Abstract
Purpose
It is not clear that Blastocystis remains without damage to the digestive tract or has a pathogenic effect in relation to subtypes in immunocompromised people, such as cancer patients. The present study aimed to investigate the frequency and subtype distribution of Blastocystis in cancer patients who were followed-up and treated in the Oncology clinic of Firat University Hospital and to determine the clinical signs of infected sufferers.
Methods
201 patients aged ≥ 18 with a diagnosis of cancer were enrolled in this cross-sectional study. Patients’ stool samples were examined between September 2017 and August 2019 by native-Lugol, trichrome staining. Microscopy-positive stool samples were subjected to DNA isolation and subtyped by Sequence Tagged Site (STS)-PCR analysis. The symptoms and demographic characteristics of the patients were also evaluated.
Results
Totally, 29 (14.4%) samples were positive for Blastocystis after all methods. 15 (51.7%) out of 29 samples were successfully subtyped by the sequenced-tagged site(STS)-PCR, while 14 (48.3%) could not be typed. Three subtypes of Blastocystis were detected: ST3 (40%), ST2 (33%), ST1 (20%), and one mixed infections with ST1/ST2 (6%). There was no statistically significant difference in terms of clinical findings and demographic characteristics.
Conclusion
The outcomes of our study promote the idea that Blastocystis could be an asymptomatic and harmless commensal organism. However, more comprehensive molecular and clinical studies are needed to fully determine the pathogenicity and epidemiology of Blastocystis in cancer patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Blastocystis is an anaerobic unicellular eukaryotic parasite located in the large intestine that can be commonly found in humans and animals that is associated with various gastrointestinal and extraintestinal disorders [1, 2]. Blastocystis has been classified in the phylum Stramenopiles [3,4,5]. At the morphological level, four main forms of Blastocystis have been identified, including the vacuole, granular, multi-vacuolar, and ameboid form, by culture and direct microscopy (DM) [6]. The prevalence of Blastocystis has been reported to be between 1.5 and 20% in developed countries and 30–60% in developing countries worldwide [7, 8]. In a recent study on children living in rural areas of Senegal, the prevalence of the parasite was found to be 100% [9]. This has been related to unsuitable substructure conditions, low sanitation, contact to animals, and uptake of contaminated food or water [3, 10]. Recently, Blastocystis prevalence has been determined at rates ranging from 1.4–23.5% in studies in Turkey [1].
Blastocystis has a highly polymorphic genome and there are many genotypes called subtype (ST) by molecular phylogenetic analyzes [11]. One of the two commonly preferred approaches to identify Blastocystis subtypes is the partial sequencing of the small-subunit ribosomal RNA (SSU-rRNA) encoding gene, and the other is the sequence-tagged site polymerase chain reaction (STS-PCR), using primers specific to the subtypes [12]. Recently, there have been 17 established subtypes, along with possibly five new subtypes (ST21, ST23–26), based on the SSU rRNA gene analysis of Blastocystis that were mostly identified in domesticated and animal wildlife species [7, 12,13,14,15]. Although the host specificity of each subtype remains unclear, reporting of nine of the 22 subtypes, ST1-ST8 and ST12, in both humans and animals indicates the zoonotic transmission of this parasite [14, 16]. It has been revealed that ST9 is only isolated from humans [17]. ST1-ST4 includes more than 90% of all reported subtypes. Besides, these four subtypes have been reported more widely in humans than in other hosts. Other subtypes (ST5–ST9) are uncommon in humans [18]. ST3 was reported to be the most common in human Blastocystis isolates [19].
Although the presence of Blastocystis in both symptomatic and asymptomatic patients leads to a dilemma as to whether it is a pathogen, the parasite is now accepted as a pathogen as well as a parasite included in “Water Sanitation and Health Program” of the World Health Organization [20, 21]. Blastocystis pathogenicity and clinical findings have been tried to be explained with many factors. The most important of these are subtypes, presence of ameboid form, and parasitic load [5, 6]. Despite conflicting results between subtypes and clinical findings, ST1, ST4, and ST7 are generally reported to be associated with symptoms, while ST2 and ST3 are reported as nonpathogenic subtypes [22].
