and Machinification
I have described this three-trend syndrome from the perspective of complexity. But there is another way to understand it, in terms of what I call “machinifcation” and its near opposite, what Rosslenbroich (2014) in his recent book calls “autonomy”.
Autonomy
Rosslenbroich’s treatment comes out of the mid-twentieth century “systems biology” of Weiss, Bertalanffy, Wiener, Ashby, Rashevsky, Rosen, and others as well as later discussions of autopoesis pioneered by Maturana and Varela (1987). At the heart of this discourse is a notion of an organism as an entity, a whole, that arises from its parts, and that simultaneously governs the behavior of those parts. Parts and whole are both cause and consequence of each other. This back and forth between parts and whole sets the entity apart from its environment, to some extent, a separation that creates a kind of a “self” that is distinct from its environment and that has the robustness to persist in the face of changes in that environment, in other words, autonomy. In organisms, autonomy is achieved in part by boundaries, which insulate it, and further by the entity’s ability to respond to environmental changes with internal changes that compensate. Rosslenbroich is interested in evolution, in particular in the notion that autonomy increases over time. And he lists some of the phenotypic indicators of increased autonomy: (1) increase in body size; (2) increase in physical separation from the environment; (3) increase in homeostatic capacity; (4) internalization of structures and/or functions; and (5) increase in flexibility of responses, including greater behavioral flexibility.
In this framework, the origin of a new level and the differentiation of its parts constitutes a clear—even paradigmatic—case of increasing autonomy. For example, the transformation of a single-celled protist into a multicellular individual produces an increase in body size (indicator #1), moves many of the cells inside the organism, buffering them from the environment (indicators #2 and #3) and enabling them to specialize. A consequence is that functions like metabolism formerly performed by a protist in diffusional contact with the environment, are now performed internally, in relative isolation (indicator #4). Finally, moving functions inside allows for greater internal control over them, setting the stage for the evolution of control systems, which in turn leads to an increase in the variety of behavioral options and therefore to greater responsive flexibility (indicator #5). Arguably the same is true of all transformations in hierarchy: one level down, in the transformation from bacterium to eukaryotic cell, and one level up, in the origin of colonies and societies from multicellular individuals.
Rosslenbroich would describe Trends 1 and 2 as increases in autonomy, and they are. With the emergence of a new level (Trend 1), and the specialization of its parts (Trend 2), the whole gains autonomy. However, I would add that as a result of the complexity drain (Trend 3), there is also a loss of autonomy at the level of the component individuals. The cells of a multicellular organism have less autonomy than a free-living protist. And the hierarchical transition is—in addition to representing an increase in autonomy at the level of the whole—also a shift in autonomy, upward, away from the parts. As the whole comes into existence and becomes autonomous, it transforms from a non-entity into an organism. It becomes organismal. And at the same time the parts are stripped down, rendered inflexible and machine-like, or in my terms, “machinified”.
Machinification
Typically, machines are brittle. A single part breaks, and the whole is at least partly disabled. And the disabling is usually permanent, at least without the assistance of a repair person. A bent needle on a sewing machine is fatal to the operation of the machine. Machines do not self-repair. Also in machines, typically the relationships among the parts do not change. The pistons, driveshaft, and wheels of a car are connected to each other in a particular way. They do not, even temporarily, find themselves in some other relation. Or if they do, it is fatal. And indeed, the rigidity of the parts is partly a design strategy for preventing part relationships from varying much. Also typically in machines, errors in construction are not just rarely advantageous. They are essentially never advantageous (Wagner and Altenberg 1996). A laptop cpu that suffers an accident, a mutation, and that does not function according to specs, is never a better-performing cpu. Machines do not adapt. They do not evolve, in the usual sense of the word. I say “typically” for all of these limitations because, of course, in some cases they can be partly overcome. Redundancy, self-repair, flexibility in connectedness, and even the capacity to evolve can be engineered into the design, to some degree. But of course, the redundancy and flexibility are accomplished by devices that are themselves machines and are therefore quite brittle. So the odds of fatal error may be reduced, but the fundamental limitations arising out of machine architecture remain.