Blastocystis has also been suggested to be an opportunistic pathogen in transplant recipients, acquired immune deficiency syndrome (AIDS) and cancer patients [23]. Blastocystis studies on ST distribution and pathogenic roles in immunocompromised individuals have been limited to AIDS patients, and there are quite insufficient studies related to these parameters in other immunocompromised individuals such as cancer patients [7, 23]. A study with cancer patients revealed that the infection indicated itself with abdominal pain, diarrhea, and bloating [24]. Subtypes 3 and 4 isolates were reported to be quite common in cancer patients [22, 23, 25]. In another study conducted with cancer patients with Blastocystis subtype distribution in Turkey, ST3, ST1, and ST2 were found to be the most common subgroups, respectively [7]. Chandramathi et al. reported that Blastocystis had also been in breast and colorectal cancer patients receiving chemotherapy. In the light of they demonstrated that the parasite can resist high cytotoxic drugs and increases colonization by weakening the immunosuppressive state induced by chemotherapy [26]. In a study, severe Blastocystis infection was detected in four people with bowel obstruction due to neoplasm. In addition it was recommended in the same study that the intestinal obstruction and concomitant stool retention, in addition to hemorrhage from the neoplasm, may have allowed hypergenesis of Blastocystis [7]. All these findings suggest that the parasite develops in cancer patients [22].
Until now, no studies have been conducted on the distribution of Blastocystis subtypes in the province of Elazığ located in southern part of Turkey. This is the first study investigating the subtype distribution of Blastocystis in cancer patients in our region. In the light of the information presented above, the main aim of the present study was to determine the frequency and subtype distribution of Blastocystis in cancer patients. The present study also aims at investigating their relation to demographic factors and the clinical symptoms of Blastocystis infection.
Materials and Methods
Design of Experiment
The design of the experiment is shown in Fig. 1.
Sample Collection and Microscopical Examination
This cross-sectional study was carried out between September 2017 and August 2019. Stool specimens of 201 cancer patients (one each) were collected from the Medical Oncology Department of Fırat University Faculty of Medicine, Elazığ, Turkey. Written informed consent was obtained from each of the cancer patients receiving inpatient and outpatient treatment. Each patient was filled a form including demographic information, patient oncological characteristics, and gastrointestinal symptoms. These cancer patients were diagnosed with breast cancer, lung cancer, gastrointestinal cancer, ovarian cancer, and other cancers. These samples were examined by DM with saline and Lugol’s iodine and using trichrome staining. Besides, the remaining fresh stool samples were stored at − 20 °C until DNA isolation.
DNA Extraction
Total DNA from stool samples was conducted using the Presto™ Stool DNA Extraction Kit (Genaid, Taiwan) in line with the manufacturer's instructions. Briefly, stool samples homogenization and lysis were performed with Bead Beating Tube and ST1 Buffer. ST2 Buffer was added to the removal step of PCR inhibitors. Residual inhibitors were further removed by passing through a specialized PCR inhibitor removal column. The flow-through was saved in the 2 ml centrifuge tube for DNA Binding. ST3 Buffer was added to flow-through and then was placed a GD Column in a 2 ml Collection Tube. The sample mixture was added to GD Column for DNA binding. It was added to Wash Buffer for removal of contaminants while DNA remains bound to membrane. The dry GD Column was transferred to a new 1.5 ml microcentrifuge tube and pre-warmed elution buffer was added. The resultant DNA was stored at − 20 °C until PCR analysis.
Detection of Subtypes Using PCR Method
In this study, SSU rDNA (barcoding) PCR was performed with to demonstrate the presence of the barcode region of Blastocystis [3, 4]. Furthermore, it was demonstrated with sequence-tagged site-PCR using seven pairs of the sequence-tagged site (STS) to investigate the presence of seven subtypes of Blastocystis [27]. The primer pairs names, sequences, and predicted product sizes used in this study are shown in Table 1.
PCR targeting 600 bp product of Blastocystis SSU-rDNA barcoding region was performed as described using the primers BhRDr and RD5 [12]. The 20 μl reaction mixture included 2.5 μl template DNA, the primers (0.5 μM each), 1U Taq DNA polymerase (Thermo Scientific) 200 mΜ dNTPs, 3.5 mM MgCl2 and 1 × Taq reaction buffer. PCR was carried out in Arktik™ Thermal Cycler (Thermo Scientific, Massachusetts, ABD) using the following condition: 2 min initial denaturation step at 95 °C followed by 35 cycles of 30 s at 94 °C 30 s 60 °C and 30 s 72 °C and final extension of 5 min at 72 °C. All PCR amplifications with ST-specific primers were carried out Arktik™ Thermal Cycler (Thermo Scientific, Massachusetts, ABD) using the following condition: 2 min initial denaturation step at 95 °C followed by 35 cycles of 30 s at 94 °C 30 s 60 °C and 1 min 72 °C and final extension of 7 min at 72 °C. All PCR products were separated by %2 agarose gel electrophoresis stained by SafeView Classic (Applied Biological Materials Inc., Richmond, Canada) and visualized under Alpha Imager HP (Alpha Innotech).
Statistical Analysis
Statistical analyses of the collected data were performed using Statistical Package for Social Science (SPSS) 22. The level of significance of the relationship between the incidence of Blastocystis and the demographic characteristics, clinical findings, location of cancer was determined by the chi-square test or Fischer’s exact test. For discrete and continuous variables, descriptive statistics (mean, standard deviation, median, minimum value, maximum value, and percentile) were performed. A value of p < 0.05 was considered as statistically significant.
Results
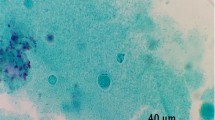
A total of 201 stool samples were enrolled from the cancer patients who were 123 males (61.19%) and 78 females (38.8%) with a mean age (± standard deviation) of 55.44 ± 12.07 years (range 27–84 years). In our study, 48 (23.8%) of the stool samples were found positive by native-Lugol and trichrome staining (Fig. 2). All the microscopy-positive stool samples were examined using barcoding PCR and amplification of expected size (~ 600 bp) was observed in 29 (60.4%) samples. Some of the Blastocystis isolates in the present study are shown in Fig. 3. These samples that were found to be positive by barcoding PCR method were screened with the PCR using specific seven types of STS primers to identify subtypes and amplification with subtype-specific primers was observed in 15 (51.7%) of 29 isolates. The remaining 14 isolates found to be positive for barcoding PCR could not be subtyped. The most common subtype was ST3 (n = 6, 40%) followed by ST2 (n = 5, 33%) and ST1 (n = 3, 20%), respectively. In addition, one patient revealed combined infections with ST1/ST2 (n = 1, 6%) and ST4-7 could not be detected in our study. (Fig. 4).
Partial SSU rRNA gene of Blastocystis were electrophoresed on 2% agarose gel. All samples amplified 600 bp amplicon. (Lane 1) shows 100 bp DNA ladder; (Lane 2) represents positive control; (Lanes 3, 4, 7, 8, 9, 10, 11, 12, 13, 14, 18, 19, 21, 22, 24, 26) represent positive results for Blastocystis; (Lanes 5, 6, 15, 16, 17, 20, 23, 25, 27) represent negative results for Blastocystis; (Lane 28) represents negative control
Isolated of Blastocystis were electrophoresed on 2% agarose gel. All samples amplified by the sequence-tagged site (STS) primers. Lanes 1, 100 bp DNA ladder; Lane 2, 7, 11 positive control; Lanes 3–6, subtype 1 (351 bp); Lanes 8–10, subtype 2 (704 bp); Lanes 12–13 subtype 3 (526 bp); Lane 14 negative control
Demographic characteristics of cancer patients with Blastocystis infection are presented in Table 2 and no significant difference was found (p > 0.05). When Blastocystis infected cancer patients were analyzed in terms of symptomatology, similarly, there was no statistically significant difference (Table 3) (p > 0.05). In our study, due to an insufficient number of subtypes, statistical analysis could not be performed between subtypes and symptoms. The incidence of Blastocystis infection according to cancer location is presented in Table 4. The gastrointestinal tract, breast, respiratory tract cancers, and urogenital tract cancers accounted for the majority (90%) of the studied patients. In the present study, the rate of Blastocystis infection was higher in urogenital tract cancers(32%) as compared to other types.
Discussion
Cancer is the second leading cause of death globally and there were 18 million cancer cases and 9.6 million cancer deaths worldwide in 2018. It is estimated that by 2040 there will be 254,179 new cancer cases and 22,609 cancer deaths in Turkey [28]. Protozoa, such as Cryptosporidium parvum, Microsporidia (Encephalitozoon spp., Enterocytozoon bieneusi)., Cyclospora cayetanensis, Isospora belli, and Giardia intestinalis are opportunistic microorganisms that cause serious manifestation in immunosuppressed patients such as cancer, HIV/AIDS patients, and transplant recipients [23].