The virtues of machines include what might be called their effectiveness, their reliability, or sometimes, their energetic efficiency. And these virtues arise from the same qualities that account for their deficiencies. For example, the solidity of the parts reduces the amount of wasted motion. Compare the action of a sewing machine—every motion furthering the project of joining two pieces of fabric—to a human seamstress doing the same job. The stability of the relationship among parts also limits wasted motion and further guarantees smooth interactions among parts. Every part’s interaction with other parts is by design. And the failure of machines to evolve means that they can always be relied on to function in the contexts for which they were designed. If I leave the vacuum cleaner in the closet for 6 months, there is no danger that it will evolve into a machine that is specialized for cleaning closets, losing its ability to clean carpets. Overall, machines work well largely because the contexts in which they operate are consistent, reliable, stable. Vacuum cleaners work well in the home because the home environment has certain consistent features that the vacuum cleaner is designed to tolerate and work with. Change the context, even a little, and the machine functions poorly. Home vacuum cleaners do not work well on a lawn. Family cars do not work well off the road. For a machine, stability of context is crucial. And to provide that stable context, we—also part of the context—go to great lengths: for cars, building and repairing roads. Another way to say this is that our machines are effective, reliable, and efficient so long as they have us to repair them and to engineer a stable interface with their environment. Most machines are not very autonomous. A machine requires an organism.
In contrast, the great virtue of organisms is their suppleness, their indifference to the details of the physical arrangement of their parts, their flexibility, their capacity to deal with a wide range of environments. A tangled bobbin is death to a sewing machine. But I can be knocked senseless or I can throw out my back, and if the concussion or dislocation are not too severe, the system eventually restores itself. Organisms are also supple in dealing with novel contexts. Unlike a car, a starfish can propel itself on its tubefeet over virtually any solid surface with which it come into contact (including the vast majority of surfaces that were never present in its evolutionary history). Starfish do not need roads. In sum, organisms—unlike machines—boast not only a great capacity for self-repair but a high level of indifference to their environments. They are autonomous.
The three-trend syndrome can now be restated in these new terms. As higher-level wholes emerge, they become organismal and their autonomy rises. And at the same time, the autonomy of their formerly autonomous parts falls. Their parts are transformed into machines. Molecular mechanisms within bacteria in the association that led to the eukaryotic cell; cells within multicellular associations that led to the first multicellular individuals; individuals within societies in the emergence of superorganisms—all these lose their suppleness, their flexibility, their capacity to deal with a wide range of environments. The driving force behind these losses was described earlier as selection on the whole favoring the greater economy of using simpler parts, the advantages of streamlining. But in present terms, we can see that the process has other aspects. The reduction in complexity is doubtless favored by the advantages to the whole of using machine parts, the efficacy and reliability of those parts. In terms of autonomy, there is another advantage: autonomous parts are dangerous to the whole. Parts with too much autonomy, with a capacity to go their own way, pursuing their own advantage, can destroy a whole. Thus, the machinification of parts is also a defensive strategy. (Of course, the strategy is not always completely successful. For example, multicellular wholes require cells that can reproduce, so a complete machinification of the cells is not possible, with the result that the whole remains vulnerable to their occasional autonomous behavior: cancer.)
It is worth noting perhaps that machinification is not restricted to organismal hierarchies, to emerging wholes as we ordinarily think of them. Some degree of machinification must also occur whenever organisms move into stable ecologies, where less suppleness and flexibility are demanded: extreme specialists, parasites, and pets.