Recently, the number of studies reporting the prevalence of Blastocystis infection in immunocompromised individuals has increased [29]. In addition, Blastocystis' impact on immunosuppressed patients has been rebated in the recent years, primarily due to its unknown pathogenicity and incidence frequency [23]. In the present study, Blastocystis was investigated among cancer patients using two different methods: DM and PCR based diagnosis. Although Blastocystis was seen in 48 of the samples by direct microscopy in our study, 29 (60.4%) of these samples by barcoding PCR gave amplicon of the expected size. A negative result was obtained in 15 isolates (31.3%). This can be attributed to the fact that some living or non-living factors such as fungi, macrophages, neutrophils, and Cyclospora sp. or fat globules in the stool cause false positivity in the DM method [30, 31]. Furthermore, the lack of consensus on which Blastocystis to be reported above which threshold value (> 5 cells, in a 40X objective area) also reduces the reliability of the results obtained from direct microscopy [11]. Some studies have also reported false-negative results with PCR due to inhibitors in the feces [32, 33]. It is also considered that the sensitivity of the PCR method is greatly influenced by the DNA extraction procedure [34].
In 2011, Yoshikawa et al. used different commercial DNA extraction kits in stool samples and reported that their detection sensitivity was different [35, 36]. The DNA isolation kit we used in our study contains an inhibitor removal column that absorbs PCR inhibitors. For this reason, the DNA isolation kit we use is thought to reduce or eliminate negative results from PCR inhibition. Based on this, in our study, it is thought that some patients who were found positive with the DM method were found to be negative with PCR due to false positives in the DM method. However, the BhRDr-RD5 primer pair used in the barcoding PCR method is not completely specific to Blastocystis since they amplify Blastocystis SSU-rDNA and SSU-rDNA from other eukaryotes, especially fungi, in the absence of Blastocystis. If these primers are used to screen fecal DNAs for Blastocystis, a certain false-positive rate may be predicted, and sequencing should often confirm positivity [37]. In sum, the whole prevalence of Blastocystis with barcoding PCR in our study was 14.4%, regardless of subtypes. This result is in accord with the prior studies declared a prevalence of 13–16% in cancer patients [24, 25]. Other studies in cancer patients in different regions of Turkey and the world, the prevalence ranged from 7.1–21.1% [7, 23, 38,39,40]. According to the results of the aforementioned studies, the rates are not similar in different regions. This may be due to weather conditions, public health, food, and different cultural habits, demographic factors as well as differences in diagnostic tests [41].
The Blastocystis subtype distribution in 29 isolates were listed as ST3 (6 samples 40%), ST2 (5 samples 33%), ST1 (3 samples 20%). Besides, more than one subtype (ST1 + ST2 6.6%) was detected in one sample. A thorough evaluation of the studies in the literature demonstrated that ST1, ST2, ST3, and ST4 constitute 90% of the Blastocystis infections in humans and, the most common subtype was ST3 [33, 42, 43]. Similar to the studies in the literature, the subtype distribution of our study consisted of ST1, ST2, and ST3. Some studies on Blastocystis subtype distribution in cancer patients have shown that ST3 is more common [7, 23, 40]. Consistent with these studies reported in cancer patients, the most common subtype (n = 6, 40%) was ST3 in our study. In line with the studies in the literature, Poirier et al. (2011) reported in their study that ST4 was the most common subtype followed by ST3 and ST7 [25]. Furthermore, Mohamed RT et al. (2017) reported that that ST2 was the most common subtype, followed by ST1 and ST5 [44]. However, ST4, ST5, ST6, and ST7 subtypes were not detected in our research though they were reported in the previous studies [7, 19, 32, 45].
One of the significant results is that no subtype could be detected in 14 samples (48.3%) which were found to be positive with barcoding PCR. In Turkey, this rate was found to be 27.9% by Ertug et al. [1] 35% by Korkmaz et al. [32] and 12% by Yersal et al. [7]. However, it was reported to be 3% by Yoshikawa et al. [27] 4% by Li et al. [46] and 42% by Zhan et al. [47] in different parts of the world. The researchers pointed out the PCR inhibitors in the stool as the most important factor in creating this condition [32]. Furthermore, it was thought that in the presence of new subtypes, STS primers may be due to disability [32, 37]. DNA sequence analysis is a more useful method in determining whether there is a different subtype in samples that cannot be subtyped [4]. However, DNA sequence analysis could not be performed in our study due to financial insufficiencies.
Similar to the results obtained in cancer patients by Zhang et al. [39], we found no significant correlation between the rate of infection and the demographic factors (age, gender, residence et al.) and also the rate of infection was higher in women and patients from rural in both studies. However, according to another study in cancer patients, it has been shown that infection rates are remarkably higher in men and patients in urban areas [7]. According to the survey data in our study, it was found that the patients with Blastocystis had symptoms of abdominal pain, nausea, itching, vomiting, bloating, constipation, and diarrhea, respectively. In another study conducted in patients in the oncology service, similar to our study, it was reported that abdominal pain, bloating and constipation observed more frequently in cases with Blastocystis [7]. However, in contrast to our study, Zhang et al. showed that Blastocystis was found more frequently in cancer patients with diarrhea [39]. It is accepted that the reason for this difference in clinical findings in people with Blastocystis depends on many factors such as genotype of infection, microbiota, host immune response, Blastocystis density, and other concomitant infections [11, 48, 49].
Similar to the previous reports, statistical analysis could not be performed between subtypes and symptoms due to the insufficient number of cases whose subtypes were determined in our study [7, 50]. Also, there was no significant difference between cancer type and the rate of Blastocystis infection, which was supported by previous studies [39, 44, 51]. However, Yersal et al. [7]. reported that the rate of infection in patients with lung cancer was significantly higher than in other types of cancer [41]. Besides, in this study, the highest and lowest Blastocystis infection rate was reported in urogenital system (32%) and hematological system (6.7%) cancer patients, respectively.
Conclusion
In summary, we showed that cancer patients in the present study had a Blastocystis prevalence of 14.4% and that ST3 was the most predominant subtype. In addition, the present study provides the first subtype distribution of Blastocystis detected in cancer patients residing in our region. The absence of a relationship between Blastocystis infection and any gastrointestinal symptoms supports the idea that Blastocystis infections are asymptomatic and that this protozoan parasite can exist as normal flora. However, molecular-based studies in cancer patients are very limited. Therefore, to determine Blastocystis subtype profile in cancer patients and to fully elucidate the relationship between subtypes and pathogenicity, more studies should be done in different regions of Turkey.
References
Ertug S, Malatyali E, Ertabaklar H, Caliskan SO, Bozdogan B (2015) Subtype distribution of Blastocystis isolates and evaluation of clinical symptoms detected in Aydin province. Turkey Mikrobiyol Bul 49(1):98–104. https://doi.org/10.5578/mb.8532
Denoeud F, Roussel M, Noel B, Wawrzyniak I, Da Silva C, Diogon M, Viscogliosi E, Brochier-Armanet C, Couloux A, Poulain J (2011) Genome sequence of the stramenopile Blastocystis, a human anaerobic parasite. Genome Biol 12(3):R29. https://doi.org/10.1186/gb-2011-12-3-r29
Aydin M, Yazici M, Demirkazik M, Koltas IS, Cikman A, Gulhan B, Duran T, Yilmaz A, Kara M (2019) Molecular characterization and subtyping of Blastocystis in urticarial patients in Turkey. Asian Pac J Trop Dis 12(10):450. https://doi.org/10.4103/1995-7645.269905
Cakir F, Cicek M, Yildirim IH (2019) Determination the subtypes of Blastocystis sp. and evaluate the effect of these subtypes on pathogenicity. Acta Parasitol 64(1):7–12. https://doi.org/10.2478/s11686-018-00002-y