Some Unsupported Speculation
The degree to which the previous discussion applies to humans and human societies—the degree to which we as individuals have been machinified—is an open question, one that opens up a number of entertaining lines of argument. One possibility is that there is a trade-off, paralleling the evolutionary one, between the wholeness, the organismness, of human society and the degree of machinification of the individual. Thus, in human history, the emergence of a complex societies may initially require greater complexity in the individual. Consider the staggering complexity of early social life—all those other people to deal with! But as the process proceeds, as a society emerges as an autonomous entity, as a quasi-organism, the lives of the individuals within it might become regularized, simplified, drained of the great complexity that must have characterized, say, hunter-gatherer life. Living within larger organic wholes, we become domesticated, and to some degree machinified, losing much of our flexibility, our robustness to environmental change, our autonomy. Alternatively, it could be that we retain these primitive capacities, that they become excess capacity, so to speak, in many contexts accounting for our tremendous creativity in technology, science, and the arts. Of course, this excess capacity is also diverted sometimes into unconstrained self-indulgence, paralyzing self-analysis, and entertaining but otherwise useless hyper-intellectualized forms of creativity (like this paper). In other words, excess capacity is also the ability to make unnecessary trouble for ourselves. And in this light, machinification may be a virtue.
An Unhelpful Conclusion
The finding of a syndrome often raises more questions than it answers, creates more uncertainty than it eliminates. It is true that the first trend, the increasing hierarchy maximum (rising vertical complexity), is about certain as can be. Over time, hierarchically deeper organisms have arisen. And the second and third trends are fairly certain. Within the animals, the degree of differentiation among parts within a new level increased (rising horizontal complexity at the level of parts) and those parts lost some of their autonomy, becoming somewhat machinified (falling horizontal complexity within parts). However, these trends have not yet been studied systematically in other multicellular groups (e.g., plants, fungi), or in the emergence of other hierarchical levels (i.e., in associations of bacteria or in nascent colonies and societies). Further, we know very little about causes, very little beyond the obvious just-so stories about what selection could be expected to be favored. Finally, notions like autonomy and machinification are quite useful, I think, for evolutionary studies in its speculative mode. But for real progress, we need to be able to apply them in hypothesis-testing mode, and for that we need to operationalize them.
References
Adamowicz, S. J., Purvis, A., & Wills, M. A. (2008). Increasing morphological complexity in multiple parallel lineages of the Crustacea. Proceedings of the National Academy of Sciences, 105, 4786–4791.
Anderson, C., & McShea, D. W. (2001). Individual versus social complexity, with particular reference to ant colonies. Biological Reviews (Cambridge), 76, 211–237.
Boardman, R. S., & Cheetham, A. H. (1973). Degrees of colony dominance in stenolaemate and gymnolaemate Bryozoa. In R. S. Boardman, A. H. Cheetham, & W. A. Oliver Jr (Eds.), Animal colonies: Development and function through time (pp. 121–220). Pennsylvania, Dowden: Hutchinson & Ross.
Bonner, J. T. (1998). The origins of multicellularity. Integrative Biology: Issues, News, and Reviews, 1, 27–36.
Bonner, J. T. (2003). On the origin of differentiation. Journal of Biosciences, 28, 523–528.
Chaisson, E. J. (2010). Energy rate density as a complexity metric and evolutionary driver. Complexity, 16, 27–40.
Doolittle, W. F. (2012). A ratchet for protein complexity. Nature, 481, 270–271.
Finnigan, G. C., Hanson-Smith, V., Stevens, T. H., & Thornton, J. W. (2012). Evolution of increased complexity in a molecular machine. Nature, 481, 360–364.
Fleming, L., & McShea, D.W. (2013). Drosophila mutants suggest a strong drive toward complexity in evolution. Evolution & Development, 15, 53–62.
Gould, S. J. (1996). Full house: The spread of excellence from Plato to Darwin. New York: Harmony.
Marcot, J. D., & McShea, D. W. (2007). Increasing hierarchical complexity throughout the history of life: Phylogenetic tests of trend mechanisms. Paleobiology, 33, 182–200.
Maturana, H. R., & Varela, F. (1987). Tree of knowledge. Boston: Shambhala Publications.
Maynard Smith, J., & Szathmáry, E. (1995). The major transitions in evolution. New York: Oxford University Press.