Poirier P, Wawrzyniak I, Vivarès CP, Delbac F, El Alaoui H (2012) New insights into Blastocystis spp.: a potential link with irritable bowel syndrome. PLoS Pathog 8(3):e1002545. https://doi.org/10.1371/journal.ppat.1002545
El Safadi D, Cian A, Nourrisson C, Pereira B, Morelle C, Bastien P, Bellanger A-P, Botterel F, Candolfi E, Desoubeaux G (2016) Prevalence, risk factors for infection and subtype distribution of the intestinal parasite Blastocystis sp. from a large-scale multi-center study in France. BMC Infect Dis 16(1):451. https://doi.org/10.1186/s12879-016-1776-8
Yersal O, Malatyali E, Ertabaklar H, Oktay E, Barutca S, Ertug S (2016) Blastocystis subtypes in cancer patients: analysis of possible risk factors and clinical characteristics. Parasitol Int 65(6):792–796. https://doi.org/10.1016/j.parint.2016.02.010
Abdulsalam AM, Ithoi I, Al-Mekhlafi HM, Al-Mekhlafi AM, Ahmed A, Surin J (2013) Subtype distribution of Blastocystis isolates in Sebha. Libya PLoS One 8(12):e84372. https://doi.org/10.1371/journal.pone.0084372
El Safadi D, Gaayeb L, Meloni D, Cian A, Poirier P, Wawrzyniak I, Delbac F, Dabboussi F, Delhaes L, Seck M (2014) Children of Senegal River Basin show the highest prevalence of Blastocystis sp. ever observed worldwide. BMC Infect Dis 14(1):164. https://doi.org/10.1186/1471-2334-14-164
Eassa SM, Masry S (2016) Blastocystis hominis among immunocompromised and immunocompetent children in Alexandria. Egypt Ann Clin Lab Res 4(2):92. https://doi.org/10.21767/2386-5180.100092
Stensvold CR, Clark CG (2016) Current status of Blastocystis: a personal view. Parasitol Int 65(6):763–771. https://doi.org/10.1016/j.parint.2016.05.015
Stensvold CR (2013) Blastocystis: genetic diversity and molecular methods for diagnosis and epidemiology. Trop Parasitol 3(1):26. https://doi.org/10.4103/2229-5070.113896
Stensvold CR, Clark CG (2020) Pre-empting pandora’s box: Blastocystis subtypes revisited. Trends Parasitol 36(3):229–232. https://doi.org/10.1016/j.pt.2019.12.009
Maloney JG, Molokin A, da Cunha MJR, Cury MC, Santin M (2020) Blastocystis subtype distribution in domestic and captive wild bird species from Brazil using next generation amplicon sequencing. Parasite Epidemiol Control 9:e00138. https://doi.org/10.1016/j.parepi.2020.e00138
Lhotská Z, Jirků M, Hložková O, Brožová K, Jirsová D, Stensvold CR, Kolísko M, Jirků Pomajbíková K (2020) A Study on the prevalence and subtype diversity of the intestinal protist Blastocystis sp. in a gut-healthy human population in the Czech Republic. Front Cell Infect Microbiol. https://doi.org/10.3389/fcimb.2020.544335
Ramírez JD, Sánchez A, Hernández C, Flórez C, Bernal MC, Giraldo JC, Reyes P, López MC, García L, Cooper PJ (2016) Geographic distribution of human Blastocystis subtypes in South America. Infect Genet Evol 41:32–35. https://doi.org/10.1016/j.meegid.2016.03.017
Yoshikawa H, Tokoro M, Nagamoto T, Arayama S, Asih PB, Rozi IE, Syafruddin D (2016) Molecular survey of Blastocystis sp. from humans and associated animals in an Indonesian community with poor hygiene. Parasitol Int 65(6):780–784. https://doi.org/10.1016/j.parint.2016.03.010
Villanueva-Garcia C, Gordillo-Chavez EJ, Lopez-Escamilla E, Rendon-Franco E, Muñoz-Garcia CI, Gama L, Martinez-Flores WA, Gonzalez-Rodriguez N, Romero-Valdovinos M, Diaz-Lopez H (2017) Clarifying the cryptic host specificity of Blastocystis spp. isolates from Alouatta palliata and A. pigra howler monkeys. PLoS ONE 12(1):e0169637. https://doi.org/10.1371/journal.pone.0169637
Dagci H, Özgür K, Demirel M, Mandiracioglu A, Aydemir S, Ulas S, Aldert B, Tom V (2014) Epidemiological and diagnostic features of Blastocystis infection in symptomatic patients in Izmir province. Turkey Iran J Parasitol 9(4):519
Yazar S, Kuk S, Miman Ö, Saygı G (2016) SAYGI’nın Temel Tıbbi PARAZİTOLOJİ’si, vol 1. Erciyes Üniversitesi, Kayseri
WHO (2011) Guidelines for Drinking-water Quality. Water Sanitation Health, FOURTH edn.,
Mehlhorn H, Tan KS, Yoshikawa H (2012) Blastocystis: pathogen or passenger?: an evaluation of 101 years of research, vol 4. Springer Science & Business Media, London
Tan T, Ong S, Suresh K (2009) Genetic variability of Blastocystis sp. isolates obtained from cancer and HIV/AIDS patients. Parasitol. Res 105(5):1283. https://doi.org/10.1007/s00436-009-1551-5
Tasova Y, Sahin B, Koltas S, Paydas S (2000) Clinical significance and frequency of Blastocystis hominis in Turkish patients with hematological malignancy. Acta Med Okayama 54(3):133–136. https://doi.org/10.18926/AMO/32298
Poirier P, Wawrzyniak I, Albert A, El Alaoui H, Delbac F, Livrelli V (2011) Development and evaluation of a real-time PCR assay for detection and quantification of Blastocystis parasites in human stool samples: prospective study of patients with hematological malignancies. J Clin Microbiol 49(3):975–983. https://doi.org/10.1128/JCM.01392-10
Chandramathi S, Suresh K, Anita ZB, Kuppusamy UR (2012) Infections of Blastocystis hominis and microsporidia in cancer patients: are they opportunistic? Trans R Soc Trop Med Hyg 106(4):267–269. https://doi.org/10.1016/j.trstmh.2011.12.008
Yoshikawa H, Wu Z, Kimata I, Iseki M, Ali IKM, Hossain MB, Zaman V, Haque R, Takahashi Y (2004) Polymerase chain reaction-based genotype classification among human Blastocystis hominis populations isolated from different countries. Parasitol Res 92(1):22–29. https://doi.org/10.1007/s00436-003-0995-2
Tatar M, Tatar F (2010) Colorectal cancer in Turkey: current situation and challenges for the future. Eur J Health Econ 10(Suppl 1):S99-105. https://doi.org/10.1007/s10198-009-0197-7
Tasić N, Milenković T, Bujić V, Zdravković D, Tasić A (2016) Blastocystis hominis: a mysterious and commonly disregarded parasite. Facta Univ Med Biol. https://doi.org/10.22190/FUMB161027001T
Wawrzyniak I, Poirier P, Viscogliosi E, Dionigia M, Texier C, Delbac F, Alaoui HE (2013) Blastocystis, an unrecognized parasite: an overview of pathogenesis and diagnosis. Ther Adv Infect Dis 1(5):167–178. https://doi.org/10.1177/2049936113504754
Parija SC, Jeremiah S (2013) Blastocystis: taxonomy, biology and virulence. Trop Parasitol 3(1):17. https://doi.org/10.4103/2229-5070.113894
Adiyaman Korkmaz G, Dogruman Al F, Mumcuoglu I (2015) Diski örneklerinde Blastocystis spp. varliginin mikroskobik, kültür ve moleküler yöntemlerle arastirilmasi. Mikrobiyol Bul. https://doi.org/10.5578/mb.8439
Souppart L, Sanciu G, Cian A, Wawrzyniak I, Delbac F, Capron M, Dei-Cas E, Boorom K, Delhaes L, Viscogliosi E (2009) Molecular epidemiology of human Blastocystis isolates in France. Parasitol Res 105(2):413. https://doi.org/10.1007/s00436-009-1398-9
Parkar U, Traub R, Kumar S, Mungthin M, Vitali S, Leelayoova S, Morris K, Thompson R (2007) Direct characterization of Blastocystis from faeces by PCR and evidence of zoonotic potential. Parasitology 134(3):359–367. https://doi.org/10.1017/S0031182006001582
Santos HJ, Rivera WL (2013) Comparison of direct fecal smear microscopy, culture, and polymerase chain reaction for the detection of Blastocystis sp. in human stool samples. Asian Pac J Trop Med. 6(10):780–784. https://doi.org/10.1016/S1995-7645(13)60138-8
Yoshikawa H, Dogruman-Ai F, Turk S, Kustimur S, Balaban N, Sultan N (2011) Evaluation of DNA extraction kits for molecular diagnosis of human Blastocystis subtypes from fecal samples. Parasitol Res 109(4):1045–1050. https://doi.org/10.1007/s00436-011-2342-3
Stensvold CR (2013) Comparison of sequencing (barcode region) and sequence-tagged-site PCR for Blastocystis subtyping. J Clin Microbiol 51(1):190–194. https://doi.org/10.1128/jcm.02541-12
Koltas I, Ozcan K, Tanrtverdi S, Paydas S, Paydas S, Baslamtslt F (1999) The prevalence of Blastocystis hominis in immunosupressed patients. Ann Med Sci 8(2):117–119