McShea, D. W. (1993). Evolutionary changes in the morphological complexity of the mammalian vertebral column. Evolution, 47, 730–740.
McShea, D. W. (1994). Mechanisms of large-scale evolutionary trends. Evolution, 48, 1747–1763.
McShea, D. W. (1996). Metazoan complexity and evolution: Is there a trend? Evolution, 50, 477–492.
McShea, D. W. (2001). The hierarchical structure of organisms: A scale and documentation of a trend in the maximum. Paleobiology, 27, 405–423.
McShea, D. W. (2002). Complexity drain on cells in the evolution of multicellularity. Evolution, 56, 441–452.
McShea, D. W., & Brandon, R. N. (2010). Biology’s first law. Chicago: University of Chicago Press.
McShea, D. W., & Changizi, M. A. (2003). Three puzzles in hierarchical evolution. Integrative and Comparative Biology, 43, 74–81.
McShea, D. W., & Simpson, C. G. (2011). The miscellaneous transitions in evolution. In B. Calcott & K. Sterelny (Eds.), The Major Transitions in Evolution Revisited. Vienna Series in Theoretical Biology (pp. 19–33). Cambridge, MA: The MIT Press.
McShea, D. W., & Venit, E. P. (2001). Testing for bias in the evolution of coloniality: A demonstration in cyclostome bryozoans. Paleobiology, 28, 308–327.
Morris, J. J., Lenski, R. E., & Zinser, E. R. (2012). The black queen hypothesis: Evolution of dependencies through adaptive gene loss. mBio, 3(2), e00036–e000112.
Pettersson, M. (1996). Complexity and evolution. Cambridge: Cambridge University Press.
Rosslenbroich, B. (2014). On the origin of autonomy: A new look at the major transitions in evolution. New York: Springer.
Ruse, M. (1996). Monad to man: The concept of progress in evolutionary biology. Cambridge: Harvard University Press.
Salthe, S. N. (1985). Evolving hierarchical systems. New York: Columbia University Press.
Salthe, S. N. (2009). A hierarchical framework for levels of reality: Understanding through representation. Axiomathes, 19, 87–99.
Salthe, S. N. (2012). Hierarchical structures. Axiomathes, 22, 355–383.
Sidor, C. A. (2001). Simplification as a trend in synapsid cranial evolution. Evolution, 55, 1142–1419.
Simon, H. A. (1962). The architecture of complexity. Proceedings of the American Philosophical Society, 106, 467–482.
Sterelny, K. (1999). Bacteria at the high table. Biology and Philosophy, 14, 459–470.
Valentine, J. W., Collins, A. G., & Meyer, C. P. (1994). Morphological complexity increase in metazoans. Paleobiology, 20, 131–142.
Valentine, J. W., & May, C. L. (1996). Hierarchies in biology and paleontology. Paleobiology, 22, 23–33.
Wagner, G. P., & Altenberg, L. (1996). Perspective: Complex adaptations and the evolution of evolvability. Evolution, 50, 967–976.
Wimsatt, W. C. (1974). Complexity and organization. In K. F. Schaffner & R. S. Cohen (Eds.), Philosophy of science association 1972 (pp. 67–86). Dordrecht: D. Reidel.
Wimsatt, W. C. (1976). Reductionism, levels of organization, and the mind-body problem. In G. G. Globus (Ed.), Consciousness and the brain (pp. 205–267). New York: Plenum Press.
Wimsatt, W. C. (1994). The ontology of complex systems: Levels of organization, perspectives, and causal thickets. Canadian Journal of Philosophy, 20(supp), 207–274.
Wimsatt, W. C. (2007). Re-engineering philosophy for limited beings: Piecewise approximations to reality. Cambridge: Harvard University Press.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
McShea, D.W. Three Trends in the History of Life: An Evolutionary Syndrome. Evol Biol 43, 531–542 (2016). https://doi.org/10.1007/s11692-015-9323-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11692-015-9323-x