Zhang W, Ren G, Zhao W, Yang Z, Shen Y, Sun Y, Liu A, Cao J (2017) Genotyping of Enterocytozoon bieneusi and subtyping of Blastocystis in cancer patients: relationship to diarrhea and assessment of zoonotic transmission. Front Microbiol 8:1835. https://doi.org/10.3389/fmicb.2017.01835
Kumarasamy V, Roslani AC, Rani KU, Govind SK (2014) Advantage of using colonic washouts for Blastocystis detection in colorectal cancer patients. Parasit Vectors 7(1):162. https://doi.org/10.1186/1756-3305-7-162
Shaker D, Anvari D, Hosseini SA, Fakhar M, Mardani A, Hezarjaribi HZ, Gholami S, Gholami S (2019) Frequency and genetic diversity of Blastocystis subtypes among patients attending to health centers in Mazandaran, northern Iran. J Parasit Dis. https://doi.org/10.1007/s12639-019-01123-5
Özyurt M, Kurt Ö, Mølbak K, Nielsen HV, Haznedaroglu T, Stensvold CR (2008) Molecular epidemiology of Blastocystis infections in Turkey. Parasitol Int 57(3):300–306. https://doi.org/10.1016/j.parint.2008.01.004
Alfellani MA, Stensvold CR, Vidal-Lapiedra A, Onuoha ESU, Fagbenro-Beyioku AF, Clark CG (2013) Variable geographic distribution of Blastocystis subtypes and its potential implications. Acta Trop 126(1):11–18. https://doi.org/10.1016/j.actatropica.2012.12.011
Mohamed AM, Ahmed MA, Ahmed SA, Al-Semany SA, Alghamdi SS, Zaglool DA (2017) Predominance and association risk of Blastocystis hominis subtype I in colorectal cancer: a case control study. Infect Agent Cancer 12(1):21. https://doi.org/10.1186/s13027-017-0131-z
Boral ÖB, Payçu DGÇ, Akgül A, İşsever H (2017) Blastocystis spp. saptanan 50 semptomatik hastada subtip dağilimin saptanmasi. KOU Sag Bil Derg 3(1):6–10. https://doi.org/10.30934/KUSBED.359174
Li L-H, Zhou X-N, Du Z-W, Wang X-Z, Wang L-B, Jiang J-Y, Yoshikawa H, Steinmann P, Utzinger J, Wu Z (2007) Molecular epidemiology of human Blastocystis in a village in Yunnan province. China Parasitol Int 56(4):281–286. https://doi.org/10.1016/j.parint.2007.06.001
Zhan T, Liu T, Shi H, He S, Yan H, Liu D (2014) PCR-based genotype classification of Blastocystis hominis isolates from college students of Guangxi. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi 32(3):209–211
Tan KS, Mirza H, Teo JD, Wu B, MacAry PA (2010) Current views on the clinical relevance of Blastocystis spp. Curr Infect Dis Rep 12(1):28–35. https://doi.org/10.1007/s11908-009-0073-8
Andersen LOB, Stensvold CR (2016) Blastocystis in health and disease: are we moving from a clinical to a public health perspective? J Clin Microbiol 54(3):524–528. https://doi.org/10.1128/JCM.02520-15
Dogan N, Aydin M, Tuzemen NU, Dinleyici EC, Oguz I, Dogruman-Al F (2017) Subtype distribution of Blastocystis spp. isolated from children in Eskisehir. Turkey. Parasitol Int 66(1):948–951. https://doi.org/10.1016/j.parint.2016.10.008
Asghari A, Zare M, Hatam G, Shahabi S, Gholizadeh F, Motazedian M (2020) Molecular identification and subtypes distribution of Blastocystis sp. isolated from children and adolescent with cancer in Iran: evaluation of possible risk factors and clinical features. Acta Parasitol. https://doi.org/10.2478/s11686-020-00186-2
Acknowledgements
We are grateful to Adnan Yüksel Gürüz and Mert Döşkaya for help in the field and laboratory work.
Funding
This work was funded by scientific and technological research council of Turkey (TUBITAK) (Grant 315S262).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical approval
The present study was approved by Fırat University Clinical Research Ethics Committee ( Protocol No: 2017/14-03).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mülayim, S., Aykur, M., Dağcı, H. et al. Investigation of Isolated Blastocystis Subtypes from Cancer Patients in Turkey. Acta Parasit. 66, 584–592 (2021). https://doi.org/10.1007/s11686-020-00322-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11686-020-00322-y